Back to Journals » Cancer Management and Research » Volume 12
Aurora-B Promotes Osteosarcoma Cell Growth and Metastasis Through Activation of the NPM1/ERK/NF-κβ/MMPs Axis
Authors Song H , Zhou Y, Peng A, Liu J , Wu X , Chen W, Liu Z
Received 7 March 2020
Accepted for publication 10 May 2020
Published 23 June 2020 Volume 2020:12 Pages 4817—4827
DOI https://doi.org/10.2147/CMAR.S252847
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Eileen O'Reilly
Honghai Song,1,2,* Yang Zhou,1,* Aifen Peng,3 Jiaming Liu,1,2,4 Xin Wu,1 Wenzhao Chen,1 Zhili Liu1,2,5
1Department of Orthopedic Surgery, The First Affiliated Hospital of Nanchang University, Nanchang 330006, People’s Republic of China; 2Institute of Spinal and Spinal Cord Diseases, Nanchang University, Nanchang 330031, People’s Republic of China; 3College of Humanities, Jiangxi University of Traditional Chinese Medicine, Nanchang, Jiangxi 330004, People’s Republic of China; 4Jiangxi Institute of Respiratory Disease, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, People’s Republic of China; 5Division of Science and Technology, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Zhili Liu
Department of Orthopedics, The First Affiliated Hospital of Nanchang University, Nanchang, Jiangxi 330006, People’s Republic of China
Tel/ Fax +86-79188693201
Email [email protected]
Purpose: Osteosarcoma (OS) is the most common primary malignant tumor of the bone in young adolescents and children. We explored the underlying mechanism of Aurora-B in promoting OS cell proliferation and metastasis.
Patient and Methods: Bioinformatics was employed to predict the substrate of Aurora-B. IHC and Western blot were used to confirm the correlation between Aurora-B and NPM1. ERK/NF-κβ pathway-related proteins were detected by Western blot and immunofluorescence (IF). CCK8, wound healing, transwell, and Tunel assays were used to identify the cell proliferation, migration and apoptosis potential. Spontaneous metastasis xenografts were established to confirm the role of Aurora-B and NPM1.
Results: Aurora-B promotes NPM1 phosphorylation on Ser125. The phosphorylation of NPM1Ser125 induced by Aurora-B activates the ERK/NF-κβ signaling. Further study revealed that Aurora-B promotes proliferation, migration and inhibits apoptosis via phosphorylating NPM1 in vitro and in vivo.
Conclusion: Aurora-B promotes OS malignancy via phosphorylating NPM1Ser125 and activating ERK/NF-κβ signaling.
Keywords: Aurora-B, osteosarcoma, NPM1, ERK, NF-κβ
Introduction
Osteosarcoma (OS) is the most common malignant tumor of the bone and is prevalent in both young adolescents and children.1 Standard treatment for OS mainly includes the surgical removal of localized lesions, combined with multiple chemotherapeutics.2 These approaches increase the 5-year survival rate of patients in the absence of metastasis from less than 20% to almost 70%. However, no further breakthroughs for OS management have occurred, owing to the drug resistance and metastasis.3 Novel and advanced molecular agents for targeted OS therapies are therefore urgently required.
Aurora-B forms a complex with INCEP, Survivin and Borealin and participates in a range of cell biological processes to ensure the correct interaction of kinetochores and spindle microtubules. Aurora-B deficiency will lead to the arrest in metaphase in in non-malignant cells.4 Numerous studies have demonstrated that Aurora-B is aberrantly expressed in various malignant tumors5–8 and facilitates cell cycle progression through the regulation of cell cycle-related targets. Gonzalez-Loyola et al reported that Aurora-B activates CDK1 via suppressing the p53/p21WAF1/CIP1 axis to promote cell cycle progression and cell survival.7 Guise and colleagues showed that Aurora-B phosphorylates Class IIa HDACs to induce cell cycle progression.9 In addition, Aurora-B facilitates lymphoma cell proliferation and survival through the activation of AKT/mTOR signaling.10 Although the overexpression or amplification of Aurora-B contributes to tumor cell malignancy,11–13 the role of Aurora-B in regulating OS cell growth and metastasis remains elusive.
