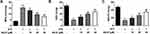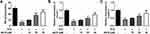Back to Journals » Diabetes, Metabolic Syndrome and Obesity » Volume 15
Astragaloside IV Ameliorates Streptozotocin Induced Pancreatic β-Cell Apoptosis and Dysfunction Through SIRT1/P53 and Akt/GSK3β/Nrf2 Signaling Pathways
Authors Lin Y , Xu Y, Zheng X, Zhang J, Liu J, Wu G
Received 3 November 2021
Accepted for publication 17 December 2021
Published 13 January 2022 Volume 2022:15 Pages 131—140
DOI https://doi.org/10.2147/DMSO.S347650
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Juei-Tang Cheng
Yuqiong Lin,1 Ying Xu,1 Xin Zheng,1 Jingwen Zhang,1 Junfeng Liu,1 Guotu Wu2
1Department of Basic Medical Science, Fujian Health College, Fuzhou, 350101, Fujian Province, People’s Republic of China; 2Department of Basic Medical Science, Fujian Medical University, Fuzhou, 350101, Fujian Province, People’s Republic of China
Correspondence: Yuqiong Lin
Department of Basic Medical Science, Fujian Health College, No. 366 Jingxi Town, Fuzhou, 350101, Fujian Province, People’s Republic of China
Email [email protected]
Background: Absolute or relative lack of insulin secretion caused by pancreatic β-cell dysfunction can lead to diabetes. Astragaloside IV (AS-IV), the main components of the traditional Chinese medicine Astragalus, has anti-oxidant, anti-inflammatory and anti-apoptotic properties, and exerts anti-diabetic pharmacological effects.
Purpose: To explore whether AS-IV can protect the apoptosis and dysfunction of pancreatic β-cells induced by streptozotocin (STZ) and its underlying molecular mechanism.
Methods: STZ-induced pancreatic β-cell line INS-1 was treated with different concentrations of AS-IV, then cell viability, apoptosis, oxidative stress and insulin secretion was assessed by CCK-8, TUNEL staining, Western blot, commercial kits and qRT-PCR, respectively. The expression of proteins involved in Sirtuin 1 (SIRT1)/p53 and Akt/glycogen synthase kinase-3 β (GSK3β)/nuclear factor E2-related factor 2 (Nrf2) signaling was measured by Western blot assay. Besides, Akt inhibitor MK-2206 and SIRT1 inhibitor EX-527 were used to co-treat STZ-induced INS-1 cells in the presence of AS-IV, and the above experiments were repeated.
Results: AS-IV increased the cell viability of INS-1 cells induced by STZ. AS-IV also reduced the increase in apoptosis rate and reversed STZ-induced down-regulation of Bcl-2 and up-regulation of Bax and Cleaved caspase 3. In addition, AS-IV significantly reduced STZ-induced malondialdehyde upregulation and reduced superoxide dismutase and glutathione peroxidase levels. Furthermore, the use of AS-IV was found to increase the insulin secretion capacity of INS-1 cells with impaired function, along with the increase of the mRNA levels of insulin 1 and insulin 2. Mechanism studies further showed that MK-2206 and EX-527 reversed the protective effect of AS-IV against STZ-induced injury on INS-1 cells.
Conclusion: AS-IV exerted cytoprotective effect on STZ-induced INS-1 cells through regulating SIRT1/p53 and Akt/GSK3β/Nrf2 signaling pathways. These findings are expected to provide new supplements to the molecular mechanism of AS-IV in the treatment of diabetes.
Keywords: astragaloside IV, pancreatic β-cell, apoptosis, dysfunction, SIRT1/p53, Akt/GSK3β/Nrf2
Introduction
Astragaloside IV (AS-IV) is extracted from the traditional Chinese medicine Astragalus. It is the main active ingredient of Astragalus, often used as a standard for evaluating the quality of Astragaloside.1 AS-IV has been found to have anti-inflammatory, anti-oxidative stress, anti-apoptotic properties, and is a potential active substance for the prevention and treatment of diabetes and its complications.2 Administration of AS-IV to a mouse gestational diabetes (GDM) model significantly reduced the levels of glucose, insulin and lipids in GDM mice while also reducing liver gluconeogenesis.3 In a rat model of diabetic cardiomyopathy (DCM), AS-IV has also been shown to protect cardiomyocytes from damage by inhibiting oxidative stress and autophagy induced by high glucose.4 Pancreatic β-cell plays an important role in the process of diabetes, and its dynamic changes have a pivotal impact on the regulation of blood sugar and the occurrence of chronic complications.5 However, as far as we know, the effect of AS-IV on pancreatic β cells has not been reported.
