Back to Journals » Medical Devices: Evidence and Research » Volume 13
Auscultation System for Acquisition of Vascular Sounds – Towards Sound-Based Monitoring of the Carotid Artery
Authors Sühn T , Spiller M , Salvi R , Hellwig S, Boese A , Illanes A , Friebe M
Received 27 June 2020
Accepted for publication 23 September 2020
Published 30 October 2020 Volume 2020:13 Pages 349—364
DOI https://doi.org/10.2147/MDER.S268057
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Thomas Sühn,1 Moritz Spiller,1 Rutuja Salvi,2 Stefan Hellwig,2 Axel Boese,1 Alfredo Illanes,1 Michael Friebe1
1INKA - Innovation Laboratory for Image Guided Therapy, Medizinische Fakultät, Otto-Von-Guericke-Universität, Magdeburg, Sachsen-Anhalt, Germany; 2IDTM GmbH, Castrop-Rauxel, Nordrhein-Westfalen, Germany
Correspondence: Thomas Sühn Email [email protected]
Introduction: Atherosclerotic diseases of the carotid are a primary cause of cerebrovascular events such as stroke. For the diagnosis and monitoring angiography, ultrasound- or magnetic resonance-based imaging is used which requires costly hardware. In contrast, the auscultation of carotid sounds and screening for bruits – audible patterns related to turbulent blood flow – is a simple examination with comparably little technical demands. It can indicate atherosclerotic diseases and justify further diagnostics but is currently subjective and examiner dependent.
Methods: We propose an easy-to-use computer-assisted auscultation system for a stable and reproducible acquisition of vascular sounds of the carotid. A dedicated skin-transducer-interface was incorporated into a handheld device. The interface comprises two bell-shaped structures, one with additional acoustic membrane, to ensure defined skin contact and a stable propagation path of the sound. The device is connected wirelessly to a desktop application allowing real-time visualization, assessment of signal quality and input of supplementary information along with storage of recordings in a database. An experimental study with 5 healthy subjects was conducted to evaluate usability and stability of the device. Five recordings per carotid served as data basis for a wavelet-based analysis of the stability of spectral characteristics of the recordings.
Results: The energy distribution of the wavelet-based stationary spectra proved stable for measurements of a particular carotid with the majority of the energy located between 3 and 40 Hz. Different spectral properties of the carotids of one individual indicate the presence of sound characteristics linked to the particular vessel. User-dependent parameters such as variations of the applied contact pressure appeared to have minor influence on the general stability.
Conclusion: The system provides a platform for reproducible carotid auscultation and the creation of a database of pathological vascular sounds, which is a prerequisite to investigate sound-based vascular monitoring.
Keywords: stenosis, screening, computer-assisted auscultation, vascular disease, bruit, long-term monitoring, heart sound
Introduction
According to data from the World Health Organization (WHO) mortality database, cerebrovascular diseases (CVD) account for a total number of 1.0 million deaths per year in Europe with around 85% of all CVD cases represented by ischemic stroke (IS).1 A cerebrovascular ischemic event occurs if atherosclerotic stenosis or abrupt occlusion of a large vessel or a cerebral artery occludes the arterial blood supply to parts of the brain.2 This event can either be temporary, yielding a transient ischemic attack, or permanent resulting in an IS. Especially atherosclerosis of the common carotid arteries is an important cause of such events.3 The two common carotid arteries are present on the left and right side of the neck and supply the head and brain with oxygenated blood. Major medical conditions associated with the carotid arteries such as dissection and atherosclerosis can lead to carotid stenosis (CS) and are specified by WHO classification “ICD-10-CM Diagnosis Code 65.2 - Occlusion and stenosis of carotid artery”.4 A stenosis evolves when fibrous material such as fat, cholesterol or calcium deposits on the inner layer of the artery. The caused narrowing alters the blood flow in the particular vessel and potentially reduces the blood supply of the associated anatomic area, eventually causing an IS.5 Diagnosis and monitoring of atherosclerotic conditions of the carotid is currently based on non-invasive imaging technology such as carotid ultrasonography (US), computed tomography angiography and magnetic resonance angiography, all of which are performed in an ambulatory setting and are indicated in symptomatic and high-risk patients. The invasive catheter angiography remains reserved for patients for whom imaging is contraindicated or with inconclusive initial evaluation results due to its inherent risk of complications. While these diagnostic methods allow an accurate determination of the severity of the stenosis and the examination of anatomical details, they come with major drawbacks. Bulky and expensive hardware is required that needs to be operated by specifically trained and experienced physicians and staff in a clinical setup. Further, some of the imaging modalities employ ionizing radiation and/or nephrotoxic contrast agent.5–7
In contrast to that, auscultation – listening to internal sounds of the body by means of a stethoscope – is an affordable and widely available diagnostic method in clinical practice. It requires substantial skill, experience, and a well-trained sense of hearing, while the assessment of the audible body sounds is difficult to quantify and with that remains, to a large extent, subjective. However, screening of carotid vascular sounds for bruits – audible sounds related to turbulent blood flow in the vessel – is recommended as a substantial part of the routine physical examination of patients with risk factors for vascular disease.8,9 Those sounds can be associated with vascular pathologies or changes of vessel status and range in the audible frequency band between 20 … 500 Hz.10–12 The majority of studies and common guidelines on the management of atherosclerotic vascular disease agree on the value of carotid bruit sounds as an indicator for CS and a justification for further diagnostic imaging.12–14 In contrast to today’s simple evaluation of the presence of such bruits, recent developments in sensor technology, signal processing and machine learning potentially allow a more specific assessment of the sounds along with associated pathology related characteristics. Periodically performed auscultation measurements, preferably in a point-of-care or home-based fashion could provide a cost-effective and simple alternative to the previously mentioned expensive diagnostic imaging. Further, it might be used as baseline parameter for the implementation of personalized management strategies or the continuous monitoring of progressive vascular diseases. It has been shown previously that diagnostically relevant parameters related to the blood flow inside the carotid artery such as the pressure waveform can be derived from the acquired vascular sounds.15 To enable further investigations regarding clinically relevant information derived from the vascular sounds, a reproducible and stable signal acquisition is required to create high-quality curated datasets.
