Back to Journals » Clinical Ophthalmology » Volume 17
Association of Retinal Pigment Epithelium Reflectivity on Optical Coherence Tomography with Recurrence of Vogt–Koyanagi–Harada Disease: A Retrospective Observational Study
Authors Hirota Y, Muraoka Y , Kogo T, Ishikura M, Kadomoto S, Nishigori N, Ishihara K, Morooka S, Uji A, Tsujikawa A
Received 17 May 2023
Accepted for publication 16 June 2023
Published 21 July 2023 Volume 2023:17 Pages 2071—2079
DOI https://doi.org/10.2147/OPTH.S419546
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Yoshimichi Hirota, Yuki Muraoka, Takahiro Kogo, Masaharu Ishikura, Shin Kadomoto, Naomi Nishigori, Kenji Ishihara, Satoshi Morooka, Akihito Uji, Akitaka Tsujikawa
Department of Ophthalmology and Visual Sciences, Kyoto University Graduate School of Medicine, Kyoto, Japan
Correspondence: Yuki Muraoka, Department of Ophthalmology, Kyoto University Graduate School of Medicine, Shogoin Kawahara Cho 54, Sakyo Ku, Kyoto City, Kyoto Prefecture, 606-8507, Japan, Tel +81-75-751-3248, Fax +81-75-752-0933, Email [email protected]
Purpose: Despite the necessity of optical coherence tomography (OCT) for diagnosis and longitudinal monitoring in patients with Vogt–Koyanagi–Harada (VKH) disease, no studies have identified useful OCT markers for predicting recurrence in these patients. Although the precise reason for this remains unclear, one possibility is that infiltration of inflammatory cells into the choroid attenuates the OCT signal, making it difficult to precisely assess the structure of the choroid. Therefore, this study aimed to investigate changes in retinal pigment epithelium (RPE) reflectivity immediately above the choroid in eyes with acute VKH disease, as well as the association between RPE reflectivity and VKH disease recurrence.
Patients and Methods: This single-centered retrospective observational study included 20 treatment-naïve patients with acute VKH disease presenting with serous retinal detachment (SRD) in the posterior pole at the initial visit between October 2015 and January 2020, as well as 15 healthy control eyes. All patients were followed up for at least 6 months and received treatment with intravenous methylprednisolone followed by oral administration of prednisolone. Swept-source OCT images through the fovea were used to measure central retinal thickness, central choroidal thickness, and RPE reflectivity.
Results: During an observation period of 37.2 ± 30.8 months, recurrence of inflammation was observed in 11 patients (55.0%). Initial visual acuity was worse in patients who developed recurrence than in those who did not (P=0.024). On initial OCT images, RPE reflectivity differed significantly between patients with and without recurrence (1.75 ± 0.42 vs 1.35 ± 0.20; P=0.018), while there were no significant differences in other chorioretinal parameters, such as central retinal thickness and choroidal thickness.
Conclusion: RPE reflectivity on OCT images may be useful for predicting the recurrence of inflammation in patients with VKH disease.
Keywords: Vogt-Koyanagi-Harada disease, optical coherence tomography, retinal pigment epithelium, inflammation recurrence
Introduction
Vogt–Koyanagi–Harada (VKH) disease is an immune-mediated disease that affects melanocyte-containing organs1,2 and tends to occur in patients with more pigmented skin, especially those of Asian, Hispanic, and American Indian descent.3,4 In the acute phase, patients with VKH disease often exhibit bilateral and granulomatous pan-uveitis, systemic symptoms, such as headache and neck stiffness, and auditory disturbances associated with meningitis and inflammation of the inner ear.4,5
Moreover, the recurrence of VKH disease often results in symptoms that are more severe than those experienced during its initial onset. This increased severity can significantly raise the potential for visual function impairment, emphasizing the importance of careful steroid tapering in cases prone to recurrence.6 In addition, recurrence not only worsens visual prognosis but also raises the possibility of reinitiating or escalating steroid treatment, potentially exposing patients to the associated side effects.
