Back to Journals » Journal of Experimental Pharmacology » Volume 12
Attenuation of Visceral and Somatic Nociception by Ghrelin Mimetics
Authors N Mohammadi E, Louwies T, Pietra C, Northrup SR, Greenwood-Van Meerveld B
Received 14 February 2020
Accepted for publication 10 June 2020
Published 5 August 2020 Volume 2020:12 Pages 267—274
DOI https://doi.org/10.2147/JEP.S249747
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Bal Lokeshwar
Ehsan N Mohammadi,1 Tijs Louwies,1 Claudio Pietra,2 S Robert Northrup,3 Beverley Greenwood-Van Meerveld1
1Oklahoma Center for Neuroscience, Department of Physiology, University of Oklahoma Health Science Center, Oklahoma City, OK, USA; 2Helsinn Healthcare SA, Lugano 6915, Switzerland; 3Helsinn Therapeutics Inc., Iselin, NJ 08830, USA
Correspondence: Beverley Greenwood-Van Meerveld
Oklahoma Center for Neuroscience, Department of Physiology, University of Oklahoma Health Science Center, O’Donoghue Building, Room 332, 1122 NE 13 th Street, Oklahoma City, OK 73117, USA
Tel +1 405 456-3547
Email [email protected]
Purpose: The anti-nociceptive properties of ghrelin have been demonstrated in alleviating inflammatory and neuropathic pain. Whether a ghrelin receptor-mediated mechanism attenuates visceral and somatic pain in the absence of active inflammation remains to be explored. Here, we investigate the efficacy of peripherally restricted (ipamorelin) and a globally active (HM01) selective ghrelin receptor agonist in an experimental model of non-inflammatory visceral hypersensitivity and somatic mechanical allodynia.
Materials and Methods: Visceral hypersensitivity was induced by dilute acetic acid (0.6%) infusion in the colon of rats in the absence of colonic epithelial inflammation. Ghrelin mimetics HM01 and ipamorelin were administered orally or intravenously, respectively. The ghrelin receptor antagonist H0900 was administered orally. Colonic sensitivity was assessed via a visceromotor behavioral response (VMR) quantified as the number of abdominal contractions in response to graded isobaric pressures (0– 60 mmHg) of colorectal distension (CRD). Somatic mechanical allodynia was quantified by the number of ipsilateral paw withdrawals in response to a calibrated von Frey filament.
Results: Compared to vehicle controls, ghrelin mimetics HM01 and ipamorelin significantly attenuated colonic hypersensitivity and somatic allodynia. The anti-nociceptive effects of the ghrelin mimetics were blocked after administration of the ghrelin receptor antagonist H0900.
Conclusion: We have shown that ghrelin receptor-mediated mechanisms are involved in visceral and somatic hypersensitivity in the absence of active colonic inflammation. Furthermore, visceral and somatic hypersensitivity could be attenuated by a peripherally restricted ghrelin mimetic. These results highlight a potential novel approach for treating acute visceral and somatic pain by ghrelin mimetics.
Keywords: ghrelin mimetics, HM01, ipamorelin, rat, visceral hypersensitivity, somatic allodynia
Introduction
Ghrelin, a 28-amino acid peptide, is mainly produced by endocrine cells in the gastric mucosa. The acylated form of ghrelin (ghrelin) can serve as a neuroendocrine signal when it binds the growth hormone secretagogue receptor (GHS-R1a). These receptors are found throughout the body but are abundantly expressed in certain regions of the central nervous system, such as the hypothalamus, ventral tegmental area, hippocampus, and substantia nigra. Ghrelin binding in the brain stimulates not only the secretion of growth hormone but also regulates appetite and feeding behavior. In addition, ghrelin activity can influence carbohydrate and lipid metabolism as well as, gastric motility and acid secretion.1 Apart from its role in controlling food intake and energy expenditure, ghrelin can also bind to GHS-R on immune cells and shift their cytokine expression profile towards an anti-inflammatory state.2 Following this discovery, it was found that ghrelin could attenuate neuropathic and inflammatory pain. For example, in animal models of neuropathic pain, ghrelin inhibited the release of pro-inflammatory cytokines, which alleviates neuropathic pain.3–5 In a model of formalin-induced inflammatory pain, ghrelin administration shifted immune cell-mediated cytokine production towards an anti-inflammatory profile, reducing the intensity of early and late phase pain.6 Interestingly, studies have shown that the analgesic effects of ghrelin in models of inflammatory pain can also be mediated through central pathways. For instance, in a rodent model of carrageenan-induced acute inflammation, intracerebrovascular (i.c.v.) or systemic administration of ghrelin attenuated hyperalgesia through a mechanism involving the central opioid system.7,8 Taken together, these studies demonstrate the beneficial role of ghrelin in regulating inflammatory and neuropathic pain, however, whether ghrelin and its receptor are also involved in visceral and somatic pain remains to be elucidated.