While computer assisted auscultation systems (CAAS) are commonly used in cardiovascular and pulmonary applications,16,17 such systems are not necessarily suitable for the auscultation of vascular sounds. The sensor setup, signal conditioning strategy, frequency bandwidth, applied filters and quantization are tailored toward the particular application. However, CAAS come with substantial advantages such as integrated signal preprocessing, storage and administration of recordings in a database, automated analysis and visualization of results and the subsequent documentation and sharing of digital records.18,19
The aim of this work was to introduce a dedicated CAAS to address the specific needs of vascular auscultation at the neck. This system is designated as the first step toward the investigation of bruit sounds for the diagnosis and monitoring of atherosclerotic disease of the carotid. A reproducible and simple way to perform signal acquisition is a prerequisite to allow the creation of a database of vascular sounds from healthy and pathological subjects in a clinical setting. This database is needed to identify end explore the characteristics of the sound signal that can be linked to the blood flow inside the vessel. Further, it facilitates the development of algorithms for the automatic detection of bruit sounds and the challenging separation from intrinsic sounds of the circulatory system.12,20 Stability and reproducibility of the signal acquisition with the proposed CAAS was evaluated in a first experimental study comprising 5 voluntary subjects.
Materials and Methods
Hardware Design
The proposed AS comprises the following three main units: 1) a skin-transducer-interface as part of an auscultation device, 2) a handheld auscultation device and 3) a proprietary desktop application. The skin-transducer-interface has a crucial role in the acquisition of carotid sounds, converting the mechanical deflection of the skin into an electrical signal. For the timebeing it is embedded into the handheld device, which allows a convenient and reproducible auscultation of the carotid via control elements. Subsequently, the acquired signal is transmitted wirelessly in real-time to a custom-built desktop application. The application acts as the user interface and provides signal visualization, quality assessment, manual entry of subject and examination related information as well as administration and retrieval of auscultation data in a convenient way. Figure 1 depicts the system components of the auscultation device (left) and the desktop application (right) as parts of the CAAS with their respective interfaces. The three main components of the CAAS were continuously tested, evaluated, and improved throughout the whole development process.
Skin-Transducer-Interface Design
The acquisition of natural body sounds in contact with the skin imposes certain challenges for the transducer interface. As the analogous input signal, the mechanical deflection of the skin originates from a dilatation of the carotid artery due to the blood flow and related pressure change inside the vessel. This dilatation propagates as sound wave through the surrounding compressible tissue, causing the deflection at the skin surface. A microphone is used as transducer to convert the mechanical sound energy into electrical energy and subsequently into a digital signal. Two different transducer-interface setups can be distinguished. Conventional contact microphones come with two major drawbacks for the acquisition of natural body sounds from the skin. The microphone needs to be in firm contact with the skin surface, which is difficult to achieve due to the nature of the skin. Further, the mechanical load and thus weight of the transducer needs to be minimal to allow an easy displacement and with that a high sensitivity.