Further pathologic changes, such as bullous serous retinal detachment (SRD),7,8 undulation of the choroid and retinal pigment epithelium (RPE) layer,9–14 and marked choroidal thickening10,12,15–19 are frequently observed in patients with severe inflammation at the posterior pole. These pathologic changes involving the macula can cause severe visual impairments. Although steroid therapy is especially effective for rapidly ameliorating these changes, inflammation can recur when the steroid dose is reduced or its administration is discontinued.4,5
Optical coherence tomography (OCT) is essential in clinical practice for diagnosing VKH disease, longitudinal monitoring, and assessing treatment efficacy.8,20–22 Recent technological advancements in OCT, particularly enhanced depth imaging (EDI), and swept-source (SS) OCT, have greatly facilitated the evaluation of the chorioretinal characteristics in VKH patients. Notably, OCT findings, such as SRD,7 choroidal folds,12,13 and choroidal thickening,10,21 may suggest the severity of VKH disease. However, the full potential of OCT in predicting the recurrence of VKH disease remains unelucidated.
Ganesh et al reported that VKH cases with a considerable initial subfoveal choroidal thickness are more likely to have a recurrence of inflammation.6 However, in clinical practice, a large number of cases with significant initial subfoveal choroidal thickness may not experience a recurrence. The underlying reason for the limited utility of OCT markers remains uncertain. One possible explanation is that the infiltration of inflammatory cells into the choroid weakens the OCT signal, which impedes a precise assessment of the choroid structure.23 Although OCT provides valuable insights, it may not entirely capture the complex pathophysiology of VKH disease recurrence. Therefore, further research is required to identify more reliable markers of recurrence.
This study aimed to investigate changes in the RPE layer immediately above the choroid in eyes with acute VKH disease, and the association between RPE reflectivity and VKH disease recurrence.
Materials and Methods
Patients
The current observational study was approved by the Institutional Review Board of Kyoto University Graduate School of Medicine (Kyoto, Japan) (approval number: 0352) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients.
The study included treatment-naïve patients with acute VKH disease, characterized by the initial onset of symptoms, presenting with SRD in the posterior pole (SRD type) at the initial visit and who could be followed up for at least 6 months. Patients with VKH disease other than the SRD type (disc or anterior segment type) and those undergoing steroid treatment for other systemic diseases were excluded. Additional exclusion criteria were as follows: ocular trauma; other forms of uveitis; posterior scleritis; and other chorioretinal diseases, such as pachychoroid spectrum diseases, including central serous chorioretinopathy, acute posterior multifocal placoid pigment epitheliopathy, and age-related macular degeneration. Eventually, 20 patients (40 eyes) with SRD-type VKH disease who had visited the Department of Ophthalmology at Kyoto University Hospital between October 2015 and January 2020 met our eligibility criteria.
Additionally, we included 15 healthy eyes from 15 age-matched volunteers (mean age: 52.6 years [30–70 years]) in our database as a control group.
At the initial examination, all patients with VKH disease underwent a complete systemic examination (blood test, spinal fluid test, and hearing test) and an extensive ophthalmic assessment to measure refraction, decimal best-corrected visual acuity based on 5-m Landolt charts, and intraocular pressure. Slit-lamp biomicroscopy, color fundus photography, fluorescein and indocyanine green angiography (Spectralis HRA+OCT; Heidelberg Engineering, Heidelberg, Germany), and swept-source EDI of SS-OCT (Topcon 3D-2000 OCT; Topcon Corporation, Tokyo, Japan) were also performed.
After the diagnosis of VKH disease, patients received intravenous methylprednisolone (200 mg/day for 3 days, followed by 150 mg/day for 3 days, followed by 100 mg/day for 3 days), followed by a tapering oral prednisolone regimen (starting dose of 0.6–0.7 mg/kg/day). This treatment protocol has been used in our institution and other centers,12,24 even though it might be considered suboptimal compared to regimens that begin with 1g/day of intravenous methylprednisolone. We acknowledge that variability in steroid treatment regimens can influence the results, including the recurrence rate, and this is a potential limitation in our study. Immunomodulatory agents were not used in this study due to our standard practice for first-episode acute VKH, although we acknowledge that the addition of such agents might have influenced the results. Treatment effects were monitored with weekly OCT imaging until the resolution of SRD.
Measurements of Central Retinal and Choroidal Thickness
To assess the retinal and choroidal condition, we obtained the horizontal and vertical SS-OCT B-scan images with a width of 12 mm and a depth of 2.6 mm through the center of the fovea. We measured central retinal thickness (CRT) and central choroidal thickness (CCT) in each B-scan image and averaged them. CRT was defined as the distance from the inner limiting membrane to the inner border of RPE layer, and CCT was defined as the distance from the outer border of the RPE layer to the choroid–sclera interface. When the central choroid was so thick that the choroid–sclera interface was obscure, the CCT was assigned a value of 1000 μm.