Visceral hypersensitivity is one of the defining symptoms of the functional gastrointestinal disorder irritable bowel syndrome (IBS). In IBS patients, visceral hypersensitivity is observed as a lowered pain threshold in response to distention of a balloon catheter in the distal rectosigmoid colon.9,10 Some epidemiological studies have shown that up to 60% of IBS patients also suffer from fibromyalgia, a disorder characterized by deep muscle pain, making it one of the most prevalent comorbidities of IBS.11–13 This enhanced nociceptive sensitivity in IBS patients occurs at least in part through viscero-somatic convergence at the level of the spinal cord.14,15 In this way, sensitization of the colonic afferents may lead to abnormal processing of sensory input from somatic afferent nerves, leading to a sensitization of the somatic afferents. Although no perfect animal model of IBS exists, advances in the understanding of the pathophysiology of IBS have facilitated the development of preclinical rodent models that recapitulate key symptoms of IBS such as visceral and somatic hypersensitivity.16 One such model uses a very low concentration of acetic acid directly administered into the colon of a rat to induce acute IBS-like visceral hypersensitivity, in absence of overt inflammation. In this rodent model, a transient sensitization of colonic sensory afferents occurs, in absence of colonic inflammation, which leads to colonic hypersensitivity in response to luminal distention.17–19
Based on the aforementioned studies on the analgesic effects of ghrelin, we hypothesized that ghrelin is capable of exerting an anti-nociceptive activity in a non-inflammatory rat model of acute visceral and somatic pain. For this purpose, we investigated the efficacy of two ghrelin mimetics, HM01 and ipamorelin (IPAM). The ghrelin receptor agonist HM01 is a synthetic small-molecule compound that displays high receptor binding affinity and a longer plasma half-life compared with endogenous ghrelin. In addition, HM01 is known to have high brain permeability, which allows it to mimic the central effects of ghrelin.20 IPAM is a synthetic peptide-mimetic that also selectively stimulates the ghrelin receptor, but is peripherally restricted.21 In order to determine whether the analgesic effects of the ghrelin mimetics were purely mediated through the ghrelin receptor, we used the selective ghrelin receptor antagonist H0900 in order to block the effects of HM01 and IPAM.
Materials and Methods
Animals
Male Sprague Dawley rats (300–400 g, 76–84 days old on arrival) were purchased from Charles River Laboratories (Wilmington, MA). Rats were housed two-per-cage with free access to food and water at 21°C-23°C and a 12-hr light/dark cycle within the University of Oklahoma Health Sciences Center (OUHSC) Department of Comparative Medicine’s animal facility in Oklahoma City, OK, USA (AAALAC international accredited facility, D16-00104, A3165-01) Rats were acclimated to the animal facility for 1 week, and to the laboratory/experimenter for another week in order to reduce stress, induced by exposure to a novel environment. After this 2-week acclimation period, experiments were performed during the light phase of the light/dark cycle. For experiments designed to investigate IPAM, which is not orally bioavailable, the rats were purchased from Charles River Laboratories with indwelling catheters implanted in the right jugular vein for administration of drugs or vehicle. The catheters were maintained patent by gently flushing with 0.3 mL of heparinized saline every 3–4 days. A total of 101 rats were used to complete this study. Upon arrival from the vendor, all animals were acclimated to the animal facility for a minimum of 1 week. To further minimize experimental stress, the rats were brought to the laboratory for an additional week to acclimate to the laboratory environment and animal handlers. The experimental protocol was approved by the University of Oklahoma Health Sciences Center (OUHSC) Institutional Animal Care and Use Committee (IACUC Animal Protocol # 13–140). The guidelines for the care and use of laboratory animals issued by the National Institute of Health (NIH) were followed.