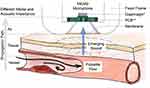
In contrast, air-coupled microphones use air as transmission medium for the propagation of the sound wave. Figure 2 depicts the generating mechanism, acoustic path and reception of vascular sounds with an air-coupled skin transducer interface. While such an interface ensures a minimal load to the skin, it results in additional challenges such as an increased susceptibility to environmental noise. Further it requires a fixed reference frame to ensure a defined path and confined volume for the propagation through the air. The deflection of a pressure-sensitive diaphragm of an electrostatic microphone (eg, electret microphone) subsequently induces a voltage change which can be converted into a digital signal. Limiting factor of such a skin transducer setup is the mismatch between the acoustic impedance of the two transmitting media tissue and air. Thus, the reflection of the sound at the interface causes only a fraction of the sound energy to be transmitted to the transducer. To mitigate those losses an acoustic membrane can be used to confine the air-volume for the propagation of sound and match the acoustic impedances. In addition, a membrane increases the contact area through which a deflection of the skin can cause sensible sound waves for the transducer. As a tradeoff, the mechanical impedance of the membrane causes a load to the skin. A combination of two air-coupled skin-transducer interfaces with one utilizing an acoustic membrane is used in the proposed CAAS to mitigate the aforementioned drawbacks.
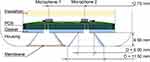
To determine the appropriate physical parameters (eg, shape, surface area, transmission path, acoustic membrane) of the interface, a preliminary phantom study was conducted for different configurations.21,22 The comparison of various bell-shaped structures similar to an analog stethoscope and with and without incorporated acoustic membrane resulted in the design shown in Figure 3. The proposed interface consists of two bell-shaped structures of similar dimensions located next to each other. They act as reference frame to ensure a defined propagation path and air volume between skin and transducer. One incorporates an additional and commercially available stethoscope membrane (3M Company, Littmann Stethoscope Membrane, Saint Paul, Minnesota, USA) to match the acoustic impedance. This complementary setup will be used to determine the influence of the membrane on signal quality, susceptibility to environmental noise and long-term stability in a realistic auscultation scenario.
A custom built printed circuit board (PCB) with two bottom-port electret-type micro-electro-mechanical system (MEMS) microphones (Knowles Electronics LLC, SPH0645LM4HB, Itasca, Illinois, USA) is mounted on the opposite site of the interface. It is firmly attached to the structure with double-sided adherent foam tape which acts as acoustically opaque gasket to seal to the PCB and prevent noise from passing to the acoustic path. A layer of acoustic damping material was added to shield the microphone from additional environmental noise. The used microphones come with an internal gain amplifier and an 18-bit sigma-delta analog-digital-converter, providing a digital output signal via I2S (inter integrated circuit sound) codec. This communication codec allows a simultaneous and synchronized recording with two microphones. The sampling frequency is set to 16 kHz and the acquired signals are stored in stereo.wav file-format.
Auscultation Device
The purpose of the handheld auscultation device is the convenient and reproducible acquisition of vascular sounds for subsequent analysis. It needs to allow for simple handling in day-to-day operation by non-expert and non-technical users such as a patient or a nurse in a general practitioner’s medical office. The proposed design comprises a single-board computer as host system (Raspberry Pi Foundation, Raspberry Pi Zero W, Cambridge, United Kingdom) and a lithium-ion polymer battery (Shenzhen Pkcell Battery Co., Ltd., LIPO804257 2000mAh, Shenzhen, China) with suitable power management board (Pimoroni Ltd., Lipo Shim, Sheffield, United Kingdom) to allow wireless and mobile use. Two custom-designed electronic circuits and PCBs were built for the control interface and the previously described transducers. The transducer PCB in conjunction with the mechanical interface to the skin acts as the distinct sensing unit. It can be connected and detached easily to the handheld device to allow the investigation of different interface configurations (eg, surface area size, type of acoustic membrane) without changing the general setup or operation of the device. Further, it allows the use of the sensing unit as a disposable component to ensure hygienic requirements if necessary. The control interface was limited to a total of two LEDs and two tactile switches as a tradeoff between convenient handling of the device and a small form factor and complexity.
To power on the device, the battery is connected to the power management circuit. After the boot up process it automatically connects to the preset local Wi-Fi network hosted by the device running the desktop application and enters the idle state. The button switches (B1/B2) are used to enter the visualization state for the real-time visualization of the acquired audio signal. This state allows the user to manually select and verify an adequate position of the sensing unit at the neck, to ensure high signal quality and amplitude. In addition, the streamed audio signal can be played back to listen to the carotid sound in real-time, to confirm the position and manually search for existing bruit sounds. When a good location or bruit is confirmed B1 starts the actual record state. The audio stream is then stored in parallel in the database linked to the application and in the auscultation device’s local file system in wave file-format. Figure 4 summarizes the usage of the control interface and the different states of the auscultation process. The control software of the device is implemented in Python 3.6 (Python Software Foundation, Python 3.6, Wilmington, Delaware, USA) and automatically updated via a remote git repository as part of the desktop application.
