Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Association of rs1544410 and rs7975232 Polymorphisms and Serum Vitamin D Levels with Psoriasis Susceptibility and Severity: A Case–Control Study in Egyptian Patients
Authors Mohamed AA, Elhussain E, Fawzy N, Sakr Y, Salah El-dien M, Abbas AM, Hussein MS, Nassar N, Ezzat O, El-Amir RY, Ibrahim S, Bedair NI
Received 27 February 2022
Accepted for publication 17 June 2022
Published 7 July 2022 Volume 2022:15 Pages 1271—1281
DOI https://doi.org/10.2147/CCID.S364267
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Amal Ahmed Mohamed,1 Eman Elhussain,2 Naglaa Fawzy,3 Yasser Sakr,3 Marwa Salah El-dien,4 Abbas Mohammed Abbas,5 Maha S Hussein,6 Nourelhuda Nassar,7 Omnia Ezzat,8 Reham Yousry El-Amir,9 Sarah Ibrahim,10 Nermeen Ibrahim Bedair11,12
1Department of Biochemistry and Molecular Biology, National Hepatology and Tropical Medicine Research institute, Cairo, Egypt; 2Department of Clinical and Chemical Pathology, Faculty of Medicine, Cairo University, Giza, Egypt; 3Department of Clinical Pathology, National Institute of Diabetics and Endocrinology, Cairo, Egypt; 4Department of Community, Environmental and Occupational Medicine, Faculty of Medicine, Benha university, Banha, Egypt; 5Department of Biochemistry, Faculty of Medicine, Cairo University, Giza, Egypt; 6Department of Dermatology and Andrology, Medical Research and Clinical Studies Institute, National Research Center, Cairo, Egypt; 7Department of Clinical Pathology, Elsahel Teaching Hospital, Cairo, Egypt; 8Department of Biochemistry, Faculty of Pharmacy, Egyptian Russian University, Cairo, Egypt; 9Department of Public Health, Faculty of Medicine, Cairo University, Giza, Egypt; 10Department of Dermatology, Faculty of Medicine, Cairo university, Cairo, Egypt; 11Department of Dermatology, Andrology, Sexual Medicine and STDs, Faculty of Medicine, Helwan University, Cairo, Egypt; 12Department of Dermatology and Andrology, Armed Forces College of medicine, Cairo, Egypt
Correspondence: Nermeen Ibrahim Bedair, Department of Dermatology, Andrology, Sexual Medicine and STDs, Faculty of Medicine, Helwan University, Cairo, Egypt, Email [email protected]
Background: Vitamin D is a regulatory factor for skin immune functions through vitamin D receptor, which is expressed on many immune cells. Vitamin D receptor is located on chromosome 12q 13.11 and has many single nucleotide polymorphisms. Some of them were hypothesized to be associated with psoriasis. Psoriasis is a genetic disease that is greatly affected by environmental factors.
Methods: A total of 135 psoriasis patients and 114 healthy controls were recruited. Both had a measurement of serum vitamin D and two vitamin D receptor variants:, rs1544410: G > A (HGVS:NC_000012.12:g.47846052) and rs7975232: C > A (HGVS: NC_000012.12:g.47845054). We assessed the relationship between vitamin deficiency as well as the two gene polymorphisms with psoriasis susceptibility and severity.
Results: Serum vitamin D levels were not significantly different between cases and controls. However, a significant association between vitamin D levels and severity was observed. We attributed this to our finding that rs7975232 was more significantly polymorphic among cases than controls, while rs1544410 polymorphism did not show a significant difference among the 2 groups.
Conclusion: We did not find a significant difference in serum vitamin D levels between cases and controls. Yet, psoriasis severity was significantly associated with serum vitamin D levels. We attributed this to other findings that the vitamin D receptor rs7975232 gene is polymorphic in psoriasis patients. At the same time, rs1544410 was not significantly more polymorphic in psoriasis patients. Both genes’ polymorphisms were associated with severe psoriasis.
Keywords: vitamin D deficiency, vitamin D receptors polymorphisms, single nucleotide polymorphism, ApaI, BsmI, rs7975232, rs1544410, psoriasis
Background
Skin immune cells are capable of both synthesizing and responding to vitamin D,1 through the expression of vitamin D receptor (VDR).2 VDR is expressed by both innate and humoral immunity cells, which can explain the role of vitamin D in suppressing inflammatory cytokines.3–5 Many vitamin D receptor polymorphisms have been previously reported. Single Nucleotide Polymorphisms (SNP) in intron 8 are particularly implicated in autoimmunity. This can affect the vitamin D, VDR signaling pathway, and diminish the vitamin D effect on immune cells.6
Psoriasis is a polygenic multifactorial cutaneous autoimmune chronic disease with altered immune cell functions and cytokines.7 The association between serum vitamin D levels and the presence of psoriasis as well as psoriasis severity has been studied intensively for the last decades, and the evidence is widely controversial.8,9 Receptor gene polymorphisms that hypothetically explain such variable outcomes were studied among different populations and ethnicities and showed variable associations with psoriasis and psoriasis severity.10 The most studied polymorphisms in psoriasis were four SNPs. They are located at exon 2 (rs2228570: C > T, detected with the FokI restriction enzyme); exon 9 (rs731236: T > C, detected with the TaqI restriction enzyme), and two SNPs on intron 8 (rs1544410:G > A, detected with the BsmI restriction enzyme and rs7975232:C > A (HGVS: NC_000012.12:g.47845054), detected with the ApaI restriction enzymes).11
Because of these widely variable findings from the previous studies, this study was designed to investigate the association among serum vitamin D levels and the severity of psoriasis. Moreover, we aimed to detect association with two VDR receptor single-nucleotide polymorphisms located at intron 8, and these are rs7975232: C > A (HGVS: NC_000012.12:g.47845054), detected with ApaI restriction enzymes, and rs1544410: G > A (HGVS:NC_000012.12:g.47846052) detected with the BsmI restriction enzyme.