Induction of Acute Visceral and Somatic Hypersensitivity
After an overnight fast, rats were brought to the laboratory and anesthetized with 5% isoflurane/100% oxygen. Visceral and somatic hypersensitivity were induced by infusing dilute (1.5 mL at 0.6%) acetic acid into the rat colon via a catheter (Intramedic PE 205 tubing, BD and Co., Franklin Lakes, NJ) inserted via the anus to the level of the mid-colon. The dilute acetic acid was prepared from concentrated glacial acetic acid stock (Sigma, St. Louis, MO). Rats receiving an infusion of normal saline into the colon served as control.
Colonic Sensitivity
Colonic sensitivity was assessed as the visceromotor response (VMR) to colorectal distension (CRD). The VMR is a protective reflex of the abdominal muscle, which is activated when CRD triggers nociceptive neuronal pathways from the colorectum.22,23 Colonic sensitivity was assessed 60 min following the infusion of the dilute acetic acid, between 10:00 AM and 2:00 PM. Immediately after the acetic acid enema, a 5-cm latex balloon was inserted approximately 11-cm past the anus into the colon, secured to the base of the tail with surgical tape and connected to a Distender Series IIR barostat (G & J Electronics Inc., Toronto, Ontario, Canada). Colonic sensitivity was measured by counting the number of abdominal contractions in response to graded pressures of isobaric CRD (randomized 0–60 mmHg). Each pressure was maintained for a period of 10 min during which the number of abdominal muscle contractions were counted. A 10-min recovery period was allowed between each distension.
Somatic Sensitivity
In a different cohort, somatic sensitivity also was assessed 60 min after acetic acid administration. Somatic sensitivity was measured by quantifying of the number of paw withdrawals in response to a calibrated von Frey filament.24,25 The von Frey Anesthesiometer (IITC Life Science Inc., Woodland Hills, CA) measures the level of somatic sensitivity by recording the minimal force required to elicit hind paw withdrawal. The animals were placed in a mesh-bottomed von Frey caging apparatus and a nylon filament was steadily pushed against the rat hind paw until withdrawal of the hind paw. The force in grams which elicited hind paw withdrawal was immediately recorded and removed. The von Frey probing was repeated two additional times using the same point on the same paw with 5-min intervals between each testing session, and an average is taken of the 3 pressures to give the average withdrawal pressure.
Test Compounds and Dose
HM01 and IPAM were supplied by Helsinn Healthcare SA, Lugano, Switzerland, and were stored at 4º C. HM01 was prepared as a suspension in a 1% methylcellulose solution and was dosed orally via gavage at a volume of 0.5 mL per 100 g body weight. The following doses of HM01 were used in the experiments: 1, 3, 10, and 30 mg/kg. IPAM was dissolved in sterile saline, pH to 7 and dosed intravenously at a volume of 0.1 mL per 100 g body weight. The following doses of IPAM were used in the experiments: 0.1, 1.0, and 10 mg/kg. The doses for this study were selected based on previous experiments and publications on the efficacy of ghrelin mimetics.26,27 The ghrelin antagonist, H0900 was supplied by Helsinn Healthcare SA, Lugano Switzerland, and administered via oral gavage as a suspension in a 1% methylcellulose solution at a volume of 0.5 mL per 100 g body weight. For all experiments with the antagonist H0900, a dose of 30 mg/kg was used. H0900 was developed as a potent and selective ghrelin antagonist from a lead optimization project. H0900 showed ghrelin receptor antagonism with an IC50 of 7.3 nM in the HEK293 cells stably expressing human ghrelin GHSR1a receptor by using a fluorometric imaging plate reader assay, without evidence for any agonistic or inverse agonist activity up to 10 µM. No relevant inhibition was observed in a panel screen conducted with H0900 at 10 µM against 73 mouse, rat, and human receptors.