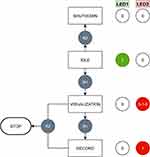 |
Figure 4 Control states of the auscultation process and respective control elements (button switches B1, B2) and feedback interface (LED: 0 = off, 1 = on, 1-0-1 = blinking). |
The electric circuits and PCBs as well as the skin-transducer-interface and the mechanical housing of the auscultation device were designed, manufactured and assembled in the on-site prototyping lab. The mechanical components were additionally manufactured (Formlabs GmbH, Form2, Berlin, Germany).
Desktop Application
The desktop application was designed to provide a real-time visualization of the acquired audio signal, to add patient related data such as demographic information or comorbidities and to organize and retrieve acquired signals. For the exchange of control commands and data in real-time, the device running the desktop application hosts a local Wi-Fi network to which the auscultation device connects automatically after start-up. Authentication takes place via access credentials stored in the auscultation device’s software, so no further communication is required. For communication, the application uses the Transmission Control Protocol (TCP). In contrast to communication protocols such as the User Datagram Protocol (UDP), TCP was designed to provide an error-checked delivery of data streams. Thereby it ensures the integrity of the data transmission between the auscultation device and the desktop application running on the host device. A real-time visualization along with play-back of the acquired sound signal starts when the device enters visualization state and the socket is receiving data from the device. As a result, the user can visually and auditorily inspect the auscultation signal, search and verify an appropriate sensing location at the neck or the presence of carotid bruits and ensure a sufficient signal quality. The play-back can be muted or listened to via headphones to not corrupt the audio acquisition.
The application further provides the possibility to enter supplementary information regarding the patient as well as information regarding the measurement. Patient related information incorporates but is not limited to: comorbidities such as diabetes, hypertension or coronary disease; previously performed invasive treatment such as angioplasty or endarterectomy,23 prescribed medication or the grading of an already diagnosed stenosis eg, according to the “North American Symptomatic Carotid Endarterectomy Trial” (NASCET) grading system24 or the “European Carotid Surgery Trial” (ECST) definition.25 Measurement related information include the sensing location eg, anatomical reference points, left/right carotid and heart rate at the beginning and at the end of the measurement. Such information is important for a subsequent annotation of the audio signal and later analysis. The entries are stored along with the wave audio file in the database linked to the desktop application.
The application was designed and implemented to run on an off-the-shelf computer with a standard operating system (Microsoft Corporation, Windows 10, Redmond, Washington, USA or various Unix-based distributions) and uses MySQL (Oracle Corporation, MySQL Community Server 8.0.15, Redwood City, USA) as a relational database management system (RDBMS). The device running the desktop application connects via Wi-Fi directly to the prototype, which is configured as a wireless access point. Since MySQL is a commonly used open-source RDMBS, which is compatible with various system platforms and developer interfaces, the database can be easily migrated to a network server. This will facilitate the interaction and data acquisition with multiple remote auscultation devices in the future. Such adaptability is an important requirement of the proposed design, to ensure backward compatibility in case of changes in system architecture, hardware or software of the auscultation device.
Experimental Study
The system was tested in a first experimental study to evaluate stability and reproducibility of vascular auscultation as well as usability of the device. For the study the vascular sounds of both carotids of a total of five voluntary subjects were acquired ranging in age between 28–42 years. To the best of our knowledge, the carotid arteries of all subjects were in a healthy state without a diagnosed atherosclerotic or cardiovascular disease or any other related comorbidity or medication presumably influencing the acquired vascular sounds. Two out of the 5 subjects were women. All volunteers who used the proposed CAAS to record auscultation signals independently were asked to breath normally during the recordings. As an example, Figure 5 depicts the auscultation at the neck of a volunteer with the final handheld device.
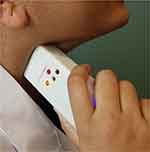 |
Figure 5 Auscultation of the carotid vascular sound of a voluntary subject with the final CAAS. |
The subjects repeated 5 consecutive measurements per carotid, each measurement with a duration of at least 10 seconds and with a minimum of 8 recorded heart cycles. Per measurement the auscultation device was placed at the neck and a good auscultation position was localized by the user with help of the desktop application. To visually confirm an adequate vascular sound signal quality, the intrinsic heart sounds S1 and S2 were used. Since the auscultation position at the neck is in sufficient distance to the heart, the presence of S1 and S2 in the recording was used to confirm the origin of the sounds from the cardiovascular system. At the end of each measurement the device was removed and placed again at the neck. With that, the influence of a manually performed positioning of the device on the stability of the signal recording was assessed. Further, no indicator was used to limit the contact force applied by the user. Along with the aforementioned measures, this allowed the evaluation of reproducibility of signal acquisition in a realistic use scenario and to assess the influence of small variations of related measurement parameters on the stability of the recordings.