In this study, we found that NPM1 is overexpressed in osteogenetic OS tissues. Aurora-B was found to promote OS cell growth and metastasis through its ability to phosphorylate NPM1Ser125 and activate ERK/NF-κβ signaling. These data suggest that targeting the Aurora-B/NPM1/ERK/NF-κβ axis represents a novel therapeutic candidate for the prevention of OS metastasis.
Materials and Methods
Bioinformatics
The bioinformatics prediction tool Oncomine was used to examine NPM1 expression in OS (https://www.oncomine.com/resource/login.html), database 200063 s at were selected; The Molecular INTeraction Database was used to explore the correlation between NPM1 and Aurora-B.
Cell Culture and Transfection
The OS cell lines U2-OS and 143B were ordered from the cell bank of Type Culture Collection of Chinese Academy of Sciences (Shanghai, China). Cells were maintained in DMEM (Gibco) plus 10% FBS. Lentivirus-Vectors were compound by GENECHEM (Shanghai, China). The sequence was as follows: shAurora-B, 5ʹ-AGAGCTGCACATTTGACGA-3ʹ; shRNA-NC, 5ʹ-TTCTCCGAACGTGTCACGT-3ʹ. 143B and U2-OS cells were infected with 5×105 PFU Lentivirus-Vectors (MOI=50) and treated with 0.6 μg/mL puromycin for cell selection.
Specimens and Immunohistochemical Analysis
A total of 87 tissue sections histologically diagnosed with OS were obtained from the First Affiliated Hospital of Nanchang University, China. No patients received therapy prior to biopsy. All experiments were approved by the ethics committee of the First Affiliated Hospital of Nanchang University (Jiangxi, China; NO.Y2019-126) and followed the Declaration of Helsinki. All the subjects were informed of the contents, latent risks, objectives and signed written informed consents.
Immunoperoxidase procedures (Maxvasion procedure) were performed to detect relative protein expression. Briefly, sections were heated in citrate buffer for 20 min and probed with anti-Aurora-B (1:500, Abcam, MA, USA) and pNPM1Ser125 (1:1000, Abcam) antibodies overnight. Stained sections were scored by two doctors, respectively. Tissues showing yellow and brown nuclear staining were used to indicate a positive result, and tissues lacking staining in the nucleolus were considered negative. Immunohistochemical expression of Aurora-B and pNPM1Ser125 were determined as “-”, “+”, “++”, and “+++” according to the coloring intensity and the percentage of positively stained tumor cells.
qPCR
Total RNA from OS cells was extracted with Trizol (Invitrogen). Aurora-B expression was evaluated by qRT-PCR. GAPDH was used as an endogenous reference gene. The final reaction mixture of all the amplification reactions was 20 μL. Primer sequences were listed as follows: Aurora-B sense 5ʹ-AGAAGGAGAACTCCTACCCCT-3ʹ, Aurora-B antisense 5ʹ-CGCGTTAAGATGTCGGGTG-3ʹ; GAPDH sense 5ʹ-CAGGGCTGCTTTTAACTCTGGT-3ʹ, GAPGH antisense 5ʹ-GATTTTGGAGGG ATCTCGCT-3ʹ. The comparative Ct method was used to calculate relative gene expression.
Wound Healing Assays
Cells (5×106) were seeded into six-well plates and cultured for 12 h. Confluent cells were scratched using a slender pipette tip and washed using PBS and complete DMEM. Images were taken 24 h later and compared to T=0. Image J (NCBI) was used to quantify the migrated distances.