Quantification of Retinal Pigment Epithelium Reflectivity on Optical Coherence Tomography
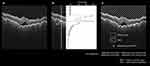
For evaluating the degree of the inflammation in the RPE layer, we measured the reflectivity of RPE in the vertical SS-OCT B-scan images (Figure 1a) using ImageJ software (National Institutes of Health, Bethesda, Maryland, USA, http://rsb.info.nih.gov/ij/index.html). RPE was defined as the point with the highest reflectivity in the depth direction of the RPE layer by using plot profile as shown in Figure 1b. We measured and averaged the reflectivity at 10 randomly selected RPE points. According to previously published methods, we used the reflectivity of the vitreous body and retinal nerve fiber layer (RNFL) as the standard in each image, and we defined the level in the vitreous as 0 and in the RNFL as 1 after measuring each averaged reflectivity (Figure 1c).25 RPE reflectivity was calculated as follows:
RPE reflectivity = (reflectivity of the RPE − reflectivity of the vitreous body)/(reflectivity of the RNFL − reflectivity of the vitreous body).
We used the data from the eye with a higher RPE reflectivity for the statistical comparisons.
Definition of Recurrence in Patients with Vogt–Koyanagi–Harada Disease
We defined VKH disease recurrence as the presence of ocular findings, such as keratic precipitates; a Standardization of Uveitis Nomenclature (SUN) Working Group Grade of 2+ in anterior chamber cells;26 and re-development of SRD, RPE-undulation, and choroidal thickening on OCT.
Statistical Analysis
Statistical analysis was conducted using PASW Statistics version 18.0 (SPSS, Chicago, IL). Descriptive statistics are presented as the mean ± standard deviation. For the comparison of variables between different groups, including the measurements of RPE reflectivity in the high RPE reflectivity eye among VKH patients and the normal control group, unpaired t-tests were performed.
Regarding eye selection, we included one eye per patient in our study. Specifically, we selected the eye with higher RPE reflectivity for analysis. As a result, among VKH cases with recurrence, all patients exhibited recurrence findings in the eye with higher RPE reflectivity.
Furthermore, we explicitly stated that normality (Shapiro–Wilk test) and homoscedasticity (Levene’s test) were assessed prior to conducting the unpaired t-tests. This step was taken to ensure the validity of the statistical analysis.
Results
This study included 20 treatment-naïve patients (9 men and 11 women) with VKH disease accompanied by SRD in the posterior pole at the initial visit. The mean age of the included patients was 60.9 ± 17.5 years (31–85 years; Table 1). During a mean observational period of 37.2 ± 30.8 months, recurrence of inflammation was observed in 11 patients (55.0%), while no cases of recurrence were observed among the remaining 9 patients (45.0%). In all 11 patients with recurrence of inflammation, the eye with a higher RPE reflectivity showed the signs of the recurrence.
 |
Table 1 Clinical Characteristics of Included Patients with Vogt–Koyanagi–Harada Disease |
Table 2 shows comparisons of various parameters between patients with and without recurrence of VKH disease. At the initial visit, visual acuity was worse in patients who developed recurrence than in those who did not (P=0.024). SS-OCT images obtained at the initial visit revealed that the RPE reflectivity was 1.35 ± 0.20 in patients without recurrence, which was not significantly different from that observed in control participants (1.34 ± 0.15, P=0.896). In contrast, the RPE reflectivity was 1.75 ± 0.42 in patients with recurrence, which was significantly greater than the value observed in patients without recurrence (P=0.018, Figure 2). There were no significant differences in the duration from onset, CRT, or CCT between patients with and without VKH disease recurrence (Table 2).
 |
Table 2 Comparisons of Clinical and Optical Coherence Tomography Parameters Among Healthy Controls and Patients with or without Recurrence of Vogt–Koyanagi–Harada Disease |
At the final visit, patients with recurrence had poorer visual acuity and greater CRT than patients without (P=0.045 and P=0.048, respectively). However, there were no significant differences in CCT or RPE reflectivity (Table 2).