Vascular Sound Signal Analysis
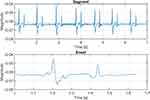
To determine the reproducibility of the vascular sound signal acquisition, the spectral characteristics of the five measurements per carotid per individual were inspected using the recordings acquired without acoustic membrane as data basis. The analysis was conducted in two steps. First, the spectral characteristics of the five measurements of one carotid were compared using a 7 second segment of each measurement. Subsequently, the characteristics of the segments were compared against the 5 measurements of the second carotid of the identic subject. By utilizing the heart sounds S1 and S2 it was ensured, that each segment contained at least 7 cardiac cycles. In a second step, each cardiac cycle comprising S1 and S2 within one measurement was defined as one event. The location of the events was annotated manually via visual inspection of the signal. Subsequently, 8 consecutive events per measurement were selected and compared with respect to their spectral properties. After analysis of the 8 events of one measurement they were compared against the events of the other measurements of one carotid and the results obtained from the second carotid of the particular individual. Figure 6 depicts the time-domain representation of the acquired vascular sounds as the basis for the segment-based analysis (top) and the event-based analysis (bottom).
For the signal processing, MATLAB (The MathWorks, Inc, MATLAB R2018b, Natick, Massachusetts, USA) was used. For assessing the spectral characteristics, the scalogram as the wavelet-based time-frequency-spectrum was computed using the continuous wavelet transform (CWT). As mother wavelet, the analytic Morse wavelet with the symmetry parameter gamma equal to 3 and time-bandwidth product equal to 60 was used, which represents the default parameter of the used MATLAB function associated with CWT. The minimum and maximum scales were automatically determined based on the wavelet’s energy spread in frequency and time by the used function. Subsequently the mean as function of frequency was computed and normalized to obtain the stationary wavelet-based spectrum as basis for the evaluation of spectral stability of the vascular recordings. The analysis was conducted for the previously described segments as well as for the events and the spectra for each subject were visualized and inspected.
To determine the influence of the acoustic membrane incorporated in the skin-transducer-interface on the acquired sound, the distribution of spectral energy was analyzed. Therefore, the power-spectral density (PSD) of the zero-mean signals of one measurement was estimated using the discrete Fourier transform. Afterwards, the normalized cumulated energy of the spectra as function of frequency was computed. Since both signals were recorded simultaneously, it allows a comparison of the distribution of spectral energy in the frequency bands.
Results
Auscultation System Prototype
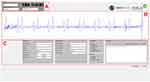
Figure 7 depicts the 3D-model (right) and the manufactured and assembled handheld device (left) of the CAAS. In the upper part, the changeable sensing unit consisting of skin-transducer-interface and transducer PCB is attached to the device. Air slots on both sides of the housing ensure heat dissipation during operation and the size and layout of the control elements allowed a convenient auscultation process during the experimental study. The graphical user interface (GUI) as the frontend of the desktop application is shown in Figure 8. The auscultation signal along with the intrinsic heart sounds S1 and S2 can be observed in the visualization panel in real-time and can be used for locating a good measurement position. A set of process related information and input fields for supplementary information was implemented. The GUI can be easily adapted for other auscultation applications. As an example, Figure 9 shows recorded auscultation signals, acquired by subject S2 from the left carotid artery without initial training. The intrinsic heart sounds of the circulatory system originating from the closing of the heart valves can be visually identified in the recordings (Figure 9A). The upper channel one (Ch 1) depicts sounds recorded with incorporated acoustic membrane, whereas channel two (Ch 2) shows simultaneous recordings without membrane. The presence of typical artifacts such as deep breathing (Figure 9B) or swallowing (Figure 9C) during the auscultation is demonstrated. While considered as artifact for the auscultation of vascular sounds, Figure 9 proves a general adaptability of the device for the acquisition of body sounds at the neck.
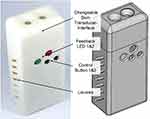 |
Figure 7 3D-model (right) and manufactured and assembled prototype of the handheld auscultation device ready to use (here: skin-transducer-interface without acoustic membrane). |
Reproducibility of Vascular Sound Acquisition
To assess the spectral stability of the vascular sounds acquired with the proposed system, the stationary wavelet-based spectra were computed for the 5 voluntary subjects S1 … S5. Figure 10 depicts the normalized and stationary spectral energy of the 10 measurements per subject as a function of frequency in logarithmic scale. Measurements 1 to 5 represent segments acquired from the left carotid while measurements 6 to 10 represent the segments of the right carotid. The odd labeling of the frequency originates from the use of CWT to compute the spectra. For each measurement the associated frequency of the particular scale with the highest contribution of energy to the scalogram is indicated in the diagram. Figure 11 depicts a similar plot individually computed for 8 consecutive events per measurement instead of the full segment. The first 40 events represent the left carotid of each subject while the second 40 events belong to the right carotid respectively.