Transwell Invasion Assays
Cell invasion assay was assessed using matrigel Chamber assays (BD Bioscience, NJ, USA) according to the manufacturer’s protocol. Briefly, cells (2×105) suspended in 250 μL of serum-free DMEM were added to the upper chamber insert and complete medium (15% FBS) was used as a chemoattractant and added to the bottom chamber. After 24 h, chambers with invaded cells were fixed and stained with Crystal Violet Solution (Beyotime, China). Five high-power images were randomly taken and the number of transmembrane cells quantified using Image J (NCBI).
CCK8 Assays
Cells (~4000) were counted and plated into 96-well plates and cultured in complete media (10% FBS) for 0 h, 24 h and 48 h, respectively. CCK-8 reagent (10 μL, Dojindo Laboratories, Japan) was added for 2 h and cell viability was defined as the absorbance at 450nm detected on a microplate reader.
Tunel Assays
Apoptotic activity in OS cells was assessed through TUNEL staining (Roche) according to the manufacturer’s protocols. Briefly, samples were fixed in freshly prepared 4% PFA and blocked in 3% H2O2 in methanol. Cells were then stained with TUNEL reagent for 1 h followed by incubation with 50 μL of converter-POD and DAB. Slides were imaged via microscopy.
Western Blot Analysis
Cells were lysed in RIPA lysis buffer with PMSF and Bradford assays were performed to quantitate total protein concentrations. Approximately 20 μg of protein was loaded onto the sample wells and transferred onto Nitrocellulose membranes. Membranes were incubated with 5% fat-free milk and probed with primary antibodies (anti-Aurora-B 1:5000; anti-NPM1, anti-pNPM1Ser125, anti-ERK1/2 and anti-pERK1/2Thr202/Tyr204 1:1000; anti-MMP9, anti-MMP2, 1:5000; anti-XIAP, anti-BCL-XL, anti-β-actin, 1:2000) for 8 h. Primary antibodies were purchased from Abcam (Cambridge, MA, USA). Species-specific secondary antibodies (dilution ratio of 1:2000) were purchased from ZSGB-BIO, China. Immune complexes were visualized using EasySee Western Blot kits (Transgen, China).
Immunofluorescence
U2-OS and 143B cells plated onto 24-well plates were grown to 70% confluency and treated with 25 µM 2-ClHyA for 30 min. Cells were fixed with 4% PFA and blocked in 5% (w/v) BSA for 30 mins. Cells were labeled with NF-κβ p65 antibodies (1:100 dilution; Abcam) and washed in PBST. Slides were incubated with Rhodamine (TRITC)-Conjugate Goat anti-rabbit IgG (1:25; ZSGB-BIO, China). PBST-washed slides were treated with VECTASHIELD Anti-fade mounting medium (VECTOR Laboratories, lnc., 30 Ingold Road, Burlingame, CA 94,010 USA). Images were obtained using a confocal laser scanning microscope (Zeiss/LSM 710, Germany). For quantification of immunofluorescence images, a minimum of 50–100 NF-κβ positive cells were counted to determine the number of NF-κβ nucleus translocation cells.
Spontaneous Metastasis Xenografts
Male BALB/c nude mice (4-weeks old) were provided by the Department of Laboratory Animal Science of Nanchang University (Nanchang, China). Spontaneous metastasis xenografts were established as previously described. Mice were randomly selected (n=12) and intratumorally injected with Aurora-B, shAurora-B, NC- Aurora-B and NC- shAurora-B lentivirus (107 PFU, 100 μL) for 7 days. Mice were sacrificed after 6 weeks of orthotopic transplantation. Tumor sizes were assessed on a graduated cylinder filled with water in which changes in water height indicated tumor volume. Lung metastatic nodules were assessed by H&E staining and stereomicroscope (Nikon SMZ1500). All animal experiments were approved by the First Affiliated Hospital of Nanchang University (Jiangxi, China; NO.Y2019-126), and performed according to the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals (1996).