The other two traditional Chinese medicines Sanbai melon seed oil (SMSO) and ginsenoside Rg1 (Rg1) also play an important role in the treatment of diabetes. According to reports, all of them can protect pancreatic β cells by regulating the Akt/ glycogen synthase kinase-3 β (GSK3β)/nuclear factor E2-related factor 2 (Nrf2) pathway and inducing the expression of antioxidant proteins.6,7 The well-recognized pro-survival factor Sulfiredoxin-1 (Srxn1) has also been found to promote the activation of the Nrf2 antioxidant signaling pathway by up-regulating the phosphorylation of Akt and GSK3β to play a protective effect on the retinal ganglion cells of diabetic patients.8 In addition, Perilipin 5 also prevents apoptosis, oxidative stress and inflammation induced by high sugar in podocytes through this pathway.9 In the current report, AS-IV has been reported to inhibit the epithelial-mesenchymal transition of hepatocellular carcinoma by targeting Akt/GSK3β signaling pathway.10 However, whether the mechanism of AS-IV treatment of diabetes involves the Akt/GSK3β/Nrf2 Pathway is still unknown.
In addition to Akt/GSK3β signaling pathway, Sirtuin 1 (SIRT1) signaling pathway can also increase Nrf2 transcription by deacetylating PGC1-α.11,12 SIRT1, as the most studied enzyme among the 7 deacetylases in the Sir family, is widely present in various cells of the body and is closely related to the energy metabolism and reduction state of cells.13,14 It is a new potential therapeutic target for diabetic nephropathy.15,16 The study found that although the SIRT1 activator SRT2104 can activate the expression of SIRT1 in Nrf2 knockout mice and inhibit P53, its protective effect on diabetic nephropathy is eliminated.17 In the process of mitochondrial-dependent apoptosis in the dorsal root ganglion of rats with diabetic peripheral neuropathy, it was detected that AS-IV can up-regulate the expression of SIRT1 and inhibit the activation of P53.18 Resveratrol protects the insulin secretion of INS-1 cells induced by ethanol through the SIRT1/UCP2 axis.19 Whether the mechanism of AS-IV treatment of diabetes involves the SIRT1 signaling pathway remains to be explored.
This study aims to construct a pancreatic β-cell injury model in vitro by incubating INS-1 cells with STZ to explore whether AS-IV can protect pancreatic β-cell through SIRT1/p53 and Akt/GSK3β/Nrf2 signaling pathways effect.
Materials and Methods
Cell Culture and Treatment
Pancreatic β-cell line INS-1 (Procell Life Science&Technology) was cultured in RPMI-1640 (Sigma-Aldrich) supplemented with 10% FBS (Corning), 50 μmol/L β-mercaptoethanol (Procell Life Science&Technology) and 1% penicillin-streptomycin (Beyotime Biotechnology), and maintained at 37°C for in a humidified incubator with 5% CO2. INS-1 cells were incubated with 3 mM streptozotocin (STZ; Sigma-Aldrich) for 24 h to construct a pancreatic β-cell injury model in vitro,20 and then incubated with or without AS-IV (10, 20, 40 µM; dissolved in DMSO at the final concentration of 50 mM; Shanghai Yuanye Biotechnology) for 4 h. For the signaling pathway analysis, after STZ stimulation for 24 h, INS-1 cells were pretreated with Akt inhibitor MK-2206 (100 nM; Beyotime Biotechnology) for 72 h or SIRT1 inhibitor EX-527 (10 µM; Beyotime Biotechnology) for 48 h prior to AS-IV administration.21,22
RNA Extraction, Complementary DNA (cDNA) Synthesis, Real‐time Quantitative Reverse Transcription-Polymerase Chain Reaction (qRT-PCR)