Influence of Acoustic Membrane
To assess the influence of the membrane on the acquired vascular sounds, the cumulated spectral energy was computed. Figure 12 depicts the cumulated PSD of the 10 measurements of one subject. It can be seen that 95% of the total spectral energy of the signal accumulated below 30 Hz for the recordings without membrane. The membrane appears to have shifted the spectral characteristic of the obtained vascular sounds to slightly higher frequencies. Further, the energy accumulated slower up to 10 Hz and spread over a broader frequency range of up to 50 Hz above 10 Hz. However, the membrane did not change the general characteristic of the spectral energy distribution. This general trend could be observed for all recorded subjects and carotids, although the degree of shift differed between individual carotids. With the quantity of recordings in the present study it was not possible to identify some kind of transfer function between the investigated cumulated PSD of measurements with and without membrane.
Discussion
As can be observed from Figure 10, the general spectral characteristics of the acquired vascular sound did not change significantly between measurements of one carotid. However, a difference between both carotids of one individual can be seen for certain subjects. The major part of the spectral energy was located between 3 … 40 Hz. This was confirmed by the event-based analysis of the spectral properties depicted in Figure 11. All subjects showed a clear accumulation of spectral energy in the lower frequency components with occasional events showing frequency components up to 500 Hz. For 4 out of 5 subjects a reproducible spectral pattern can be observed for each carotid in the event-based analysis. While subject S1 represented an outlier in that sense, the segment-based analysis confirms a conformity with the general observed characteristic of the acquired sound signals.
It has to be noted, that the two intrinsic heart sounds S1 and S2 were present in all acquired recordings used for the stability analysis. Both represent the predominant morphology in the time-domain representation of the vascular sounds as shown in Figure 6. As a result, it is apparent that the heart sounds propagated through the vascular system have a contribution to the spectral properties of the recorded sound. However, it can be seen from Figures 10 and 11 that the characteristics of the sounds of left and right carotid of one individual can differ significantly. Especially for subjects S1, S3 and S4, the two carotid arteries can be clearly distinguished based on the spectral profile of their sound. Since the recordings for both carotids were acquired during one measurement session, we assume that attributes of the heart sounds did not change during that time. This indicates that characteristics exist in the acquired vascular sound that can be linked to the particular vessel. Further, the results reveal that parameters related to the acquisition such as the manual localization of an auscultation position or small variations in the applied contact pressure, have a minor influence on the general spectral stability. While this holds true for the present study, the impact of contact pressure on the vascular sounds needs to be investigated in future studies. Excessive pressure could potentially deform the subcutaneous vessel and thus cause a change of the sound. As a result, signal characteristics containing information related to the status of the carotid might be covered or distorted. In the future, subsequent to the experimental study of the mentioned parameters, it is envisaged to design a wearable path like auscultation device.
Besides factors related to the acquisition and the user, a range of physical parameters related to the skin-transducer-interface have an influence on the spectral properties. Dimensions and mechanical configuration of the interface can have a considerable impact on the quality and reproducibility of acquisition.22 It has been shown in Figure 12, that the presence of an acoustic membrane does change the spectral properties of the acquired sounds, since it appears to shift spectral components toward the higher frequencies. A possible explanation is microtremors introduced by the hand of the user which result in motion and friction between membrane and skin. This might be amplified by the increased contact surface of the membrane and could be reflected in the acquired signals. Further, the membrane lowered the amplitude of the signal as can be seen in Figure 9. This might be explained by the additional load of the membrane, which causes a reduced deflection of the skin. It needs to be investigated, whether the changed spectral properties are linked to a difference in the content of information of the acquired vascular sounds. Since the stethoscope with membrane is currently used in clinical practice, it is essential to check whether the sound characteristics clinicians are screening for can be obtained without a membrane. The proposed CAAS is the prerequisite for the creation of a database in a clinical setting, incorporating pathological vascular sounds. As a result, such a database would allow clinicians to annotate the recordings and hence facilitate work in this field. Since the sensing unit is designed to be easily detachable and exchangeable, the CAAS can serve as a platform for the comparison of different skin-transducer-interfaces in a realistic clinical scenario.
We are aware that the present study comes with certain limitations. Firstly, the number of participants was comparably low and measurements were taken in one session. A future study needs to comprise a significantly higher number of subjects and should be conducted over a period of several weeks, allowing an evaluation of the long-term stability of the acquisition. Further, the absence of pathological cases of vascular sounds is a limiting factor. This is primarily due to ethical guidelines in place at the host institution. The proposed CAAS is considered a first step toward an ethical approval to include diagnosed pathological cases of CS in a future study. Since a reproducible signal acquisition was confirmed, a study with pathological cases of carotid stenosis prior and subsequent to (surgical-) treatment is envisaged to examine expected changes in the spectral characteristics of the vascular sounds.