Statistical Analysis
Non-parametric Wilcoxon rank-sum tests were used to analyze count data. The correlation between Aurora-B and pNPM1Ser125 protein levels were determined by Spearman’s rho tests. Measurement data are shown as the mean ± standard deviation. A Student’s t-test was used for two-sample analysis. A one-way ANOVA was used for multiple-sample analysis. Data were analyzed using SPSS statistical software (Version 13.0).
Results
Aurora-B Promotes the Phosphorylation of NPM1
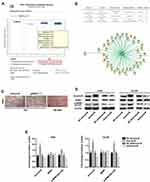
To explore NPM1 expression in OS samples and the potential interaction between Aurora-B and NPM1, bioinformatics was performed using the Molecular INTeraction Database; Oncomine Database). The results indicated that NPM1 was up-regulated in osteogenetic OS samples and that Aurora-B interacts with NPM1 (Figure 1A-B). In addition, we detected the Aurora-B and p-NPM1 expression in 87 samples of osteogenetic OS by immunohistochemistry (Figure 1C). The correlation between Aurora-B and phosphorylated NPM1 expression was analyzed using bivariate Spearman assays (Table 1). The results indicated that Aurora-B was positively related to phosphorylated NPM1. We further investigated the effects of Aurora-B alterations on NPM1 phosphorylation. Cells were infected with Aurora-B, NC-Aurora-B, shAurora-B, and NC-shAurora-B lentivirus, respectively. Aurora-B, NPM1 and p-NPM1Ser125 expression were confirmed by Western blot. The results indicated that p-NPM1Ser125 expression was higher in cells infected with Aurora-B compared to control groups. Lower p-NPM1Ser125 levels were observed in cells infected with shAurora-B lentivirus compared to control groups. However, the total levels of NPM1 did not differ between the groups (Figure 1D and E). The reason for the different bands of Aurora-B may be related to different splice variants/isoforms. Collectively, these results suggest that Aurora-B promotes NPM1 phosphorylation on Ser125.
 |
Table 1 Correlation Between Aurora-B and pNPM1Ser125 Expression in Osteosarcoma |
Aurora-B Activates ERK/NF-κβ Signaling Through the Phosphorylation of NPM1Ser125
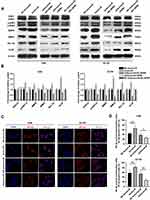
To explore the downstream effects of Aurora-B-mediated NPM1 phosphorylation in OS cells, ERK1/2, pERK1/2, MMP2, MMP9, and apoptotic Bcl-xL and XIAP levels were assessed in U2-OS and 143B cells transfected with Aurora-B and scrambled controls. Western blot analysis revealed that pERK1/2, MMP2, MMP9, Bcl-xL and XIAP levels were significantly higher in Aurora-B vs control groups. In contrast, pERK1/2, MMP2, MMP9, Bcl-xL and XIAP level were significantly lower in cells infected with LV/shAurora-B compared to the control group. This loss of protein expression could be recovered by NPM1 overexpression (Figure 2A and B). Furthermore, the nuclear translocation of NF-κβ was examined by confocal microscopy. The results revealed that NF-κβ expression and nucleus translocation were enhanced in OS cells infected with LV/Aurora-B compared to the control group. An opposing phenotype was observed in cells transfected with NV/shAurora-B (Figure 2C and D). These results suggest that Aurora-B activates ERK/NF-κβ signaling via phosphorylating NPM1Ser125.
Aurora-B-Induced NPM1Ser125 Phosphorylation Promotes OS Malignant Phenotypes in vitro
To investigate the role of Aurora-B-mediated NPM1 phosphorylation in OS progression. Wound healing, transwell invasion, CCK8 and Tunel assays were performed to evaluate the malignant phenotypes induced by Aurora-B and NPM1 alterations. The results indicated that the metastasis and proliferation ability of OS cells significantly increased in the Aurora-B group, whilst the opposite phenotype was observed in Aurora-B silenced groups. In addition, the rates of apoptosis were significantly reduced in cells transfected with LV/Aurora-B. These effects could be reversed by the restoration of NPM1 (Figure 3A-D). These results indicate that Aurora-B promotes OS malignancy in-part through the phosphorylation of NPM1.