Total RNA was extracted from the cultured INS-1 cell line using an RNA isolation kit (HaiGene Biotech Co., Ltd.), according to the manufacturer’s instructions. Total RNA was reversed into cDNA using a PrimeScript™ RT reagent kit (Perfect Real Time) (Takara Bio, Inc.). qPCR was subsequently performed on an StepOnePlus™ Real-Time PCR system (Thermo Fisher Scientific, Inc.) using a One Step TB Green® PrimeScript™ plus RT-PCR kit (Perfect Real Time) (Takara Bio, Inc.), according to the manufacturer’s protocol. The following thermocycling conditions were used for the qPCR: Initial denaturation at 42°C for 5 min and 95°C for 10 sec, followed by 40 cycles of 95°C for 5 sec and 60°C for 30 sec. The following primers pairs for Insulin 1, Insulin 2 and GAPDH were used for the qPCR:23 Insulin 1 forward, 5ʹ-GTCCTCTGGGAGCCCAAG-3ʹ and reverse, 5ʹ-ACAGAGCCTCCACCAGG-3ʹ; Insulin 2 forward, 5ʹ-ATCCTCTGGGAGCCCCGC-3ʹ and reverse, 5ʹ-AGAGAGCTTCCACCAAG-3ʹ; and β-actin forward, 5ʹ-ACCCGCCACCAGTTCGC-3ʹ and reverse, 5ʹ-CACGATGGAGGGGAAGACG-3ʹ. β-actin was used for normalization.
Western Blot (WB) Analysis
After indicated treatments with STZ, AS-IV, EX527 and MK2206, the expression of proteins in INS-1 cells were measured by WB analysis as previously reported.24 The total protein was prepared by adding Cell Lysis (ThermoFisher), after that they were isolated by using sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE; Beyotime Biotechnology) and transferred onto PVDF membranes (Millipore). After incubating in 5% skimmed milk powder dissolved in 0.1% Tween-20 triethanolamine buffered saline solution (TBST) for 2 h at room temperature, incubate at 4°C with the corresponding primary antibody overnight. Washed in TBST 3 times, HRP-conjugated secondary antibody was used for further incubation for 1 h at room temperature, chemiluminescent Western blot reagents SuperSignal West Pico Plus (ThermoFisher) was used for detection. The image was scanned with a Bio-Rad GS800 densitometer scanner, and ImageJ 1.46 software was used for data analysis. Primary antibodies: rabbit anti-Bcl-2 (1:2000, ab196495, Abcam); rabbit anti-Bax (1:8000, ab32503, Abcam); rabbit anti-Cleaved caspase 3 (1:1000, 9664, Cell Signaling Technology); rabbit anti-caspase 3 (1:1000, 9662, Cell Signaling Technology); rabbit anti-β actin (1:5000, ab8227, Abcam); rabbit anti-SIRT1 (1:1000, ab189494, Abcam); rabbit anti-p53 (1:800, ab131442, Abcam); rabbit anti-Akt (1:1000; 9272, Cell Signaling Technology); rabbit anti-p-Akt (1:2000, 4060S, Cell Signaling Technology); rabbit anti-GSK3β (1:1000, 9315, Cell Signaling Technology); and rabbit anti-Phospho-GSK3β (1:1000, 9315, Cell Signaling Technology). HRP-conjugated secondary antibodies: goat anti-rabbit IgG H&L (HRP) (1:20,000, ab6721, Abcam); goat anti-mouse IgG H&L (HRP) (1:10,000, ab6789, Abcam).
Terminal Deoxynucleotidyl Nick-End Labeling (TUNEL) Assay
Cell apoptosis was evaluated using TUNEL assay.25 5×104 INS-1 cells/well were seeded into 24-well plates and cultured overnight for attachment, followed by indicated treatments with STZ, AS-IV, EX527 and MK2206. Subsequently, One Step TUNEL Apoptosis Assay Kit (cat. no C1088; Beyotime Biotechnology) was used to determine the cell apoptosis according to the manufacturer’s commendation. The normal INS-1 cells without treatment were used as control. The levels of apoptosis were estimated as the ratio of the number of TUNEL-positive cells to the total number of DAPI-positive cells using a fluorescence microscope (Olympus Corporation) at 200 times magnification.