Materials and Methods
Selection of Participants
This study was conducted in the Dermatology Department, Badr University hospital, Helwan University. A total of 135 patients with psoriasis vulgaris were collected. Alongside, 115 sex-matched healthy controls were recruited. The study was approved by the Helwan University, Faculty of Medicine ethical committee and fulfilled all the ethical aspects required in human research that complies with the declaration of Helsinki. All participants received full information about the study objectives, and they all provided informed consent. We excluded patients who had any systemic treatment or phototherapy less than 6 months prior to enrollment, women who are pregnant or breastfeeding, any participant who is genetically related to another participant within the studied groups, patients that were diagnosed with any concomitant dermatological disease and individuals with prior vitamin D supplementations and any healthy controls who reported positive family history of psoriasis All participants underwent complete history taking and full examination, and psoriasis severity was determined using Psoriasis Area and Severity Index (PASI) score.12
Anthropometric Measurements
We measured the weight and height, then we calculated body mass index (BMI) as body weight (kg)/height2 (m2), and calculated BMI Z scores online.13
Blood Samples
A 10 mL venous sample was drawn. About 4 mL was taken in coagulant-free sterile tubes to be used in the analysis of biochemical markers. About 3 mL was taken in EDTA tubes for complete blood count (CBC) and 3 mL for DNA extraction and gene polymorphism analysis. Samples were centrifuged for coagulation, and serum was obtained immediately and stored at −80° to determine serum Vit. D by ELIZA ELISA, according to the manufacturer’s instructions.
Routine Biochemical Analysis
Glucose, Creatinine, Cholesterol, and Triglycerides were performed by an automatic autoanalyzer.
Genetic Analysis of SNPs
Human Genomic DNA Extraction: It was carried out using QIA amp® DNA Blood Mini Kit (QIAGEN GmbH, Hilden, Germany), according to recommended instructions. We measured the concentration of the extracted DNA using the Nano Drop® (ND-1000) Spectrophotometer (Nano Drop Technologies Inc., Washington, USA). The ratio of DNA extracted absorbance was 1.7–1.9 at 260 /280 nm.
VDR rs1544410: G > A and rs7975232: C > A, SNPs polymorphism: Genotyping of VDR rs1544410, G > A and rs7975232, C > A, SNPs were carried out using real-time polymerase chain reaction with TaqMan® allelic discrimination assay software using (Applied Biosystems Step One TM Real-Time PCR system Thermal Cycling Block, Singapore), and according to the manufacturer instructions using dual-labeled fluorogenic TaqMan® probes.
Statistical Analysis
Data were statistically described as mean±standard deviation (± SD), median and range, or frequencies (number of cases) and percentages when appropriate. Because the groups are large enough, a comparison of numerical variables between the study groups was performed using the Student’s t-test for independence. For comparing categorical data, Chi-square (χ2) test was performed. The exact test was used instead when the expected frequency was less than 5. Correlation between various variables was conducted using Pearson moment correlation equation for linear relation of normally distributed variables and Spearman rank correlation equation for non-normal variables/non-linear monotonic relation. Multivariate linear regression analysis was used to test the association between vitamin D level and severity after adjusting the effect of age and gender. Two-sided p-values less than 0.05 were considered statistically significant. All statistical calculations were carried out by the computer program IBM SPSS (Statistical Package for the Social Science; IBM Corp, Armonk, NY, USA), release 22 for Microsoft Windows.
Results
Demographics Clinical and Laboratory Characteristics
The present study was conducted on 135 patients with psoriasis vulgaris, and 116 healthy sex-matched (p= 0.924) controls who were slightly older than the cases (p= 0.000). We summarized all participants’ demographic, clinical, and laboratory characteristics in Table 1. There was no statistically significant difference between cases and controls regarding serum vitamin D levels (P= 0.161), and this applied to all subgroups of vitamin D levels (sufficient, insufficient, and deficient). Vitamin D deficiency was present in 35.6% of cases vs 32.8% of controls (p<0.200). There was no significant difference between cases and controls in CRP levels (p= 0.161).