Figure 3a shows a scatter plot of the RPE reflectivity at the initial visit. In most eyes with high RPE reflectivity, the recurrence of inflammation was observed. We created a receiver operating characteristic curve, wherein the RPE reflectivity at the initial visit was set as the independent variable, and the presence or absence of future recurrence of inflammation was set as the dependent variable (Figure 3b). The Youden index showed an RPE reflectivity of 1.4700; the sensitivity and specificity at this time point were 72.73% and 88.89%, respectively.
Discussion
This study examined the reflectivity of the RPE on SS-OCT in eyes with first-episode acute VKH disease, and its association with the recurrence of inflammation during the observation period. At the initial visit, there were no significant differences in CRT or CCT; however, RPE reflectivity was significantly greater in patients who eventually developed VKH recurrence than in those who did not (P=0.018).
VKH disease is a T-cell-associated autoimmune disease that targets melanocytes,2 leading to inflammation of the meninges, inner ear, hair follicles, skin, and uvea. The disease is a relatively common cause of bilateral and granulomatous uveitis, especially in Asian populations.27 Although VKH disease responds well to steroid therapy, the associated inflammation often recurs when treatment is discontinued or when the dose is tapered.15,16 It is also difficult to predict the likelihood of recurrence based on general clinical findings at the initial visit.
Within the fundus, melanocytes are present in the RPE and choroid, although the majority are contained in the choroid given that its volume is greater than that of the RPE.28 Therefore, we believe that the immune response to melanocytes in patients with VKH disease, including the infiltration of inflammatory cells, is greater in the choroid than in the RPE. OCT is quite useful for non-invasive assessment of the degree of inflammation. Previously, OCT findings, such as choroidal thickening17,19,22,28 and undulations of the inner choroid and RPE,11,12 have been identified as indicators of disease activity. However, the use of OCT markers to accurately predict recurrence in patients with VKH disease remains unelucidated.
Reduced blood flow at the choroidal level in LSFG may be suggestive of VKH disease recurrence.29 However, LSFG parameters can be affected by the opacity of the medium in individual patients,30 indicating that comparisons of choroidal pathology based on LSFG may not always lead to accurate results. In contrast, OCT is considered a more reliable modality for comparisons among patients.20 Previous studies using OCT for the assessment of VKH disease have demonstrated that choroidal thickening and undulation in the inner choroid are useful for examining disease activity.9–11,15–17 Ganesh et al proposed the potential utility of a large initial SFCT prior to treatment as a predictor of recurrent inflammation.6 However, it is important to note that SFCT exhibits high variability, contingent upon the inflammatory state. In clinical practice, even patients with thick SFCT often do not exhibit recurrence. In this study, the initial SFCT at the initial visit was not associated with recurrence (Table 2). Although the reasons for this discrepancy remain unclear, there are several possible explanations. Among them, choroidal thickening was significant even in patients without recurrence, and measurements of significant choroidal thickening may be technically difficult even using SS-OCT. Furthermore, choroidal thickening alone may not be sufficient for detecting more severe inflammation that extends beyond the choroid.9,12
Furthermore, pathological examination for VKH disease revealed that infiltration of inflammatory cells extended beyond the choroid and occasionally into the RPE; other changes observed in the RPE included the proliferation of the RPE itself and hyperaccumulation of melanin pigment.31 In our study, patients with recurrence of VKH disease during the observation period had significantly higher reflectivity in the RPE and poorer visual acuity at the initial examination than those without recurrence (Tables 2; Figure 2). This association may indicate that severe inflammation cannot be adequately detected based on choroidal thickness alone.
Jacob et al previously tried to evaluate the RPE reflectivity in eyes with VKH disease and reported an increase in RPE reflectivity during the resolution of VKH,32 which differed from the results presented in our manuscript. The differences in our findings can be attributed to several factors. First, previous reports assessing RPE reflectivity did not adequately account for influences, such as the choroidal shadow or signal strength, nor did they employ any method to control these effects. In contrast, our study utilized EDI to minimize the impact of SRF and employed correction methods for the reflectivity of RNFL and vitreous to mitigate the effects of the choroidal shadow and signal strength. Furthermore, our method of evaluating RPE reflectivity involved considering the highest reflectivity point in the RPE layer, providing an objective and consistent approach before and after treatment. Second, the timing of the post-treatment evaluation differs between our study and that of Jacob et al. In their study, they assessed RPE reflectivity during the inactive stage of VKH disease, while our study focused on long-term assessment, with the recurrence group evaluated at 46.4 months and the non-recurrence group at 26.0 months from the beginning of treatment. This extended evaluation period allowed us to better capture the changes in RPE reflectivity as inflammation subsided and the ocular tissues approached a state closer to normal.