Determination of Intracellular Malondialdehyde (MDA), Superoxide Dismutase (SOD) and Glutathione Peroxidase (GSH-Px)
5×104 INS-1 cells/well were seeded into 24-well plates and cultured overnight for attachment, followed by indicated treatments with STZ, AS-IV, EX527 and MK2206. To measure levels of oxidative stress in INS-1 cells, Lipid Peroxidation (MDA) Assay Kit (cat. no ab118970; Abcam), Superoxide Dismutase (SOD) Activity Assay Kit (cat. no ab65354; Abcam) and GSH/GSSG Ratio (GSH-Px) Detection Assay Kit (cat. no ab138881; Abcam) were used, according to manufacturers’ protocol.
ELISA Assay
The insulin (INS) secretion in culture medium of INS-1 cells were detected by Rat insulin (INS) ELISA Kit (cat. no JN5756; Shanghai Jining Shiye). Briefly, 2×106 INS-1 cells were seeded into 6-well plates and cultured overnight for attachment, followed by indicated treatments with STZ, AS-IV, EX527 and MK2206. Subsequently, cells were collected and centrifuged at 1000×g for 20 min to obtain the supernatant, the release of INS in which was then determined according to the manufacturer’s commendation.
Cell Counting Kit-8 (CCK8) Assay
CCK8 assay Kit (cat. no C0038; Beyotime Biotechnology) was used to determine cell viability. A total of 2×104 INS-1 cells/well were seeded into a 96-well and cultured overnight for attachment, followed by indicated treatments with STZ, AS-IV, EX527 and MK2206. Cells were then incubated with CCK-8 regent for 2 h at 37°C, the absorbance at a wavelength of 450 nm was measured using an automated microplate reader (Beckman DU6400 spectrophotometer).
Statistics
Statistical analysis was performed using GraphPad Prism 8.0 (GraphPad Software, Inc.) software. Continuous variables are presented as the mean ± standard deviation from three independent experiments. P-values were calculated using unpaired Student’s t-test for comparisons between two groups or one-way ANOVA with Tukey’s test for comparisons among multiple groups. P-values<0.05 were considered significant.
Results
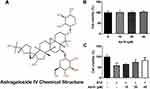
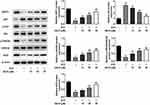
AS-IV Improves STZ-Induced INS-1 Cell Viability
AS-IV is a cycloalkane-type triterpene glycoside chemical substance (Figure 1A). In order to screen out the appropriate concentration of AS-IV, we first incubated INS-1 cells with different concentrations of AS-IV (0, 10, 20, 40 µM) for 4 h and tested the corresponding cell viability. The results showed that 0–40 µM AS-IV will not affect cell viability (Figure 1B). Next, we incubated INS-1 cells with STZ for 24 h and continued treatment with different concentrations of AS-IV for 4 h. The results showed that STZ treatment significantly reduced the cell viability of INS-1 cells, while after the addition of AS-IV, the cell viability increased in a dose-dependent manner (Figure 1C). These data indicated that AS-IV has a significant protective effect on STZ-induced INS-1 cell viability.
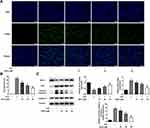
AS-IV Inhibits STZ-Induced Apoptosis of INS-1 Cells
To investigate the protective effect of AS-IV on STZ-induced INS-1 cell apoptosis, different concentrations of AS-IV (0, 10, 20, 40 µM) were added to STZ-pretreated cells. TUNEL assay and Western blot analysis were used to determine the percentage of apoptotic cells in total cells and the changes of apoptosis-related proteins. The results showed that STZ significantly caused INS-1 cell apoptosis, with an apoptotic rate of more than 20%, accompanied by a decrease in the expression of Bcl-2 and an increase in the expression of Bax and Cleaved-caspase 3. The use of AS-IV treatment significantly reversed the apoptosis caused by STZ and the apoptotic rate continued to decrease with the increase of AS-IV’s concentration (Figure 2). These data suggested that AS-IV has a protective effect on STZ-induced INS-1 cell apoptosis, and the protective effect increases with the increase of AS-IV’s concentration within a certain range.