Experimental Design
Colonic or somatic sensitivity was assessed 1 h after acetic acid enema. Rats in the HM01 group, received HM01 via oral gavage immediately after the dilute acetic acid enema. Rats in the IPAM group received IPAM intravenously 5 min prior to colonic or somatic sensitivity assessment. Vehicle controls rats were treated at the same time points with their respective vehicle (1% methylcellulose for HM01 and saline for IPAM). The ghrelin receptor antagonist H0900 was administered 30 min before colonic or somatic sensitivity assessment.
Data Analysis
Data are shown as mean ± SEM. To determine statistical significance, data were compared using two-way ANOVA followed by a Bonferroni multi-comparison post-test (visceral sensitivity) and one-way ANOVA followed by a Tukey’s multi-comparison post-test (somatic sensitivity). Results were deemed significant when p-values were less than 0.05 (GraphPad Prism 6.0c; La Jolla, CA). A sample size calculation, based on our previous experiments and experience, was performed prior to experimentation to determine the minimum number of animals required for each experiment (n=4 for colonic sensitivity and n=9 for somatic sensitivity). All experiments were conducted in a randomized order. The experimenter was not blinded to the treatment groups.
Results
Inhibition of Colonic Hypersensitivity by Ghrelin Mimetics
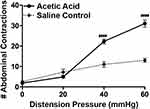
Our findings revealed main effect of treatment (F(1, 108)=50.15, p<0.0001), a main effect of pressure (F(3, 108)=54.15, p<0.0001), and a treatment X pressure interaction (F(3, 108)=23.10, p<0.0001). As illustrated in Figure 1, rats treated with acetic acid had a significantly higher number of abdominal contractions at distention pressures of 40 mmHg (p<0.0001) and 60 mmHg (p<0.0001). Treatment with the selective ghrelin mimetic HM01 (1–30 mg/kg p.o) induced a dose-dependent decrease in the number of abdominal contractions (F(4, 156) = 41.44, p<0.0001) at 40 mmHg (p=0.0173) and 60 mmHg (p=0.0115) (Figure 2A). To confirm that colonic hypersensitivity was attenuated due to the interaction between the ghrelin mimetics and the ghrelin receptors, we employed the selective ghrelin receptor antagonist H0900. We investigated whether administration of H0900 could block the anti-nociceptive effects of HM01 (1 mg/kg) since this dose showed efficacy in decreasing acetic acid-induced colonic hypersensitivity in our previous experiments. Administration of H0900 prevented the anti-nociceptive effects of HM01 (1 mg/kg) as we observed a main effect of pressure (F(3, 48)=371.9, p<0.0001), but no main effect of treatment (F(1, 48)=1.494, p=0.2276) on the number of abdominal contractions (Figure 2B). We conducted a similar set of experiments to test the efficacy of the peripherally restricted ghrelin mimetic IPAM. Our findings revealed a main effect of pressure (F(3, 112)=240.1, p<0.0001) and treatment (F(3, 112)=20.4, p<0.0001) after IPAM (0.01–1.0 mg/kg i.v.). As illustrated in Figure 3A, rats that received the highest dose of IPAM (1 mg/kg i.v.) had a significantly lower number of abdominal contractions at 40 mmHg (p<0.0001) and 60 mmHg (p<0.0001). Administration of the ghrelin antagonist H0900 prevented the anti-nociceptive effects of IPAM (1 mg/kg) as we observed an effect of pressure (F(3, 48)=146.2, p<0.0001), but not treatment (F(1, 48)=0.3262, p=0.5706) (Figure 3B).