This study had limitations. First, this study was retrospective in nature. Thus, the actual dosage of steroids and duration of follow-up varied slightly among patients, and it is not possible to eliminate completely the effects of these factors on the results. However, there were no statistically significant differences in these factors between patients with and without recurrence of VKH disease (Table 2). Therefore, we believed that the degree of bias was not large enough to distort our study conclusions. Second, we only included patients with acute VKH disease exhibiting SRD at the posterior pole, which is the most common phenotype of the disease; however, our findings cannot be applied to patients with the anterior segment type or disc type of VKH. Third, RPE reflectivity could not be measured using the existing OCT software and was instead determined using ImageJ software. The widespread adoption of the markers identified in the current study will require an upgrade to the OCT software. Finally, we did not eliminate the effect of individual variations in RPE reflectivity.
Nevertheless, the current findings indicate that RPE changes on OCT may be useful for predicting the recurrence of VKH disease. Given the preliminary nature of our investigation, future prospective studies with a larger number of patients are necessary to confirm the reproducibility of the current findings.
Conclusion
Using SS-OCT, we studied the correlation between chorioretinal factors and the recurrence of VKH disease. Our findings revealed a significant association between high RPE reflectivity prior to steroid treatment and the recurrence. RPE reflectivity on OCT images may be useful for predicting the recurrence of inflammation in patients with VKH disease.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Ethics Approval and Consent to Participate
The current observational study was approved by the Institutional Review Board of Kyoto University Graduate School of Medicine (Kyoto, Japan) (approval number: 0352) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all patients.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported in part by a grant-in-aid for scientific research (no. 20K09771) from the Japan Society for the Promotion of Science (Tokyo, Japan) and by Canon INC. (Tokyo, Japan). The funders had no role in the study design, data collection/analysis, decision to publish, or preparation of the article.
Disclosure
None of the authors has proprietary interest in any product described in the article. Y. Muraoka: personal fees from Bayer Yakuhin, Novartis Pharma, Canon, Santen Pharmaceutical, Alcon Japan, Senju Pharmaceutical, Japan Focus, Findex, Kowa Pharmaceutical, Pfizer, AMO Japan, Wakamoto Pharmaceutical, Alcon Pharma, Otsuka Pharmaceutical, Tomey Corporation, Taiho Pharma, Hoya, Chugai Pharmaceutical, Astellas, Eisai, Daiichi-Sankyo, Janssen Pharmaceutical, Kyoto Drug Discovery & Development, Allergan Japan, MSD, Ellex, Sanwa Kagaku Kenkyusho, Nitten Pharmaceutical, AbbVie GK, outside the submitted work. S. Kadomoto: Canon, Santen Pharmaceutical, Senju Pharmaceutical, Japan Focus. K. Ishihara: Bayer Yakuhin, Santen Pharmaceutical. A Uji: Bayer Yakuhin, Novartis Pharma, Canon, Santen Pharmaceutical, Senju Pharmaceutical. A. Tsujikawa: grants and/or personal fees from Canon, Findex, Santen Pharmaceutical, Sumitomo Pharma, Kowa Pharmaceutical, Pfizer, AMO Japan, Senju Pharmaceutical, Wakamoto Pharmaceutical, Alcon Japan, Alcon Pharma, Otsuka Pharmaceutical, Tomey Corporation, Taiho Pharma, Hoya, Bayer Yakuhin, Novartis Pharma, Kyowa Kirin, Nidek, Chugai Pharmaceutical, Rohto Nitten, Nippon Boehringer Ingelheim, Rohto Pharmaceutical, Johnson & Johnson, Nikon Solutions, Astellas, Eisai, Daiichi-Sankyo, Janssen Pharmaceutical, Kyoto Drug Discovery & Development, Allergan Japan, MSD, Ellex, Sanwa Kagaku Kenkyusho, Nitten Pharmaceutical, AbbVie GK. The authors report no other conflicts of interest in this work.