AS-IV Reduces STZ-Induced Oxidative Stress in INS-1 Cells
To test whether AS-IV could alleviate STZ-induced oxidative stress in INS-1 cells, we detected the lipid peroxidation, superoxide dismutase activity and GSH-Px of STZ pretreated cells incubated with different concentrations of AS-IV (0, 10, 20, 40 µM). Pretreatment of INS-1 cells with STZ does caused cellular oxidative stress, which was manifested by an increase in MDA levels and a decrease in SOD and GSH-Px levels. After the addition of AS-IV, these conditions were reversed in a dose-dependent manner (Figure 3). These data indicated that AS-IV also has a relieving effect on STZ-induced oxidative stress in INS-1 cells.
AS-IV Improves STZ-Induced Insulin Secretion in INS-1 Cells
As a rat pancreatic islet cell line, INS-1 cells have the function of insulin secretion. Our research confirmed that treatment of INS-1 cells with STZ significantly inhibits their ability to secrete insulin. With different concentrations of AS-IV (0, 10, 20, 40 µM) for treatment, INS-1 cells partially restored their insulin secretion ability, and the amount of secretion was positively correlated with the concentration of AS-IV (Figure 4). In general, the use of AS-IV helped to partially restore the INS-1 cell insulin secretion dysfunction caused by STZ.
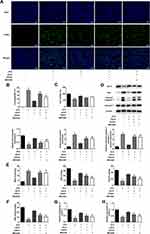
AS-IV Regulates SIRT1/P53 and Akt/GSK3β/Nrf2 Signaling Pathways
To further study the therapeutic effect of AS-IV on STZ-induced apoptosis and dysfunction of INS-1 cells, we examined the expression of SIRT1/p53 and Akt/GSK3β/Nrf2 signaling pathways relative protein by Western blot analysis. We found that after STZ treatment, the expression levels of SIRT1 and Nrf2, as well as the phosphorylation levels of Akt and GSK3p53 in INS-1 cells were significantly down-regulated along with the up-regulated expression of p53. Therefore, we determined that SIRT1/p53 and Akt/GSK3β/Nrf2 signaling pathways are involved in STZ-induced INS-1 cell apoptosis and dysfunction. By adding different concentrations of AS-IV (0, 10, 20, 40 µM) for treatment, the expression levels and phosphorylation levels of the above-mentioned pathway proteins were significantly reversed (Figure 5). These results suggested that SIRT1/p53 and Akt/GSK3β/Nrf2 signaling pathways may be involved in the therapeutic effect of AS-IV on STZ-induced inS-1 cell apoptosis and dysfunction.
EX527 (SIRT1 Inhibitor) or MK2206 (Akt Inhibitor) Reversed the Protective Effect of as-IV on STZ-Induced INS-1 Cells
To further test our hypothesis, EX527 (SIRT1 inhibitor) or MK2206 (Akt inhibitor) was used. The addition of EX527 and MK2206 to STZ-pretreated INS-1 cells both weakened the role of AS-IV in protecting cells from apoptosis, and the apoptotic rate of INS-1 cells treated with EX527 was higher than that treated with MK2206 (Figure 6A and B). The experimental data obtained by Western blot analysis were consistent with this, both inhibitors induced up-regulation of Bax and Cleaved caspase 3 expression and down-regulation of Bcl-2 expression. Additionally, EX527 had a greater effect on Bcl-2 than MK2206 (Figure 6D). From the results of the CCK-8 assay, the use of EX527 partially weakened the protective effect of AS-IV on INS-1 cell viability, while the use of MK2206 had no significant effect on cell viability (Figure 6C). In addition, compared with AS-IV treated STZ-induced INS-1 cells, INS-1 cells treated with both EX527 and MK2206 showed increased oxidative stress level, as evidenced by an increase in MDA and a decrease in SOD and GSH-Px, suggesting that the inhibitory effect of AS-IV on STZ-induced oxidative stress in INS-1 cells was reversed by both EX527 and MK2206 (Figure 6E). Finally, we explored the effects of two inhibitors on the insulin secretion capacity of INS-1 cells. The results showed that the use of EX527 and MK2206 to treat STZ pre-incubated INS-1 cells limited the therapeutic effect of AS-IV. The expression of INS, Insulin 1 and Insulin 2 were all significantly reduced, and MK2206 had a greater impact (Figure 6F). In conclusion, these results suggested that AS-IV may protect INS-1 cells from STZ-induced apoptosis mainly through activation of SIRT1, and may alleviate STZ-induced oxidative stress and insulin secretion dysfunction through activation of Nrf2 antioxidant pathway by Akt.