Inhibition of Somatic Hypersensitivity by Ghrelin Mimetics
Intracolonic infusion of dilute acetic acid also induced somatic hypersensitivity, as our findings revealed that the withdrawal threshold of animals that received acetic acid was significantly lower when compared to animals receiving intracolonic saline (p<0.0001) (Figure 4A and B). Since our previous experiments showed that HM01 at 1 mg/kg and IPAM at 1 mg/kg effectively decreased visceral hypersensitivity, the same doses were used to assess whether these treatments also improved acetic acid-induced somatic hypersensitivity. We observed a main effect of treatment with HM01 (1 mg/kg p.o.) on withdrawal threshold (F(3, 32)=18.55, p<0.0001). Treatment with HM01 after acetic acid significantly increased the withdrawal threshold (p=0.0001). Administration of H0900 (30 mg/kg, p.o.) significantly decreased the withdrawal threshold when compared to animals treated with HM01 (p=0.0057) to levels comparable to acetic acid treatment (Figure 4A). We observed similar effects when rats were treated with IPAM. Our findings revealed a main effect of treatment with ipamorelin after acetic acid on withdrawal threshold (F(3, 32)=71.85, p<0.0001), as IPAM (1 mg/kg, i.v.) significantly increased withdrawal threshold (p<0.0001). Administration of H0900 (30 mg/kg, p.o.) blocked the anti-nociceptive effects of IPAM, as evidenced by a significant decrease in withdrawal threshold (p<0.0001) which was comparable to acetic acid-treated animals (Figure 4B).
Discussion
In this study, we investigated the anti-nociceptive properties of two ghrelin receptor agonists/ghrelin mimetics (HM01 and IPAM) in a non-inflammatory rat model of acute visceral and somatic hypersensitivity. Our results demonstrate that the two ghrelin mimetics significantly attenuated visceral hypersensitivity and somatic allodynia. In addition, administration of the ghrelin receptor antagonist H0900 prevented the anti-nociceptive effects of the ghrelin mimetics, suggesting that the anti-nociceptive properties of ghrelin mimetics are mediated through a ghrelin receptor-mediated mechanism.
Our results are supported by previous reports showing the anti-nociceptive effects of ghrelin in models of inflammatory, neuropathic, and acute pain, which could be blocked by antagonizing GHSR-1a.6,7,15 In some of these studies, ghrelin exerted its anti-nociceptive effects by reducing the release of pro-inflammatory cytokines and/or reducing overall inflammation.3,28 In a model of neuropathic pain, administration of ghrelin in the dorsal horn of the spinal cord inhibited phosphorylation of p38 MAPK and the activation of NF-kBp65, which are known to be involved in the upregulation of pro-inflammatory cytokines.5 In a model of acute pain, the anti-nociceptive effects of i.c.v. administered ghrelin were attenuated by the opioid receptor antagonist naloxone in the tail withdrawal and hot-plate test, showing that the central opioid receptor system could also be involved in the anti-nociceptive effects of ghrelin.29 Further evidence for the interaction between ghrelin and the opioid system comes from a study by Mao et al (2017). In a model of chronic visceral pain, induced by maternal deprivation during early life, subcutaneous ghrelin administration was capable of inhibiting TRPV1 expression and increasing opioid receptor expression in peripheral and central sites. As a result, ghrelin administration attenuated chronic visceral pain.30
In contrast to some of the aforementioned studies, our model of hypersensitivity was established in the absence of an active inflammatory reaction. Therefore, the observed anti-nociceptive effects of the ghrelin mimetics were likely not due to their interaction with active immune cells. Using an enema of low-concentrated acetic acid, we were able to induce transient visceral hypersensitivity in absence of active colonic inflammation. This concentration of acetic acid has been previously shown to sensitize visceral afferents.17,19 In this study, we also observed that transient sensitization of colonic visceral afferents also leads to somatic hypersensitivity, measured as a decrease in withdrawal threshold of the hind-paw. These observations are explained by convergence of visceral and somatic afferents, for instance at the level of the spinal cord or higher brain centers. In this way, sensitization of visceral afferents can alter central processing of both visceral and somatic inputs, leading to increased visceral and somatic nociception.31–34 In this model of visceral and somatic hypersensitivity, ghrelin mimetics were able to attenuate visceral and somatic hypersensitivity. The anti-nociceptive effects of these ghrelin mimetics were mediated through their interaction with the ghrelin receptors, as administration of the ghrelin receptor antagonist H0900 prevented amelioration of visceral and somatic hypersensitivity.