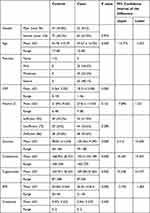 |
Table 1 Baseline Demographic, Clinical, and Laboratory Characteristics of Cases and Controls |
Among the cases with psoriasis, the prevalence of mild, moderate, and severe conditions was 18.5%, 33.3%, and 48.1%, respectively. The vitamin D level decreased with increasing severity of psoriasis (95% CI −0.021- −0.007, p= 0.000). No significant association was found between severity and sex or age (p= 543 and 230, respectively).
rs1544410 and rs7975232 Genes Polymorphisms
Allele frequencies of rs7975232C>T (HGVS: NC_000012.12:g.47845054) and rs1544410 G > A (HGVS:NC_000012.12:g.47846052) genotypes are shown in Tables 2 and 4. The absence of ApaI and BsmI restriction sites was described as C and G, respectively. At the same time, T and A were used to describe the presence of the sites (polymorphism), with CC and GG considered the wild type where restriction sites are absent on both alleles, CT and GA were the heterozygotic genotypes with restriction sites present on only one allele, and TT and AA were the mutant types with the presence of restriction sites on both alleles. A statistically significant difference was found among genotype frequencies between psoriasis patients and healthy controls for rs7975232C>T (HGVS: NC_000012.12:g.47845054) and rs1544410 G > A (HGVS:NC_000012.12:g.47846052) sites where p= 0.001 and 0.002, respectively. However, the significance was lost after Bonferroni’s adjustment (Tables 3 and 5).
 |
Table 2 Comparison Between the Two Studied Groups Regarding rs 7975232 Gene Polymorphism |
 |
Table 3 rs 7975232 Genotypes. Bonferroni Correction for Multiple Comparisons |
 |
Table 4 Comparison Between the Two Studied Groups Regarding rs1544410 Gene Polymorphism |
 |
Table 5 rs1544410 Genotypes After Bonferroni Correction for Multiple Comparisons |
rs7975232C>T (HGVS: NC_000012.12.g.47845054) was polymorphic in 89 (65%) of cases compared to 56 (48.5%) healthy controls, and this was statistically significant (p= 0.005). Genotype CT was the highest among psoriasis cases compared to the wild genotype CC, which was the highest among controls, and this too was also statistically significant (p= 0.001). While rs1544410 G > A (HGVS:NC_000012.12:g.47846052) was polymorphic in 77 (57%) cases compared to 66 controls (56.9%), and this was not statistically significant (p= 0.982). The frequency of genotype AA was significantly higher in cases than in controls (p= 0.002).
Within the psoriasis cases, rs7975232C>T (HGVS: NC_000012.12:g.47845054) was polymorphic in 38 (42.7%) cases with deficient vitamin D, 29 (32.6%) insufficient, and 22 (24.7%) cases with sufficient vitamin D, which was statistically significant (p= 0.019). Genotype CT was also the most prevalent among the vitamin D deficient group, which was also statistically significant (p= 0.038). Rs1544410 G > A (HGVS:NC_000012.12:g.47846052) was polymorphic in 29 (37.7%) cases with deficient vitamin D, 25 (32.5%) insufficient, and 23 (29.9%) cases with sufficient vitamin D, and this was not statistically significant (p= 0.800). Genotype GG was the most prevalent among the vitamin D deficient group, which was not statistically significant (p= 0.965) (Tables 6 and 7).
 |
Table 6 Association Between Vitamin D Status and rs 7975232 Gene Polymorphism in Cases with Psoriasis |
 |
Table 7 rs1544410 Gene Polymorphism in Association with Vitamin D Levels Among Psoriasis Cases |
Association Between rs1544410 and rs7975232 Polymorphisms and Psoriasis Severity
Among the psoriasis patients group, polymorphic rs7975232C>T(HGVS: NC_000012.12:g.47845054) presented in 57 of the 65 cases with severe psoriasis (87.7% of severe cases), 28 out of the 45 moderate cases (62.2% of the moderate cases), and only 4 out of the 25 mild cases (16%), which was statistically significant (p=0.000). The most prevalent genotype within moderate and severe psoriasis groups was CT, while the wild CC genotype was the most prevalent among the mild cases (84% of mild cases) (Table 8). While polymorphic rs1544410 G > A (HGVS:NC_000012.12:g.47846052) presented in 49 of the 65 cases with severe psoriasis (75.4% of severe cases), 21 out of the 45 moderate cases (46.7% of the moderate cases), and only 7 out of the 25 mild cases (28%), which was statistically significant (p=0.000). The most prevalent genotype within severe psoriasis groups was AA (Table 9).