Discussion
For thousands of years, the traditional Chinese medicine Astragalus has been commonly used to treat many diseases including diabetes.1,2 Its main active substance is AS-IV, a cycloartane-type triterpene glycoside chemical. Here, in the current study, we aim to investigate the effect of AS-IV on STZ-induced pancreatic β-cell apoptosis and dysfunction and to explore the potential molecular mechanism. Interestingly, the protective effect of AS-IV on STZ-induced decrease in INS-1 cell viability showed a dose-dependent increase in a certain range. This protective effect has been observed in cardiomyocytes, renal tubular epithelial cells and retinal capillary endothelial cells exposed to high glucose.4,26,27 In addition, these reports also show the anti-apoptotic and anti-oxidant properties of AS-IV. Similarly, in the current study, we also observed that through AS-IV treatment, cell apoptosis and oxidative stress originally induced by STZ have been alleviated to a certain extent. Enzyme activities, including superoxide dismutase (SOD) and glutathione peroxidase (GSH-Px) have been significantly improved.
Through the determination of INS, Insulin 1 and Insulin 2 content in different groups of INS-1 cells, the current study also observed that AS-IV has a significant therapeutic effect on STZ-induced INS-1 cell pancreatic β-cell dysfunction. Deng et al gavage AS-IV to young rats with diabetic ketoacidosis and also found an improvement in insulin levels. Additionally, this study also analyzed the phosphorylation level of JNK and the expression level of Nrf2 protein in pancreatic tissue after administration and pointed out that its potential mechanism of action may involve the regulation of JNK/Nrf2 signal pathway by AS-IV.28 SMSO’s treatment of type 2 diabetes also seems to involve the Nrf2 antioxidant pathway. In a rat model of type 2 diabetes induced by a high-fat and high-sugar diet and STZ, SMSO induces Nrf2 expression and Akt and GSK3β phosphorylation in a dose-dependent manner to exert a protective effect on pancreatic β cells.6 Soon after, a study on another Chinese medicine Rg1 against myocardial damage caused by type 1 diabetes also pointed out that it can exert a protective effect by regulating the Akt/GSK3β/Nrf2 signaling pathway.7 The current study found that the phosphorylation level of Akt and GSK3β and the protein level of Nrf2 in INS-1 cells treated with AS-IV increased significantly. In order to further confirm whether its protective effect involves Akt/GSK3β/Nrf2 signaling pathway, in the current study, Akt inhibitor MK2206 was used, and it was found that AS-IV had protective effect on STZ-induced INS-1 cells, including apoptosis, oxidative stress and insulin secretion dysfunction, all be weakened. These indicate that the Akt/GSK3β/Nrf2 signaling pathway may be a conventional regulatory pathway for traditional Chinese medicine to treat diabetes and its complications.
The SIRT1 signaling pathway also plays an important role in the prevention and treatment of diabetes and its complications.29 Using AS-IV to treat cardiomyocytes in a high-glycemic environment, significantly improved the level of SIRT1.4 Moreover, AS-IV was also found to reverse glucose-induced podocyte epithelial-mesenchymal transition by increasing SIRT1 expression and decreasing p53 acetylation.30 Similar phenomena have also been observed in the dorsal root ganglia of rats with uncle peripheral neuropathy.18 In the current study, the regulation of SIRT1 and p53 by AS-IV was also observed. After treating STZ pre-incubated INS-1 cells with the SIRT1 inhibitor EX527, the cytoprotective effect of AS-IV was found to be significantly weakened. More importantly, it can be seen from experimental results that SIRT1/p53 and Akt/GSK3β/Nrf2 signaling pathways were both involved in the protective effects of AS-IV on STZ-induced apoptosis, oxidative stress and dysfunction of INS-1 cells (Figure 6). However, its protection against apoptosis seems to be mainly through SIRT1 signaling pathway, and its protection against oxidative stress and regulation of dysfunction seems to be mainly through Akt/GSK3β/Nrf2 signaling pathway.