Aurora-B Promotes OS Tumor Formation Through Phosphorylating NPM1
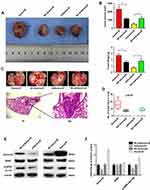
To confirm the role of Aurora-B-mediated NPM1 phosphorylation in OS initiation and metastasis, lentivirus-vectors were injected into tumor tissues. The results showed that tumor size and pulmonary nodules in nude mice intratumorally injected with LV/Aurora-B increased compared to the control group. The opposite phenotype was observed in nude mice injected with LV/shAurora-B (Figure 4A-D and Table 2). In addition, Western blot analysis indicated that the expression of Aurora-B and pNPM1Ser125 were significantly higher in nude mice transfected with LV/Aurora-B compared to the control group. The expression of NPM1 remained almost unchanged in each group (Figure. 4E and F). These results suggest that Aurora-B accelerates tumor initiation and metastases through the phosphorylation of NPM1 in vivo.
 |
Table 2 Number of Nude Mice with/Without Lung Metastasis |
Discussion
Aurora-B has been proved to play a tumor-promoting role in osteosarcoma.14 Our previous studies indicated that Aurora-B expression was higher in OS and promoted OS malignant phenotypes via the activation of PI3K/Akt signaling.15,16 However, the inhibition of PI3K/Akt alleviated the effects of Aurora-B on OS cell malignancy only partially, suggesting the existence of other mechanisms in Aurora-B-mediated OS. Herein, we explored the downstream effectors of Aurora-B and its potential molecular mechanisms in vitro and in vivo. Based on bioinformatics tools, we identified NPM1 as a substrate of Aurora-B, and hypothesized that Aurora-B interacts with NPM1, thus affecting the OS phenotype.
NPM1 is a ubiquitously expressed nucleocytoplasmic shuttling protein that plays an active role in ribosomal assembly, chromatin remodeling, DNA repair, replication, and transcription.17–19 Accumulating evidence suggest that NPM1 is directly implicated in cancer progression. High levels of NPM1 expression have been found in various malignant tumors and are associated with oncogenic progression and chemoresistance, including prostate, hematological tumors and high-grade serous ovarian adenocarcinoma.20–22 Sawazaki et al reported that nucleophosmin is strongly associated with the recurrence and prognosis of urothelial carcinoma patients with radical nephroureterectomy.23 Chen et al revealed that NPM1 inhibits apoptosis through its effects on Akt activity in breast cancer.24 However, the upstream molecular mechanisms through which NPM1 promotes cancer progression remain unclear. In this study, we investigated whether Aurora-B modulates NPM1 to promote OS metastasis. Bioinformatics predictions (the Molecular INTeraction Database; the Oncomine Database) revealed that NPM1 was overexpressed in osteogenetic OS tissue and may interact with Aurora-B. We found that Aurora-B expression was positively associated with phosphorylated NPM1 in OS tissue and cell lines. Western blot analysis also confirmed the effects of Aurora-B on the modulation of NPM1 phosphorylation. Although more assays should be performed to identify the interaction between Aurora-B and NPM1, our results were consistent with Shandilya et al who found that NPM1 can be phosphorylated by Aurora-B and participate in mitotic progression.25 Further studies demonstrated that the inhibitory effects induced by Aurora-B silencing could be partially reversed by NPM1 overexpression. These results suggest that Aurora-B promotes OS cells malignancy in-part, by phosphorylating NPM1Ser125.