Previous studies have shown that oral administration of the ghrelin mimetic HM01 can activate c-Fos neurons in the brain and spinal cord.20,35 Moreover, the ghrelin receptor is expressed in central regions related to pain transmission such as the hypothalamus and the midbrain.36,37 In our study, it is possible that HM01 could have activated central and peripheral ghrelin receptor-mediated pathways, which alleviated visceral and somatic hypersensitivity. In contrast to HM01, the ghrelin mimetic IPAM is not able to cross the blood-brain-barrier, but can still show efficacy when administrated intravenously.21,38 Interestingly, although peripherally restricted, our results indicate that IPAM administration could also attenuate visceral and somatic pain behaviors. Given the fact that a peripherally restricted ghrelin mimetic could also alleviate visceral and somatic hypersensitivity points toward a (partial) peripheral mechanism of the anti-nociceptive activity of ghrelin mimetics. A caveat of this interpretation is that we cannot exclude the potential influence of the central activity of HM01, as higher doses of HM01 showed increasing efficacy in ameliorating visceral hypersensitivity.
In conclusion, our data suggest that ghrelin receptor-mediated mechanisms are involved in acute visceral and somatic hypersensitivity. Hence, activating the ghrelin receptor with ghrelin mimetics may offer a novel approach for the treatment of acute visceral pain in IBS.
Abbreviations
IBS, irritable bowel syndrome; VMR, visceromotor behavioral response; CRD, colorectal distension; i.c.v., intracerebroventricular; HM01, (N’-[(1S)-1-(2,3-dichloro-4-methoxyphenyl)ethyl]-N-methyl-N-[1,3,3-trimethyl-(4R)]; i.v., intravenous; p.o., per os; Veh, vehicle control.
Acknowledgments
Preliminary results of this study entitled, A Study to Investigate the Effect of Ghrelin Mimetics HM01 and Ipamorelin in a Rodent Model of Colonic and Somatic Hypersensitivity, was presented as a poster presentation at “Society for Neuroscience” meeting, 2014, Washington DC, USA.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Ehsan N. Mohammadi reports grants from Helsinn Pharma, during the conduct of the study. Claudio Pietra reports having been an employee of Helsinn SA Switzerland and that they are now are consultant for them, in addition to being an employee of Helsinn Healthcare SA, Switzerland, at the time of the study. S. Robert Northrup reports being an employee of Helsinn Therapeutics Inc., USA, at the time of the study. The authors reports no other potential conflicts of interest in this work.
References
1. Avau B, Carbone F, Tack J, Depoortere I. Ghrelin signaling in the gut, its physiological properties, and therapeutic potential. Neurogastroenterol Motil. 2013;25(9):720–732. doi:10.1111/nmo.12193
2. Dixit VD, Schaffer EM, Pyle RS, et al. Ghrelin inhibits leptin- and activation-induced proinflammatory cytokine expression by human monocytes and T cells. J Clin Invest. 2004;114(1):57–66. doi:10.1172/JCI200421134
3. Guneli E, Kazikdas KC, Kolatan E. Ghrelin may attenuate proinflammatory cytokine-mediated neuropathic pain. Med Hypotheses. 2007;69(2):356–360. doi:10.1016/j.mehy.2006.12.042
4. Peng Z, Zha L, Yang M, Li Y, Guo X, Feng Z. Effects of ghrelin on pGSK-3beta and beta-catenin expression when protects against neuropathic pain behavior in rats challenged with chronic constriction injury. Sci Rep. 2019;9(1):14664. doi:10.1038/s41598-019-51140-w
5. Zhou CH, Li X, Zhu YZ, et al. Ghrelin alleviates neuropathic pain through GHSR-1a-mediated suppression of the p38 MAPK/NF-kappaB pathway in a rat chronic constriction injury model. Reg Anesth Pain Med. 2014;39(2):137–148. doi:10.1097/AAP.0000000000000050
6. Azizzadeh F, Mahmoodi J, Sadigh-Eteghad S, Farajdokht F, Mohaddes G. Ghrelin exerts analgesic effects through modulation of IL-10 and TGF-beta levels in a rat model of inflammatory pain. Iran Biomed J. 2017;21(2):114–119. doi:10.18869/acadpub.ibj.21.2.114