 |
Table 8 Association Between Psoriasis Severity and rs 7975232 Gene Polymorphism |
 |
Table 9 Association Between Psoriasis Severity and rs1544410 Gene Polymorphism |
Association Between Vitamin D Level and Psoriasis Severity
Vitamin D level among cases of psoriasis with rs7975232C>T (HGVS: NC_000012.12.g.47845054) gene polymorphism ranged between 7 and 78 mg/dl, with a mean level of 25.2± 16.257, compared to its level within cases of psoriasis with no polymorphism that ranged between 8 and 80 mg/dl with mean of 32.85 ± 18.681, which was statistically significant (95% CI= −14.110- - 1.181, p= 0.021). No statistically significant difference was found between the different genotypes regarding vitamin D levels (p= 0.053). While vitamin D levels among cases of psoriasis with rs1544410 G > A (HGVS:NC_000012.12:g.47846052) gene polymorphism ranged between 7 and 80 mg/dl, with a mean level of 27.84± 18.122, compared to its level within cases had psoriasis with no polymorphism that ranged between 8 and 78 mg/dl with mean of 27.76 ± 16.636, which was not statistically significant (95% CI= −5.933–6.104, p= 0.978). No statistically significant difference was found between the different genotypes regarding vitamin D levels (p= 0.975).
After correction of confounders, no association was found between severity and either age or sex (95% CI= - 0.012–0.003, p= 0.230) and (95% CI= - 0.176–0.332, p= 0.543) respectively. However, a significant inverse relation was found between vitamin D levels and severity of psoriasis (95% CI= −21- −0.007, p= 0.000). Spearman correlation coefficient was −0.387 and P = 0.000.
Discussion
Although the exact pathogenesis of psoriasis is unknown, it is well established that psoriasis’s genetic component plays an effective role.14,15 HLA and non-HLA genes are involved.16,17
Vitamin D has a complex role in regulating skin biology, which alters keratinocytes proliferation and differentiation,18 inhibits keratinocytes apoptosis,19 downregulates cytokines TNF-α, IL-1β, IL-6, and IL-8,20,21 inhibits T cell proliferation and modulates T regs induction,22 stimulates the expression of antimicrobial peptides,23,24 and regulates barrier functions.25 It has a role in several autoimmune skin disorders.26,27
In our patient’s cohort, vitamin D levels showed no difference between patients with psoriasis and healthy controls. This was per several other studies.28–33 On the other hand, different studies found that psoriasis patients had significantly lower serum vitamin D levels.34–37 However, our patients showed a significant inverse relationship between vitamin D levels and the severity of psoriasis. Conversely, Orgaz-Molina et al and Atwa et al found a significant association between low vitamin D levels and psoriasis, but no association between vitamin D levels and psoriasis severity.34,38 In contrast, other studies found a significant association between the presence of psoriasis and low levels of vitamin D, with a positive association between severity and vitamin D levels.39–41
The positive inverse association between vitamin D levels and psoriasis severity in our patients, in the absence of significant difference in serum vitamin D levels between cases and controls, can be hypothetically attributed to a polymorphic VDR gene (HGNC Id: 12679) that can affect vitamin D functions even in patients with normal serum levels. The finding supports this hypothesis that the VDR gene (HGNC Id:12679) showed decreased expression in lesional skin of psoriasis patients,42 and this inverse relation was severity-dependent.43 Variable evidence exists in the literature when studying different polymorphisms of VDR gene (HGNC Id:12679). The current study is concerned with intron 8 genes rs7975232C>T (HGVS: NC_000012.12:g.47845054) and rs1544410 G > A (HGVS:NC_000012.12:g.47846052).
Rs7975232C>T (HGVS: NC_000012.12:g.47845054) was significantly polymorphic among our patients with psoriasis compared to healthy controls, and this was per several other studies on psoriasis patients among Turkish,44,45 Korean,46 and Chinese populations.47 Conversely, other studies found no rs7975232C>T (HGVS: NC_000012.12:g.47845054) polymorphism among psoriasis patients in Egyptian,48 Italian,49 Chinese,51 Croatian,51 and Japanese populations.52