ERK/NF-κβ signaling plays a critical role in malignant tumor proliferation, invasion and metastasis.26 ERK1/2 are highly conserved, universal extracellular-regulated kinases that participate in a range of pathologies. The phosphorylation of ERK1/2 induces NF-κβ activation in tumor cells.27 Nuclear factor-κβ (NF-κβ) is a well-known transcription factor consisting of five components, NF-κβ1 (p50), NF-κβ2 (p52), RelA (p65), RelB and Rel.28 NF-κβ participates in cell cycle regulation, apoptosis, and cytokine secretion via initiating protein transcription. Aberrant NF-κβ expression was observed in various malignant tumors, including gastric cancer,29 breast cancer,30 and prostate cancer.31 NF-κβ activation initiates the transcription of MMP-2 and MMP-9, the increased MMPs proteins enhance the invasiveness of tumor cells through the degradation of extracellular matrix (ECM).32,33 Besides, NF-κβ silencing promotes apoptosis in hematopoietic tumors via inhibiting Bcl-xL and XIAP.34 Recent studies revealed that NPM1 silencing inhibits prostate cancer cell growth, migration and invasion via reducing the phosphorylation of ERK1/2.35 We therefore hypothesized that Aurora-B-mediated NPM1 phosphorylation affects the OS phenotype via activating ERK/NF-κβ signaling. Herein, we report that Aurora-B overexpression enhances pERK1/2, MMP9, MMP2, BCL-XL, XLAP expression and the translocation of NF-κβ/p65. In addition, the inhibitory effects of Aurora-B on pERK1/2, MMP9, MMP2, BCL-XL and XLAP can be recovered by NPM1 overexpression, confirming our hypothesis.
Conclusion
Our results show that Aurora-B promotes OS cell growth and metastasis via activating NPM1/ERK/NF-κβ. Targeting the Aurora-B and NPM1/ERK/NF-κβ axis may therefore represent a promising strategy for OS management (Figure 5).
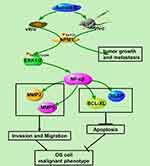 |
Figure 5 Proposed scheme of molecular basis for effect of Aurora-B/NPM1/ERK/NF-κβ axis in osteosarcoma according to our results. |
Abbreviations
OS, osteosarcoma; Aurora-B, aurora kinase B-like; NPM1, nucleophosmin 1; IHC, immunohistochemistry; IF, immunofluorescence; qRT-PCR, quantitative reverse transcriptase polymerase chain reaction; DMEM, Dulbecco’s Modified Eagle Media; shRNA, short-hairpin RNA; TUNEL, TdT-mediated dUTP Nick-End Labeling.
Author Contributions
JM L and ZL L contribute to the conception and design of the study; HH S, AF P and X W researched data; JM L and WZ C contribute to the analysis and interpretation of data. HH S and Y Z edited the manuscript; ZL L contributes to the final approval of the version to be submitted. All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hegyi M, Semsei AF, Jakab Z, et al. Good prognosis of localized osteosarcoma in young patients treated with limb-salvage surgery and chemotherapy. Pediatr Blood Cancer. 2011;57(3):415–422. doi:10.1002/pbc.23172
2. Isakoff MS, Bielack SS, Meltzer P, Gorlick R. Osteosarcoma: current treatment and a collaborative pathway to success. J Clin Oncol. 2015;33(27):3029–3035. doi:10.1200/JCO.2014.59.4895
3. Wang Z, Li B, Ren Y, Ye Z. T-cell-based immunotherapy for osteosarcoma: challenges and opportunities. Front Immunol. 2016;7:353. doi:10.3389/fimmu.2016.00353
4. Al-Khafaji AS, Davies MP, Risk JM, et al. Aurora B expression modulates paclitaxel response in non-small cell lung cancer. Br J Cancer. 2017;116(5):592–599. doi:10.1038/bjc.2016.453