References
1. Inomata H, Sakamoto T. Immunohistochemical studies of Vogt-Koyanagi-Harada disease with sunset sky fundus. Curr Eye Res. 1990;9(Suppl):35–40. doi:10.3109/02713689008999417
2. Kahn M, Pepose JS, Green WR, Miller J, Foos RY. Immunocytologic findings in a case of Vogt-Koyanagi-Harada syndrome. Ophthalmology. 1993;100(8):1191–1198. doi:10.1016/S0161-6420(93)31506-X
3. Hou S, Du L, Lei B, et al. Genome-wide association analysis of Vogt-Koyanagi-Harada syndrome identifies two new susceptibility loci at 1p31.2 and 10q21.3. Nat Genet. 2014;46(9):1007–1011. doi:10.1038/ng.3061
4. O’Keefe GA, Rao NA. Vogt-Koyanagi-Harada disease. Surv Ophthalmol. 2017;62(1):1–25. doi:10.1016/j.survophthal.2016.05.002
5. Oellers P, Jaffe GJ, Proia AD. Clinical-pathological correlation of Vogt-Koyanagi-Harada disease. JAMA Ophthalmol. 2016;134(3):343–345. doi:10.1001/jamaophthalmol.2015.5464
6. Ganesh SK, Mistry S, Nair N. Role of swept source optical coherence tomography in management of acute Vogt-Koyanagi-Harada’s disease. Indian J Ophthalmol. 2022;70(7):2458–2463. doi:10.4103/ijo.IJO_1944_21
7. Ishihara K, Hangai M, Kita M, Yoshimura N. Acute Vogt-Koyanagi-Harada disease in enhanced spectral-domain optical coherence tomography. Ophthalmology. 2009;116(9):1799–1807. doi:10.1016/j.ophtha.2009.04.002
8. Tsujikawa A, Yamashiro K, Yamamoto K, Nonaka A, Fujihara M, Kurimoto Y. Retinal cystoid spaces in acute Vogt-Koyanagi-Harada syndrome. Am J Ophthalmol. 2005;139(4):670–677. doi:10.1016/j.ajo.2004.11.053
9. Agrawal R, Li LK, Nakhate V, Khandelwal N, Mahendradas P. Choroidal vascularity index in Vogt-Koyanagi-Harada disease: an EDI-OCT derived tool for monitoring disease progression. Transl Vis Sci Technol. 2016;5(4):7. doi:10.1167/tvst.5.4.7
10. Fong AH, Li KK, Wong D. Choroidal evaluation using enhanced depth imaging spectral-domain optical coherence tomography in Vogt-Koyanagi-Harada disease. Retina. 2011;31(3):502–509. doi:10.1097/IAE.0b013e3182083beb
11. Gupta V, Gupta A, Gupta P, Sharma A. Spectral-domain cirrus optical coherence tomography of choroidal striations seen in the acute stage of Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2009;147(1):148–153 e142. doi:10.1016/j.ajo.2008.07.028
12. Hosoda Y, Uji A, Hangai M, Morooka S, Nishijima K, Yoshimura N. Relationship between retinal lesions and inward choroidal bulging in Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2014;157(5):1056–1063. doi:10.1016/j.ajo.2014.01.015
13. Tsuboi K, Nakai K, Iwahashi C, Gomi F, Ikuno Y, Nishida K. Analysis of choroidal folds in acute Vogt-Koyanagi-Harada disease using high-penetration optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2015;253(6):959–964. doi:10.1007/s00417-015-2945-y
14. Wu W, Wen F, Huang S, Luo G, Wu D. Choroidal folds in Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2007;143(5):900–901. doi:10.1016/j.ajo.2006.11.050
15. Bacsal K, Wen DS, Chee SP. Concomitant choroidal inflammation during anterior segment recurrence in Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2008;145(3):480–486. doi:10.1016/j.ajo.2007.10.012
16. Hashizume K, Imamura Y, Fujiwara T, Machida S, Ishida M, Kurosaka D. Choroidal thickness in eyes with posterior recurrence of Vogt-Koyanagi-Harada disease after high-dose steroid therapy. Acta Ophthalmol. 2014;92(6):e490–e491. doi:10.1111/aos.12384