The heterozygotic genotype was the most prevalent among our patients compared to the wild type that was the most prevalent among healthy controls, while in a previous Egyptian study, the heterozygotic genotype was the most prevalent among both patients and controls.48 The discrepancy in results between the current study and the previous one can be attributed to the larger sample size recruited. However, the wide genetic variations among Egyptians cannot be disregarded.53 Ruggiero et al suggested that the role of the VDR gene polymorphism in psoriasis can vary dramatically among different ethnic groups within the same population.54 Indeed, genotypes prevalence was widely variable among different studies, even in the same populations. Results similar to ours with the prevalence of heterozygotic type among patients while the homozygotic wild type prevalent among healthy controls were previously found in studies on Turkish,44 and Asian populations.55 However, other studies found the prevalence of the wild homozygotic genotype in both cases and controls.47,50 Heterozygotic genotype was also found prevalent in some studies,49,51 while Saeki et al found prevalent mutant genotype among both cases and controls.56
There was no significant polymorphism in the rs1544410 G > A (HGVS:NC_000012.12:g.47846052) gene among psoriasis patients in the current study. This was in accordance with several other studies among psoriasis patients in England,57 Chinese,50 Italian,49,54 Turkish,44 and Korean populations,58 while unlike ours, one study found an association between rs1544410 G > A (HGVS:NC_000012.12:g.47846052) polymorphism and psoriasis in the Japanese population.56 The homozygotic polymorphic genotype in our patient’s cohort was significantly higher among cases than in healthy controls. However, the heterozygotic genotype was the most prevalent among healthy controls, while the wild homozygotic type was the most prevalent among psoriasis cases. Other studies among Caucasian populations found similar results, with the prevalence of heterozygotic genotype being the most prevalent among healthy controls; however, they also found the same genotype mostly prevalent among psoriasis cases.44,51,54 Conversely, studies among Asian populations found the homozygotic polymorphic genotype most prevalent among both psoriasis cases and healthy controls.52,56,58,59
In the current study, both rs7975232C>T (HGVS: NC_000012.12:g.47845054) and rs1544410 G > A (HGVS:NC_000012.12:g.47846052) polymorphisms were positively correlated to psoriasis severity, and there are limited data in the literature on the association between VDR gene (HGNC Id:12679) polymorphism and psoriasis severity.10,60 We suggest that the wild genotype of both studied genes could be protective against the severe condition. Unlike our results, the previous study among Egyptian patients with psoriasis and another study among Turkish patients with psoriasis found no association between rs7975232C>T (HGVS: NC_000012.12.g.47845054) VDR polymorphism and PASI.48,52 Our results suggest that the wild gene in both rs7975232C>T (HGVS: NC_000012.12.g.47845054) and rs1544410 G > A (HGVS:NC_000012.12:g.47846052) can have a protective role against severe psoriasis cases.
The current study provided more evidence of the association between serum vitamin D levels and psoriasis and psoriasis severity and the role of VDR and its gene polymorphisms in such association. Several clinical and public health implications can be reached from these findings. It gives some insight into the potential presence of hypovitaminosis D manifestations in individuals with sufficient serum levels, as VDR gene (HGNC Id: 12679) polymorphism can affect vitamin D functions.
Our study has several methodologic and scientific strengths. We used our case-control study to estimate the prevalence of vitamin D deficiency in psoriasis patients. We adjusted for potential confounders, including age and gender, since we did not match them at the recruitment stage. In addition, we explored effect modification by vitamin D level, age, and gender, which is not frequently performed in dermatologic research. Although we did not perform a priori formal sample size and power calculation, a closer look at the narrow confidence intervals reveals that our study was powered enough.
Our findings still need to be considered with some limitations in mind. We conducted a case-control study, so there is a potential confounding. Reverse causation is a concern in all case-control studies. There is a lack of information regarding sun exposure cases and controls. Finally, we could only assemble a small sample size which might affect statistical evaluation and conclusions.
Conclusion
The current study found that serum vitamin D levels were not associated with psoriasis, although within the psoriasis cases, the Vitamin D levels were inversely related to severity. We attributed this to our other findings that showed an association between rs7975232C>T (HGVS: NC_000012.12:g.47845054) gene polymorphism and the presence of psoriasis. Although rs1544410 G > A (HGVS:NC_000012.12:g.47846052) polymorphism was not associated with the presence of psoriasis, both genes were polymorphic in severe psoriasis cases. Further studies are needed to determine the role of the VDR gene (HGNC Id: 12679) in psoriasis.
Statement of Ethics