5. Hauf S, Cole RW, LaTerra S, et al. The small molecule Hesperadin reveals a role for Aurora B in correcting kinetochore–microtubule attachment and in maintaining the spindle assembly checkpoint. J Cell Biol. 2003;161(2):281–294. doi:10.1083/jcb.200208092
6. Bogen D, Wei JS, Azorsa DO, et al. Aurora B kinase is a potent and selective target in MYCN-driven neuroblastoma. Oncotarget. 2015;6(34):35247–35262. doi:10.18632/oncotarget.6208
7. Gonzalez-Loyola A, Fernandez-Miranda G, Trakala M, et al. Aurora B overexpression causes aneuploidy and p21Cip1 repression during tumor development. Mol Cell Biol. 2015;35(20):3566–3578. doi:10.1128/MCB.01286-14
8. Huang PY, Li Y, Luo DH, et al. Expression of Aurora-B and FOXM1 predict poor survival in patients with nasopharyngeal carcinoma. Strahlenther Onkol. 2015;191(8):649–655. doi:10.1007/s00066-015-0840-4
9. Guise AJ, Greco TM, Zhang IY, Yu F, Cristea IM. Aurora B-dependent regulation of class IIa histone deacetylases by mitotic nuclear localization signal phosphorylation. Mol Cell Proteomics. 2012;11(11):1220–1229. doi:10.1074/mcp.M112.021030
10. Wang C, Chen J, Cao W, Sun L, Sun H, Liu Y. Aurora-B and HDAC synergistically regulate survival and proliferation of lymphoma cell via AKT, mTOR and Notch pathways. Eur J Pharmacol. 2016;779:1–7. doi:10.1016/j.ejphar.2015.11.049
11. Qi G, Ogawa I, Kudo Y, et al. Aurora-B expression and its correlation with cell proliferation and metastasis in oral cancer. Virchows Arch. 2007;450(3):297–302. doi:10.1007/s00428-006-0360-9
12. Hetland TE, Nymoen DA, Holth A, et al. Aurora B expression in metastatic effusions from advanced-stage ovarian serous carcinoma is predictive of intrinsic chemotherapy resistance. Hum Pathol. 2013;44(5):777–785. doi:10.1016/j.humpath.2012.08.002
13. Tuncel H, Shimamoto F, Kaneko GQH, et al. Nuclear Aurora B and cytoplasmic Survivin expression is involved in lymph node metastasis of colorectal cancer. Oncol Lett. 2012;3(5):1109–1114. doi:10.3892/ol.2012.633
14. Zhao Z, Jin G, Yao K, et al. Aurora B kinase as a novel molecular target for inhibition the growth of osteosarcoma. Mol Carcinog. 2019;58(6):1056–1067. doi:10.1002/mc.22993
15. Zhu LB, Jiang J, Zhu XP, et al. Knockdown of Aurora-B inhibits osteosarcoma cell invasion and migration via modulating PI3K/Akt/NF-kappaB signaling pathway. Int J Clin Exp Pathol. 2014;7(7):3984–3991.
16. Zhu XP, Liu ZL, Peng AF, et al. Inhibition of Aurora-B suppresses osteosarcoma cell migration and invasion. Exp Ther Med. 2014;7(3):560–564. doi:10.3892/etm.2014.1491
17. Colombo E, Marine JC, Danovi D, Falini B, Pelicci PG. Nucleophosmin regulates the stability and transcriptional activity of p53. Nat Cell Biol. 2002;4(7):529–533. doi:10.1038/ncb814
18. Borer RA, Lehner CF, Eppenberger HM, Nigg EA. Major nucleolar proteins shuttle between nucleus and cytoplasm. Cell. 1989;56(3):379–390. doi:10.1016/0092-8674(89)90241-9
19. Lindstrom MS. NPM1/B23: A multifunctional chaperone in ribosome biogenesis and chromatin remodeling. Biochem Res Int. 2011;2011:195209. doi:10.1155/2011/195209
20. Leotoing L, Meunier L, Manin M, et al. Influence of nucleophosmin/B23 on DNA binding and transcriptional activity of the androgen receptor in prostate cancer cell. Oncogene. 2008;27(20):2858–2867. doi:10.1038/sj.onc.1210942
21. Li XY, Yao X, Li SN, et al. RNA-Seq profiling reveals aberrant RNA splicing in patient with adult acute myeloid leukemia during treatment. Eur Rev Med Pharmacol Sci. 2014;18(9):1426–1433.