In general, AS-IV may protect INS-1 cells by regulating SIRT1/p53 and Akt/GSK3β/Nrf2 signaling pathways, and alleviate STZ-induced oxidative stress, apoptosis and cell dysfunction. These findings may lay the foundation for using AS-IV belonging to traditional Chinese medicine as a treatment for diabetes.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was supported by the Project of the Fujian Provincial Department of Education (JAT201217).
Disclosure
The authors declare that they have no competing interests.
References
1. Li L, Hou X, Xu R, Liu C, Tu M. Research review on the pharmacological effects of astragaloside IV. Fundam Clin Pharmacol. 2017;31(1):17–36. doi:10.1111/fcp.12232
2. Zhang J, Wu C, Gao L, Du G, Qin X. Astragaloside IV derived from astragalus membranaceus: a research review on the pharmacological effects. Adv Pharmacol. 2020;87:89–112. doi:10.1016/bs.apha.2019.08.002
3. Zhang R, Xing B, Zhao J, et al. Astragaloside IV relieves gestational diabetes mellitus in genetic mice through reducing hepatic gluconeogenesis. Can J Physiol Pharmacol. 2020;98(7):466–472. doi:10.1139/cjpp-2019-0548
4. Zhu Y, Qian X, Li J, et al. Astragaloside-IV protects H9C2 (2-1) cardiomyocytes from high glucose-induced injury via miR-34a-mediated autophagy pathway. Artif Cells Nanomed Biotechnol. 2019;47(1):4172–4181. doi:10.1080/21691401.2019.1687492
5. Remedi MS, Emfinger C. Pancreatic β-cell identity in diabetes. Diabetes Obes Metab. 2016;18(Suppl 1):110–116. doi:10.1111/dom.12727
6. Wang F, Xi Y, Liu W, et al. Sanbai melon seed oil exerts its protective effects in a diabetes mellitus model via the Akt/GSK-3β/Nrf2 pathway. J Diabetes Res. 2019;2019:5734723. doi:10.1155/2019/5734723
7. Yu H, Zhen J, Yang Y, Du J, Leng J, Tong Q. Rg1 protects H9C2 cells from high glucose-/palmitate-induced injury via activation of Akt/GSK-3β/Nrf2 pathway. J Cell Mol Med. 2020;24(14):8194–8205. doi:10.1111/jcmm.15486
8. Zhu F, Shao J, Tian Y, Xu Z. Sulfiredoxin-1 protects retinal ganglion cells from high glucose-induced oxidative stress and inflammatory injury by potentiating Nrf2 signaling via the Akt/GSK-3β pathway. Int Immunopharmacol. 2021;101:108221. doi:10.1016/j.intimp.2021.108221
9. Feng J, Xie L, Yu X, et al. Perilipin 5 ameliorates high-glucose-induced podocyte injury via Akt/GSK-3β/Nrf2-mediated suppression of apoptosis, oxidative stress, and inflammation. Biochem Biophys Res Commun. 2021;544:22–30. doi:10.1016/j.bbrc.2021.01.069
10. Qin CD, Ma DN, Ren ZG, et al. Astragaloside IV inhibits metastasis in hepatoma cells through the suppression of epithelial-mesenchymal transition via the Akt/GSK-3β/β-catenin pathway. Oncol Rep. 2017;37(3):1725–1735. doi:10.3892/or.2017.5389
11. Kerforne T, Giraud S, Danion J, et al. Rapid or slow time to brain death? Impact on kidney graft injuries in an allotransplantation porcine model. Int J Mol Sci. 2020;19:3671.