17. Hirooka K, Saito W, Namba K, et al. Significant role of the choroidal outer layer during recovery from choroidal thickening in Vogt-Koyanagi-Harada disease patients treated with systemic corticosteroids. BMC Ophthalmol. 2015;15:181. doi:10.1186/s12886-015-0171-3
18. Maruko I, Iida T, Sugano Y, Go S, Sekiryu T. Subfoveal choroidal thickness in papillitis type of Vogt-Koyanagi-Harada disease and idiopathic optic neuritis. Retina. 2016;36(5):992–999. doi:10.1097/IAE.0000000000000816
19. Maruko I, Iida T, Sugano Y, et al. Subfoveal choroidal thickness after treatment of Vogt-Koyanagi-Harada disease. Retina. 2011;31(3):510–517. doi:10.1097/IAE.0b013e3181eef053
20. Chee SP, Afrin M, Tumulak MJ, Siak J. Role of optical coherence tomography in the prognosis of Vogt-Koyanagi-Harada disease. Ocul Immunol Inflamm. 2021;29(1):118–123. doi:10.1080/09273948.2019.1655580
21. Li M, Liu Q, Luo Y, et al. Enhanced depth SD-OCT images reveal characteristic choroidal changes in patients with Vogt-Koyanagi-Harada disease. Ophthalmic Surg Lasers Imaging Retina. 2016;47(11):1004–1012. doi:10.3928/23258160-20161031-04
22. Nakayama M, Keino H, Okada AA, et al. Enhanced depth imaging optical coherence tomography of the choroid in Vogt-Koyanagi-Harada disease. Retina. 2012;32(10):2061–2069. doi:10.1097/IAE.0b013e318256205a
23. Miura M, Makita S, Yasuno Y, et al. Polarization-sensitive optical coherence tomographic documentation of choroidal melanin loss in chronic Vogt-Koyanagi-Harada disease. Invest Ophthalmol Vis Sci. 2017;58(11):4467–4476. doi:10.1167/iovs.17-22117
24. Kitaichi N, Horie Y, Ohno S. Prompt therapy reduces the duration of systemic corticosteroids in Vogt-Koyanagi-Harada disease. Graefes Arch Clin Exp Ophthalmol. 2008;246(11):1641–1642. doi:10.1007/s00417-008-0869-5
25. Horii T, Murakami T, Nishijima K, et al. Relationship between fluorescein pooling and optical coherence tomographic reflectivity of cystoid spaces in diabetic macular edema. Ophthalmology. 2012;119(5):1047–1055. doi:10.1016/j.ophtha.2011.10.030
26. Standardization of Uveitis Nomenclature (SUN) Working Group. Classification criteria for Vogt-Koyanagi-Harada disease. Am J Ophthalmol. 2021;228:205–211. doi:10.1016/j.ajo.2021.03.036
27. Beniz J, Forster DJ, Lean JS, Smith RE, Rao NA. Variations in clinical features of the Vogt-Koyanagi-Harada syndrome. Retina. 1991;11(3):275–280. doi:10.1097/00006982-199111030-00001
28. Kitamura Y, Oshitari T, Kitahashi M, Baba T, Yamamoto S. Acute posterior multifocal placoid pigment epitheliopathy sharing characteristic OCT findings of Vogt-Koyanagi-Harada disease. Case Rep Ophthalmol Med. 2019;2019:9217656. doi:10.1155/2019/9217656
29. Maruyama K, Noguchi A, Shimizu A, Shiga Y, Kunikata H, Nakazawa T. Predictors of recurrence in Vogt-Koyanagi-Harada disease. Ophthalmol Retina. 2018;2(4):343–350. doi:10.1016/j.oret.2017.07.016
30. Kuroda Y, Uji A, Yoshimura N. Factors associated with optic nerve head blood flow and color tone: a retrospective observational study. Graefes Arch Clin Exp Ophthalmol. 2016;254(5):963–970. doi:10.1007/s00417-015-3247-0
31. Borrelli E, Sarraf D, Freund KB, Sadda SR. OCT angiography and evaluation of the choroid and choroidal vascular disorders. Prog Retin Eye Res. 2018;67:30–55. doi:10.1016/j.preteyeres.2018.07.002
32. Jacob N, Tyagi M, Chhablani J, et al. Retinal pigment epithelial characteristics in acute and resolved Vogt-Koyanagi-Harada disease. J Clin Med. 2023;12(6):2368. doi:10.3390/jcm12062368
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.