22. Fan X, Wen L, Li Y, Lou L, Liu W, Zhang J. The expression profile and prognostic value of APE/Ref-1 and NPM1 in high-grade serous ovarian adenocarcinoma. Apmis. 2017;125(10):857–862. doi:10.1111/apm.12733
23. Sawazaki H, Ito K, Asano T, et al. Increased nucleophosmin expression is a strong predictor of recurrence and prognosis in patients with N0M0 upper tract urothelial carcinoma undergoing radical nephroureterectomy. World J Urol. 2017;35(7):1081–1088. doi:10.1007/s00345-016-1977-1
24. Chen S, Meng T, Zheng X, et al. Contribution of nucleophosmin overexpression to multidrug resistance in breast carcinoma. J Drug Target. 2018;26(1):27–35. doi:10.1080/1061186X.2017.1332066
25. Shandilya J, Senapati P, Dhanasekaran K, et al. Phosphorylation of multifunctional nucleolar protein nucleophosmin (NPM1) by aurora kinase B is critical for mitotic progression. FEBS Lett. 2014;588(14):2198–2205. doi:10.1016/j.febslet.2014.05.014
26. Weng MC, Wang MH, Tsai JJ, et al. Regorafenib inhibits tumor progression through suppression of ERK/NF-kappaB activation in hepatocellular carcinoma bearing mice. Biosci Rep. 2018.
27. Pan PJ, Tsai JJ, Liu YC. Amentoflavone inhibits metastatic potential through suppression of ERK/NF-kappaB activation in osteosarcoma U2OS cells. Anticancer Res. 2017;37(9):4911–4918. doi:10.21873/anticanres.11900
28. Karin M, Yamamoto Y, Wang QM. The IKK NF-kappa B system: a treasure trove for drug development. Nat Rev Drug Discov. 2004;3(1):17–26. doi:10.1038/nrd1279
29. Xu YH, Li ZL, Qiu SF. IFN-gamma induces gastric cancer cell proliferation and metastasis through upregulation of integrin beta3-mediated NF-kappaB signaling. Transl Oncol. 2018;11(1):182–192. doi:10.1016/j.tranon.2017.11.008
30. Ekambaram P, Lee JL, Hubel NE, et al. The CARMA3-Bcl10-MALT1 signalosome drives NF-kappaB activation and promotes aggressiveness in angiotensin II receptor-positive breast cancer. Cancer Res. 2017.
31. Huang S, Wa Q, Pan J, et al. Downregulation of miR-141-3p promotes bone metastasis via activating NF-kappaB signaling in prostate cancer. J Exp Clin Cancer Res. 2017;36(1):173. doi:10.1186/s13046-017-0645-7
32. Young D, Das N, Anowai A, Dufour A. Matrix metalloproteases as influencers of the cells’ social media. Int J Mol Sci. 2019;20(16):16. doi:10.3390/ijms20163847
33. Conlon GA, Murray GI. Recent advances in understanding the roles of matrix metalloproteinases in tumour invasion and metastasis. J Pathol. 2019;247(5):629–640. doi:10.1002/path.5225
34. Tsubaki M, Ogawa N, Takeda T, et al. Dimethyl fumarate induces apoptosis of hematopoietic tumor cells via inhibition of NF-kappaB nuclear translocation and down-regulation of Bcl-xL and XIAP. Biomed Pharmacother. 2014;68(8):999–1005. doi:10.1016/j.biopha.2014.09.009
35. Loubeau G, Boudra R, Maquaire S, et al. NPM1 silencing reduces tumour growth and MAPK signalling in prostate cancer cells. PLoS One. 2014;9(5):e96293. doi:10.1371/journal.pone.0096293
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.