7. Sibilia V, Lattuada N, Rapetti D, et al. Ghrelin inhibits inflammatory pain in rats: involvement of the opioid system. Neuropharmacology. 2006;51(3):497–505. doi:10.1016/j.neuropharm.2006.04.009
8. Sibilia V, Pagani F, Mrak E, Dieci E, Tulipano G, Ferrucci F. Pharmacological characterization of the ghrelin receptor mediating its inhibitory action on inflammatory pain in rats. Amino Acids. 2012;43(4):1751–1759. doi:10.1007/s00726-012-1260-8
9. Whitehead WE, Holtkotter B, Enck P, et al. Tolerance for rectosigmoid distention in irritable bowel syndrome. Gastroenterology. 1990;98(5 Pt 1):1187–1192. doi:10.1016/0016-5085(90)90332-U
10. Poitras P, Riberdy Poitras M, Plourde V, Boivin M, Verrier P. Evolution of visceral sensitivity in patients with irritable bowel syndrome. Dig Dis Sci. 2002;47(4):914–920. doi:10.1023/A:1014729125428
11. Sperber AD, Atzmon Y, Neumann L, et al. Fibromyalgia in the irritable bowel syndrome: studies of prevalence and clinical implications. Am J Gastroenterol. 1999;94(12):3541–3546. doi:10.1111/j.1572-0241.1999.01643.x
12. Veale D, Kavanagh G, Fielding JF, Fitzgerald O. Primary fibromyalgia and the irritable bowel syndrome: different expressions of a common pathogenetic process. Br J Rheumatol. 1991;30(3):220–222. doi:10.1093/rheumatology/30.3.220
13. Yang TY, Chen CS, Lin CL, Lin WM, Kuo CN, Kao CH. Risk for irritable bowel syndrome in fibromyalgia patients: a national database study. Medicine (Baltimore). 2017;96(14):e6657. doi:10.1097/MD.0000000000006657
14. Chang L. Brain responses to visceral and somatic stimuli in irritable bowel syndrome: a central nervous system disorder? Gastroenterol Clin North Am. 2005;34(2):271–279. doi:10.1016/j.gtc.2005.02.003
15. Zhou Q, Fillingim RB, Riley JL, Malarkey WB, Verne GN. Central and peripheral hypersensitivity in the irritable bowel syndrome. Pain. 2010;148(3):454–461. doi:10.1016/j.pain.2009.12.005
16. Greenwood-van Meerveld B, Prusator DK, Johnson AC. Animal models of gastrointestinal and liver diseases. Animal models of visceral pain: pathophysiology, translational relevance, and challenges. Am J Physiol Gastrointest Liver Physiol. 2015;308(11):G885–903. doi:10.1152/ajpgi.00463.2014
17. Plourde V, St-Pierre S, Quirion R. Calcitonin gene-related peptide in viscerosensitive response to colorectal distension in rats. Am J Physiol. 1997;273(1 Pt 1):G191–196. doi:10.1152/ajpgi.1997.273.1.G191
18. Gaudreau GA, Plourde V. Role of tachykinin NK1, NK2 and NK3 receptors in the modulation of visceral hypersensitivity in the rat. Neurosci Lett. 2003;351(2):59–62. doi:10.1016/S0304-3940(03)00414-2
19. Greenwood-van Meerveld B, Johnson AC, Foreman RD, Linderoth B. Attenuation by spinal cord stimulation of a nociceptive reflex generated by colorectal distention in a rat model. Auton Neurosci. 2003;104(1):17–24. doi:10.1016/S1566-0702(02)00262-X
20. Karasawa H, Pietra C, Giuliano C, et al. New ghrelin agonist, HM01 alleviates constipation and L-dopa-delayed gastric emptying in 6-hydroxydopamine rat model of Parkinson’s disease. Neurogastroenterol Motil. 2014;26(12):1771–1782. doi:10.1111/nmo.12459
21. Venkova K, Mann W, Nelson R, Greenwood-van Meerveld B. Efficacy of ipamorelin, a novel ghrelin mimetic, in a rodent model of postoperative ileus. J Pharmacol Exp Ther. 2009;329(3):1110–1116. doi:10.1124/jpet.108.149211
22. Jones RC, Otsuka E, Wagstrom E, Jensen CS, Price MP, Gebhart GF. Short-term sensitization of colon mechanoreceptors is associated with long-term hypersensitivity to colon distention in the mouse. Gastroenterology. 2007;133(1):184–194. doi:10.1053/j.gastro.2007.04.042
23. Myers B, Greenwood-van Meerveld B. Corticosteroid receptor-mediated mechanisms in the amygdala regulate anxiety and colonic sensitivity. Am J Physiol Gastrointest Liver Physiol. 2007;292(6):G1622–1629. doi:10.1152/ajpgi.00080.2007
24. Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111(4):409–419.