The ethical committee of the faculty of Medicine, Helwan University, approved this research in Oct. 2021 with serial 69/2021. Informed consent was obtained from all participants and guardians of participants less than 18-year-old. The study fulfilled all the ethical aspects required in human research.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Aranow C. Vitamin D and the immune system. J Investig Med. 2011;59(6):881–886. doi:10.2310/JIM.0b013e31821b8755
2. Rosen Y, Daich J, Soliman I, Brathwaite E, Shoenfeld Y. Vitamin D and autoimmunity. Scand J Rheumatol. 2016;45(6):439–447. doi:10.3109/03009742.2016.1151072
3. Zhang Y, Leung DY, Richers BN, et al. Vitamin D inhibits monocyte/macrophage proinflammatory cytokine production by targeting MAPK phosphatase- 1. J Immunol. 2012;188(5):2127–2135. doi:10.4049/jimmunol.1102412
4. Joshi S, Pantalena L-C, Liu XK, et al. 1, 25-Dihydroxyvitamin D3 ameliorates Th17 autoimmunity via transcriptional modulation of interleukin-17A. Mol Cell Biol. 2011;31:3653–3669. doi:10.1128/MCB.05020-11
5. Mattner F, Smiroldo S, Galbiati F, et al. Inhibition of Th1 development and treatment of chronic-relapsing experimental allergic encephalomyelitis by a non-hypercalcemic analogue of 1, 25-dihydroxyvitamin D3. Eur J Immunol. 2000;30(2):498–508. doi:10.1002/1521-4141(200002)30:2<498::AID-IMMU498>3.0.CO;2-Q
6. Sun L, Arbesman J, Piliang M. Vitamin D, autoimmunity and immune-related adverse events of immune checkpoint inhibitors. Arch Dermatol Res. 2021;313(1):1. doi:10.1007/s00403-020-02094-x
7. Rachakonda TD, Schupp CW, Armstrong AW. Psoriasis prevalence among adults in the United States. J Am Acad Dermatol. 2014;70(3):512–516. PMID: 24388724. doi:10.1016/j.jaad.2013.11.013
8. Lee YH, Song GG. Association between circulating 25-hydroxyvitamin D levels and psoriasis, and correlation with disease severity: a meta-analysis. Clin Exp Dermatol. 2018;43(5):529–535. PMID: 29341195. doi:10.1111/ced.13381
9. Pitukweerakul S, Thavaraputta S, Prachuapthunyachart S, Karnchanasorn R. Hypovitaminosis D is associated with psoriasis: a systematic review and meta-analysis. Kans J Med. 2019;12(4):103–108. PMID: 31803350; PMCID: PMC6884011. doi:10.17161/kjm.v12i4.13255
10. Lee YH. Vitamin D receptor ApaI, TaqI, BsmI, and FokI polymorphisms and psoriasis susceptibility: an updated meta-analysis. Clin Exp Dermatol. 2019;44(5):498–505. PMID: 30474246. doi:10.1111/ced.13823
11. Liu J, Wang W, Liu K, et al. Vitamin D receptor gene polymorphisms are associated with psoriasis susceptibility and the clinical response to calcipotriol in psoriatic patients. Exp Dermatol. 2020;29(12):1186–1190. doi:10.1111/exd.14202
12. Mattei PL, Corey KC, Kimball AB. Psoriasis Area Severity Index (PASI) and the Dermatology Life Quality Index (DLQI): the correlation between disease severity and psychological burden in patients treated with biological therapies. J Eur Acad Dermatol Venereol. 2014;28(3):333–337. PMID: 23425140. doi:10.1111/jdv.12106
13. Martinez-Millana A, Hulst JM, Boon M, et al. Optimisation of children z-score calculation based on new statistical techniques. PLoS One. 2018;13(12):e0208362. doi:10.1371/journal.pone.0208362
14. Raharja A, Mahil SK, Barker JN. Psoriasis: a brief overview. Clin Med. 2021;21(3):170–173. PMID: 34001566; PMCID: PMC8140694. doi:10.7861/clinmed.2021-0257
15. Nanda H, Ponnusamy N, Odumpatta R, Jeyakanthan J, Mohanapriya A. Exploring genetic targets of psoriasis using genome wide association studies (GWAS) for drug repurposing. 3 Biotech. 2020;10(2):43. PMID: 31988837; PMCID: PMC6954159. doi:10.1007/s13205-019-2038-4
16. Nair RP, Stuart PE, Nistor I, et al. Sequence and haplotype analysis supports HLA-C as the psoriasis susceptibility 1 gene. Am J Hum Genet. 2006;78(5):827–851. doi:10.1086/503821
17. Zhang XJ, Huang W, Yang S, et al. Psoriasis genome-wide association study identifies susceptibility variants within LCE gene cluster at 1q21. Nat Genet. 2009;41(2):205–210. doi:10.1038/ng.310
18. Pillai S, Bikle DD, Elias PM. 1,25-Dihydroxyvitamin D production and receptor binding in human keratinocytes varies with differentiation. J Biol Chem. 1988;263(11):5390–5395. doi:10.1016/S0021-9258(18)60729-X
19. De Haes P, Garmyn M, Carmeliet G, et al. Molecular pathways involved in the anti-apoptotic effect of 1,25-dihydroxyvitamin D3 in primary human keratinocytes. J Cell Biochem. 2004;93(5):951–967. doi:10.1002/jcb.20227
20. Calton EK, Keane KN, Newsholme P, Soares MJ. The impact of vitamin D levels on inflammatory status: a systematic review of immune cell studies. PLoS One. 2015;10(11):e0141770. PMID: 26528817; PMCID: PMC4631349. doi:10.1371/journal.pone.0141770
21. De Haes P, Garmyn M, Degreef H, Vantieghem K, Bouillon R, Segaert S. 1,25-Dihydroxyvitamin D3 inhibits ultraviolet B-induced apoptosis, Jun kinase activation, and interleukin-6 production in primary human keratinocytes. J Cell Biochem. 2003;89(4):663–673. doi:10.1002/jcb.10540