Conclusion
The developed CAAS allows a repeatable and in the spectral characteristics stable acquisition of vascular sounds from the neck without technical background or the need for complex hardware. The visual real-time feedback provided by the desktop application is appropriate to localize a good position for the auscultation without previous training. While positioning can be solved manually, the identification of pathological sounds of the circulatory and vascular system requires a substantial amount of experience and training. In future work, a real-time processing algorithm has to be developed and implemented to assess and confirm a suitable position based on the signal quality, to reduce the sensitivity to user errors. However, the proposed CAAS enables a streamlined auscultation of vascular sounds at the neck. It provides a platform for an extended study of intrinsic and extrinsic parameters influencing the acquisition and characteristics of vascular sounds. Further, it facilitates the creation and annotation of a database of pathological vascular sounds, of individual vascular sound profiles, and a library of typical artifacts related to the auscultation.
Such a database is a prerequisite for future research with respect to the auscultation-based monitoring of vascular conditions and atherosclerotic changes of the carotid. A potential long-term tracking (month, years) of individual sound profiles could result in promising applications such as monitoring of the treatment and the management of pathologies. However, the value of such sound-based monitoring needs to be verified based on clinical and pathological data first.
With a growing database and understanding of the vascular signals, an automatized annotation of pathological sounds or artefacts is envisaged. The GUI and desktop application are implemented in such a way to allow a simple incorporation of additional signal processing and real-time analysis functionalities at a later stage. Of primary interest is the implementation of an online annotation capability of sound characteristics and artifacts for clinical experts. Further, integration of a web-based user interface is considered to allow the setup of an open source database of vascular sounds and carotid bruits. This could potentially be used as an educational tool or accelerate research in the field.
The architecture of the CAAS is designed to be customizable for a range of similar auscultation applications such as the recording of vibroarthrographic signals of the knee joint.26 Besides body sounds, an adaptation of the mechanical interface of the sensing unit allows the use for other medical applications. As an example, an adapted version of the system is used for the acquisition of vibroacoustic signals originating from instrument-tissue-interactions in the field of minimally invasive surgery.27–29
Abbreviations
CAAS, computer-assisted auscultation system; CVD, cerebrovascular diseases; IS, ischemic stroke; CS, carotid stenosis; US, ultrasonography; WHO, World Health Organization; PCB, printed circuit board; MEMS, micro-electro-mechanical system; TCP, transmission control protocol; UDP, user datagram protocol; NASCET, North American Symptomatic Carotid Endarterectomy Trial; ECST, European Carotid Surgery Trial; RDBMS, relational database management system; GUI, graphical user interface; AU, arbitrary unit; SNR, signal to noise ratio; CWT, continuous wavelet transform; PSD, power spectral density.
Disclosure
TS, AB, AI and MF report a patent “MEDICAL ACOUSTIC SENSOR SYSTEM” (US 62/756487) pending. Stefan Hellwig reports a patent, Bloxton US-Patent pending. Rutuja Salvi and Stefan Hellwig are employees of IDTM GmbH. The authors report no other potential conflicts of interest in this work.
References
1. Townsend N, Wilson L, Bhatnagar P, et al. Cardiovascular disease in Europe: epidemiological update 2016. Eur Heart J. 2016;37(42):3232–3245. doi:10.1093/eurheartj/ehw334.
2. World Health Organization. Global Burden of Disease 2000: The Global Burden of Cerebrovascular Disease. Geneva; 2000. Available from: https://www.who.int/healthinfo/statistics/bod_cerebrovasculardiseasestroke.pdf.
3. Flaherty ML, Kissela B, Khoury JC, et al. Carotid artery stenosis as a cause of stroke. Neuroepidemiology. 2013;40(1):36–41. doi:10.1159/000341410.
4. World Health Organization. International statistical classification of diseases and related health problems 10th Revision. Available from: https://icd.who.int/browse10/2016/en.
5. Liem MI, Kennedy F, Bonati LH, et al. Investigations of carotid stenosis to identify vulnerable atherosclerotic plaque and determine individual stroke risk. Circ J. 2017;81(9):1246–1253. doi:10.1253/circj.CJ-16-1284.
6. Gokaldas R, Singh M, Lal S, et al. Carotid stenosis: from diagnosis to management, where do we stand? Curr Atheroscler Rep. 2015;17(2):480. doi:10.1007/s11883-014-0480-7.
7. Ooi YC, Gonzalez NR. Management of extracranial carotid artery disease. Cardiol Clin. 2015;33(1):1–35. doi:10.1016/j.ccl.2014.09.001.