25. Johnson AC, Tran L, Greenwood-van Meerveld B. Knockdown of corticotropin-releasing factor in the central amygdala reverses persistent viscerosomatic hyperalgesia. Transl Psychiatry. 2015;5:e517. doi:10.1038/tp.2015.16
26. Greenwood-van Meerveld B, Tyler K, Mohammadi E, Pietra C. Efficacy of ipamorelin, a ghrelin mimetic, on gastric dysmotility in a rodent model of postoperative ileus. J Exp Pharmacol. 2012;4:149–155. doi:10.2147/JEP.S35396
27. Mohammadi EN, Pietra C, Giuliano C, Fugang L, Greenwood-van Meerveld B. A comparison of the central versus peripheral gastrointestinal prokinetic activity of two novel ghrelin mimetics. J Pharmacol Exp Ther. 2019;368(1):116–124. doi:10.1124/jpet.118.250738
28. Guneli E, Onal A, Ates M, et al. Effects of repeated administered ghrelin on chronic constriction injury of the sciatic nerve in rats. Neurosci Lett. 2010;479(3):226–230. doi:10.1016/j.neulet.2010.05.066
29. Wei J, Zhi X, Wang XL, et al. In vivo characterization of the effects of ghrelin on the modulation of acute pain at the supraspinal level in mice. Peptides. 2013;43:76–82. doi:10.1016/j.peptides.2013.03.004
30. Mao Y, Li Z, Chen K, et al. Antinociceptive effect of ghrelin in a rat model of irritable bowel syndrome involves TRPV1/opioid systems. Cell Physiol Biochem. 2017;43(2):518–530. doi:10.1159/000480478
31. Cameron DM, Brennan TJ, Gebhart GF. Hind paw incision in the rat produces long-lasting colon hypersensitivity. J Pain. 2008;9(3):246–253. doi:10.1016/j.jpain.2007.10.017
32. Cervero F. Somatic and visceral inputs to the thoracic spinal cord of the cat: effects of noxious stimulation of the biliary system. J Physiol. 1983;337:51–67. doi:10.1113/jphysiol.1983.sp014611
33. Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. II. Noxious visceral stimuli inhibit cutaneous nociceptive neurons and reflexes. J Neurophysiol. 1991;66(1):29–39. doi:10.1152/jn.1991.66.1.29
34. Ness TJ, Gebhart GF. Interactions between visceral and cutaneous nociception in the rat. I. Noxious cutaneous stimuli inhibit visceral nociceptive neurons and reflexes. J Neurophysiol. 1991;66(1):20–28. doi:10.1152/jn.1991.66.1.20
35. Naitou K, Mamerto TP, Pustovit RV, et al. Site and mechanism of the colokinetic action of the ghrelin receptor agonist, HM01. Neurogastroenterol Motil. 2015;27(12):1764–1771. doi:10.1111/nmo.12688
36. Ferrini F, Salio C, Lossi L, Merighi A. Ghrelin in central neurons. Curr Neuropharmacol. 2009;7(1):37–49. doi:10.2174/157015909787602779
37. Zigman JM, Jones JE, Lee CE, Saper CB, Elmquist JK. Expression of ghrelin receptor mRNA in the rat and the mouse brain. J Comp Neurol. 2006;494(3):528–548. doi:10.1002/cne.20823
38. Raun K, Hansen BS, Johansen NL, et al. Ipamorelin, the first selective growth hormone secretagogue. Eur J Endocrinol. 1998;139(5):552–561. doi:10.1530/eje.0.1390552
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.