22. Fisher SA, Rahimzadeh M, Brierley C, et al. The role of vitamin D in increasing circulating T regulatory cell numbers and modulating T regulatory cell phenotypes in patients with inflammatory disease or in healthy volunteers: a systematic review. PLoS One. 2019;14(9):e0222313. PMID: 31550254; PMCID: PMC6759203. doi:10.1371/journal.pone.0222313
23. Wang -T-T, Nestel FP, Bourdeau V, et al. Cutting edge: 1,25-dihydroxyvitamin D3 is a direct inducer of antimicrobial peptide gene expression. J Immunol. 2004;173:2909–2912. doi:10.4049/jimmunol.173.5.2909
24. Weber G, Heilborn JD, Chamorro Jimenez CI, Hammarsjö A, Törmä H, Ståhle M. Vitamin D induces the antimicrobial protein hCAP18 in human skin. J Invest Dermatol. 2005;124(5):1080–1082. doi:10.1111/j.0022-202X.2005.23687.x
25. Piotrowska A, Wierzbicka J, Żmijewski MA. Vitamin D in the skin physiology and pathology. Acta Biochim Pol. 2016;63(1):17–29. PMID: 26824295. doi:10.18388/abp.2015_1104
26. Ahmed Mohamed A, Salah Ahmed EM, Farag YMK, Bedair NI, Nassar NA, Ghanem AIM. Dose-response association between vitamin D deficiency and atopic dermatitis in children, and effect modification by gender: a case-control study. J Dermatolog Treat. 2021;32(2):174–179. PMID: 31296076. doi:10.1080/09546634.2019.1643447
27. Karagün E, Ergin C, Baysak S, Erden G, Aktaş H, Ekiz Ö. The role of serum vitamin D levels in vitiligo. Postepy Dermatol Alergol. 2016;33(4):300. doi:10.5114/pdia.2016.59507
28. Zuchi MF, Pde OA, Tanaka AA, Schmitt JV, Martins LE. Serum levels of 25-hydroxy vitamin D in psoriatic patients. An Bras Derm. 2015;90(3):430–432. doi:10.1590/abd1806-4841.20153524
29. Wilson PB. Serum 25-hydroxyvitamin D status in individuals with psoriasis in the general population. Endocrine. 2013;44(2):537–539. doi:10.1007/s12020-013-9989-8
30. Solak B, Dikicier BS, Celik HD, Erdem T. Bone mineral density, 25-OH vitamin D and inflammation in patients with psoriasis. Photodermatol Photoimmunol Photomed. 2016;32(3):153–160. doi:10.1111/phpp.12239
31. Maleki M, Nahidi Y, Azizahari S, Meibodi NT, Hadianfar A. Serum 25-OH vitamin D level in psoriatic patients and comparison with control subjects. J Cutan Med Surg. 2016;20(3):207–210. doi:10.1177/1203475415622207
32. Merola JF, Han J, Li T, Qureshi AA. No association between vitamin D intake and incident psoriasis among US women. Arch Dermatol Res. 2014;306(3):305–307. doi:10.1007/s00403-013-1426-6
33. Morimoto S, Yoshikawa K, Fukuo K, et al. Inverse relation between severity of psoriasis and serum 1,25-dihydroxy-vitamin D level. J Dermatol Sci. 1990;1(4):277–282. doi:10.1016/0923-1811(90)90120-3
34. Atwa MA, Balata MG, Hussein AM, Abdelrahman NI, Elminshawy HH. Serum 25-hydroxyvitamin D concentration in patients with psoriasis and rheumatoid arthritis and its association with disease activity and serum tumor necrosis factor-alpha. Saudi Med J. 2013;34(8):806–813.
35. Mattozzi C, Paolino G, Salvi M, et al. Peripheral blood regulatory T cell measurements correlate with serum vitamin D level in patients with psoriasis. Age. 2016;56:23–85.
36. Bergler-Czop B, Brzezińska-Wcisło L. Serum vitamin D level - the effect on the clinical course of psoriasis. Postepy Dermatol Alergol. 2016;6(6):445–449. PMID: 28035222; PMCID: PMC5183783. doi:10.5114/ada.2016.63883
37. Zaher HA, El-Komy MH, Hegazy RA, El Khashab HA, Ahmed HH. Assessment of interleukin-17 and vitamin D serum levels in psoriatic patients. J Am Acad Dermatol. 2013;69(5):840–842. doi:10.1016/j.jaad.2013.07.026
38. Orgaz-Molina J, Buendía-Eisman A, Arrabal-Polo MA, Ruiz JC, Arias-Santiago S. Deficiency of serum concentration of 25-hydroxyvitamin D in psoriatic patients: a case-control study. J Am Acad Dermatol. 2012;67(5):931–938. PMID: 22387034. doi:10.1016/j.jaad.2012.01.040
39. Ricceri F, Pescitelli L, Tripo L, Prignano F. Deficiency of serum concentration of 25-hydroxyvitamin D correlates with severity of disease in chronic plaque psoriasis. J Am Acad Dermatol. 2013;68(3):511–512. doi:10.1016/j.jaad.2012.10.051
40. Kincse G, Bhattoa PH, Heredi E, et al. Vitamin D3 levels and bone mineral density in patients with psoriasis and/or psoriatic arthritis. J Dermatol. 2015;42:679–684. doi:10.1111/1346-8138.12876
41. Mattozzi C, Paolino G, Richetta AG, Calvieri S. Psoriasis, vitamin D and the importance of the cutaneous barrier’s integrity: an update. J Dermatol. 2016;43(5):507–514. doi:10.1111/1346-8138
42. Visconti B, Paolino G, Carotti S, et al. Immunohistochemical expression of VDR is associated with reduced integrity of tight junction complex in psoriatic skin. J Eur Acad Dermatol Venereol. 2015;29(10):2038–2042. doi:10.1111/jdv.12736
43. Chandra R, Roesyanto‐Mahadi ID, Yosi A. Pilot study: immunohistochemistry expressions of vitamin D receptor associated with severity of disease in psoriasis patients. Int J Dermatol. 2020;59(9):1092–1097.