8. Sandercock PAG, Kavvadia E. The carotid bruit. Pract Neurol. 2002;2(4):221–224. doi:10.1046/j.1474-7766.2002.00078.x.
9. Lanzino G, Rabinstein AA, Brown RD. Treatment of carotid artery stenosis: medical therapy, surgery, or stenting? Mayo Clin Proc. 2009;84(4):362–368. doi:10.1016/S0025-6196(11)60546-6
10. Lees RS, Dewey CF. Phonoangiography: a new noninvasive diagnostic method for studying arterial disease. Proc Natl Acad Sci. 1970;67(2):935–942. doi:10.1073/pnas.67.2.935
11. Duncan GW, Gruber JO, Dewey CF
12. Tavel ME, Bates JR. The cervical bruit: sound spectral analysis related to severity of carotid arterial disease. Clin Cardiol. 2006;29(10):463–465. doi:10.1002/clc.4960291009
13. Paraskevas KI, Hamilton G, Mikhailidis DP. Clinical significance of carotid bruits: an innocent finding or a useful warning sign? Neurol Res. 2008;30(5):523–530. doi:10.1179/174313208X289525.
14. Brott TG, Halperin JL, Abbara S, et al. ASA/ACCF/AHA/AANN/AANS/ACR/ASNR/CNS/SAIP/SCAI/SIR/SNIS/SVM/SVS guideline on the management of patients with extracranial carotid and vertebral artery disease: executive summary. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American Stroke Association, American Association of Neuroscience Nurses, American Association of Neurological Surgeons, American College of Radiology, American Society of Neuroradiology, Congress of Neurological Surgeons, Society of Atherosclerosis Imaging and Prevention, Society for Cardiovascular Angiography and Interventions, Society of Interventional Radiology, Society of NeuroInterventional Surgery, Society for Vascular Medicine, and Society for Vascular Surgery. Circulation. 2011;124(4):489–532. doi:10.1161/CIR.0b013e31820d8d78.
15. Zambrano IM, Illanes A, Boese A, et al.; Characterization of a carotid distension waveform from audio signal acquired with a stethoscope. In:
16. Zühlke L, Myer L, Mayosi BM. The promise of computer-assisted auscultation in screening for structural heart disease and clinical teaching. Cardiovasc J Afr. 2012;23(7):405–408. doi:10.5830/CVJA-2012-007.
17. Viviers PL, Kirby JAH, Viljoen JT, et al. The diagnostic utility of computer-assisted auscultation for the early detection of cardiac murmurs of structural origin in the periodic health evaluation. Sports Health. 2017;9(4):341–345. doi:10.1177/1941738117695221.
18. Swarup S, Makaryus AN. Digital stethoscope: technology update. Med Devices (Auckl). 2018;11:29–36. doi:10.2147/MDER.S135882.
19. Leng S, Tan RS, Chai KTC, et al. The electronic stethoscope. Biomed Eng Online. 2015;14:66. doi:10.1186/s12938-015-0056-y.
20. Huang A, Lee CW, Liu HM. Rolling ball sifting algorithm for the augmented visual inspection of carotid bruit auscultation. Sci Rep. 2016;6:30179. doi:10.1038/srep30179.
21. Sühn T, Mahmoodian N, Sreenivas A, et al. Computer assisted auscultation system for phonoangiography of the carotid artery. Current Directions Biomed Eng. 2019;5(1):175–178. doi:10.1515/cdbme-2019-0044.
22. Sühn T, Sreenivas A, Mahmoodian N, et al. Design of an Auscultation system for phonoangiography and monitoring of carotid artery diseases. Conf Proc IEEE Eng Med Biol Soc. 2019;2019:
23. Morris DR, Ayabe K, Inoue T, et al. Evidence-based carotid interventions for stroke prevention: state-of-the-art review. J Atheroscler Thromb. 2017;24(4):373–387. doi:10.5551/jat.38745.
24. Barnett HJM, Taylor DW, Haynes RB, et al. Beneficial effect of carotid endarterectomy in symptomatic patients with high-grade carotid stenosis. N Engl J Med. 1991;325(7):445–453. doi:10.1056/NEJM199108153250701.
25. European Carotid Surgery Trialists’ Collaborative Group. Randomised trial of endarterectomy for recently symptomatic carotid stenosis: final results of the MRC European Carotid Surgery Trial (ECST). The Lancet. 1998;351(9113):1379–1387. doi:10.1016/S0140-6736(97)09292-1..
26. Klemm L, Sühn T, Spiller M, et al. Improved acquisition of vibroarthrographic signals of the knee joint. Conf Proc IEEE Eng Med Biol Soc. 2019;2019:
27. Chen CH, Sühn T, Kalmar M, et al. Texture differentiation using audio signal analysis with robotic interventional instruments. Comput Biol Med. 2019;112:103370. doi:10.1016/j.compbiomed.2019.103370.
28. Illanes A, Boese A, Maldonado I, et al. Novel clinical device tracking and tissue event characterization using proximally placed audio signal acquisition and processing. Sci Rep. 2018;8(1):12070. doi:10.1038/s41598-018-30641-0.
29. Illanes A, Sühn T, Esmaeili N, et al.; Surgical Audio Guidance SurAG: extracting non-invasively meaningful guidance information during minimally invasive procedures. In:
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.