12. Feige JN, Lagouge M, Canto C, et al. Specific SIRT1 activation mimics low energy levels and protects against diet-induced metabolic disorders by enhancing fat oxidation. Cell Metab. 2008;8(5):347–358. doi:10.1016/j.cmet.2008.08.017
13. He W, Wang Y, Zhang MZ, et al. Sirt1 activation protects the mouse renal medulla from oxidative injury. J Clin Invest. 2010;120(4):1056–1068. doi:10.1172/JCI41563
14. Kume S, Uzu T, Horiike K, et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120(4):1043–1055. doi:10.1172/JCI41376
15. Kitada M, Kume S, Takeda-Watanabe A, Kanasaki K, Koya D. Sirtuins and renal diseases: relationship with aging and diabetic nephropathy. Clin Sci. 2013;124(3):153–164. doi:10.1042/CS20120190
16. Polak-Jonkisz D, Laszki-Szcząchor K, Rehan L, Pilecki W, Filipowski H, Sobieszczańska M. Nephroprotective action of sirtuin 1 (SIRT1). J Physiol Biochem. 2013;69(4):957–961. doi:10.1007/s13105-013-0268-1
17. Ma F, Wu J, Jiang Z, et al. P53/NRF2 mediates SIRT1’s protective effect on diabetic nephropathy. Biochim Biophys Acta Mol Cell Res. 1866;2019:1272–1281.
18. Ben Y, Hao J, Zhang Z, et al. Astragaloside IV inhibits mitochondrial-dependent apoptosis of the dorsal root ganglion in diabetic peripheral neuropathy rats through modulation of the SIRT1/p53 signaling pathway. Diabetes Metab Syndr Obes. 2021;14:1647–1661. doi:10.2147/DMSO.S301068
19. Luo G, Xiao L, Wang D, et al. Resveratrol protects against ethanol-induced impairment of insulin secretion in INS-1 cells through SIRT1-UCP2 axis. Toxicol In Vitro. 2020;65:104808. doi:10.1016/j.tiv.2020.104808
20. Liu Y, Han J, Zhou Z, Li D. Tangeretin inhibits streptozotocin-induced cell apoptosis via regulating NF-κB pathway in INS-1 cells. J Cell Biochem. 2019;120(3):3286–3293. doi:10.1002/jcb.27596
21. Bai X, Li X, Tian J, Zhou Z. Antiangiogenic treatment diminishes renal injury and dysfunction via regulation of local Akt in early experimental diabetes. PLoS One. 2014;9(4):e96117. doi:10.1371/journal.pone.0096117
22. Huang X, Sun J, Chen G, et al. Resveratrol promotes diabetic wound healing via SIRT1-FOXO1-c-Myc signaling pathway-mediated angiogenesis. Front Pharmacol. 2019;10:421.
23. Hussein AG, Mohamed RH, Shalaby SM, Abd El Motteleb DM. Vitamin K(2) alleviates type 2 diabetes in rats by induction of osteocalcin gene expression. Nutrition. 2018;47:33–38. doi:10.1016/j.nut.2017.09.016
24. Chen L, Su X, Hu Y. Berberine down-regulated myostatin expression and facilitated metabolism via smad pathway in insulin resistant mice. Diabetes Metab Syndr Obes. 2020;47:4561–4569. doi:10.2147/DMSO.S275301
25. Cong PF, Qu YC, Chen JP, et al. Growth inhibition and apoptosis induction by alternol in pancreatic carcinoma cells. World J Gastroenterol. 2015;21(15):4526–4535. doi:10.3748/wjg.v21.i15.4526
26. Qiao Y, Fan CL, Tang MK. Astragaloside IV protects rat retinal capillary endothelial cells against high glucose-induced oxidative injury. Drug Des Devel Ther. 2017;11:3567–3577. doi:10.2147/DDDT.S152489
27. Wang YN, Zhao SL, Su YY, et al. Astragaloside IV attenuates high glucose-induced EMT by inhibiting the TGF-β/Smad pathway in renal proximal tubular epithelial cells. Biosci Rep. 2020;40(6). doi:10.1042/BSR20190987.
28. Deng LL. Astragaloside IV as potential antioxidant against diabetic ketoacidosis in juvenile mice through activating JNK/Nrf2 signaling pathway. Arch Med Res. 2020;51(7):654–663. doi:10.1016/j.arcmed.2020.06.013
29. Karbasforooshan H, Karimi G. The role of SIRT1 in diabetic cardiomyopathy. Biomed Pharmacother. 2017;90:386–392. doi:10.1016/j.biopha.2017.03.056
30. Wang X, Gao Y, Tian N, et al. Astragaloside IV inhibits glucose-induced epithelial-mesenchymal transition of podocytes through autophagy enhancement via the SIRT-NF-κB p65 axis. Sci Rep. 2019;9(1):323. doi:10.1038/s41598-018-36911-1
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.