44. Kaya TI, Erdal ME, Tursen U, et al. Association between vitamin D receptor gene polymorphism and psoriasis among the Turkish population. Arch Dermatol Res. 2002;294(6):286–289. doi:10.1007/s00403-002-0326-y
45. Dayangac-Erden D, Karaduman A, Erdem-Yurter H. Polymorphisms of vitamin D receptor gene in Turkish familial psoriasis patients. Arch Dermatol Res. 2007;299(10):487–491. doi:10.1007/s00403-007-0782-5
46. Park BS, Park JS, Lee DY, et al. Vitamin D receptor polymorphism is associated with psoriasis. J Invest Dermatol. 1999;112(1):113–116. doi:10.1046/j.1523-1747.1999.00482.x
47. Zhao Y, Chen X, Li J, et al. VDR gene polymorphisms are associated with the clinical response to calcipotriol in psoriatic patients. J Dermatol Sci. 2015;79(3):305–307. doi:10.1016/j.jdermsci.2015.06.014
48. Zuel-Fakkar NM, Kamel MM, Asaad MK, et al. A study ofApaI and TaqI genotypes of the vitamin D receptor inEgyptian patients with psoriasis. Clin Exp Dermatol. 2011;36(4):355–359. doi:10.1111/j.1365-2230.2010.03970.x
49. Richetta AG, Silvestri V, Giancristoforo S, et al. A-1012G promoter polymorphism of vitamin D receptor gene is associated with psoriasis risk and lower allele-specific expression. DNA Cell Biol. 2014;33(2):102–109. doi:10.1089/dna.2013.2217
50. Zhou X, Xu LD, Li YZ. The association of polymorphisms of the vitamin D receptor gene with psoriasis in the Hanpopulation of northeastern China. J Dermatol Sci. 2014;73(1):63–66. doi:10.1016/j.jdermsci.2013.08.014
51. Rucevic I, Stefanic M, Tokic S, et al. Lack of association of vitamin D receptor gene 30-haplotypes with psoriasis in Croatian patients. J Dermatol. 2012;39(1):58–62. doi:10.1111/j.1346-8138.2011.01296.x
52. Okita H, Ohtsuka T, Yamakage A, Yamazaki S. Polymorphism of the vitamin D3 receptor in patients with psoriasis. Arch Dermatol Res. 2002;294(4):159–162. doi:10.1007/s00403-002-0314-2
53. Wohlers I, Künstner A, Munz M, et al. An integrated personal and population-based Egyptian genome reference. Nat Commun. 2020;11(1):4719. PMID: 32948767; PMCID: PMC7501257. doi:10.1038/s41467-020-17964-1
54. Ruggiero M, Gulisano M, Peruzzi B, et al. Vitamin D receptor gene polymorphism is not associated with psoriasis in Italian Caucasian population. J Dermatol Sci. 2004;35(1):68–70. doi:10.1016/j.jdermsci.2004.02.007
55. Liu JL, Zeng HM, Lin MG, et al. Association of vitamin D receptor polymorphisms with susceptibility to psoriasis vulgaris and clinical response to calcipotriol in patients with psoriasis vulgaris. Chin J Dermatol. 2017;50(12):889–893.
56. Saeki H, Asano N, Tsunemi Y, et al. Polymorphisms of vitamin D receptor gene in Japanese patients with psoriasis vulgaris. J Dermatol Sci. 2002;30(2):167–171. doi:10.1016/S0923-1811(02)00073-7
57. Mee JB, Cork MJ. Vitamin D receptor polymorphism and calcipotriol response in patients with psoriasis. J Invest Dermatol. 1998;110(3):301–302. doi:10.1046/j.1523-1747.1998.00128.x
58. Lee DY, Park BS, Choi KH, et al. Vitamin D receptor genotypes are not associated with clinical response to calcipotriol in Korean psoriasis patients. Arch Dermatol Res. 2002;294(1–2):1–5. doi:10.1007/s00403-002-0293-3
59. Zhu HQ, Xie KC, Chen LD, et al. The association between vitamin D receptor polymorphism and psoriasis. Chin J Dermatol. 2002;35:386–388.
60. Stefanic M, Rucevic I, Barisic‐Drusko V. Meta‐analysis of vitamin D receptor polymorphisms and psoriasis risk. Int J Dermatol. 2013;52(6):705–710. doi:10.1111/j.1365-4632.2012.5813.x
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
