Back to Journals » Neuropsychiatric Disease and Treatment » Volume 17
Association of CDKN2A/CDKN2B Gene Polymorphisms with Increased Susceptibility to Intracranial Aneurysm in a Chinese Han Population
Authors Cui X, Xin WQ , Wang B, Zhao Y, Hou C, Cai S , Peng C, Wang Z, Li J, Huan L, Chen L, Yang X
Received 22 February 2021
Accepted for publication 21 April 2021
Published 12 May 2021 Volume 2021:17 Pages 1443—1449
DOI https://doi.org/10.2147/NDT.S306542
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Taro Kishi
Xiaopeng Cui,1,2,* Wen-Qiang Xin,1,* Bangyue Wang,1 Yan Zhao,1 Changkai Hou,1 Shifei Cai,1 Chao Peng,1 Zhen Wang,1 Jian Li,1 Linchun Huan,3 Lei Chen,2 Xinyu Yang1
1Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin, People’s Republic of China; 2Department of Neurosurgery, Tianjin Fifth Central Hospital, Tianjin, People’s Republic of China; 3Department of Neurosurgery, Linyi People’s Hospital, Shandong, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Xinyu Yang
Department of Neurosurgery, Tianjin Medical University General Hospital, Tianjin Medical University, 154 Anshan Road, Peace Zone, Tianjin, 300041, People’s Republic of China
Email [email protected]
Lei Chen
Department of Neurosurgery, Tianjin Fifth Central Hospital, 41 Zhejiang Road, Binhai New Area, Tianjin, 300450, People’s Republic of China
Email [email protected]
Objective: Several studies have reported that single-nucleotide polymorphisms (SNPs) of the CDKN2A/CDKN2B gene on chromosome 9p21.3 are associated with increased risk of intracranial aneurysm (IA). However, the association between IAs and SNPs of CDKN2A/CDKN2B in Chinese Han people is yet to be evaluated. This study examined the association of the SNPs rs10811661 and rs4977574 with IA in the Chinese Han population.
Methods: A total of 595 IA patients and 600 sex- and age-matched controls were enrolled in the study. Peripheral blood was collected and stored at – 80°C until use. CDKN2A/CDKN2B was identified using polymerase chain reaction–ligase detection reaction. SNP genotyping was performed for rs10811661 and rs4977574 using a MassArray system. Associations between these two SNPs and IAs was tested with χ2 or Fisher’s exact tests and multivariate logistic regression.
Results: rs10811661 and rs4977574 were significantly associated with IA. The frequency of rs10811661-T in IA was higher than in controls (OR 1.26, 95% CI 1.07– 1.49; P< 0.01). There was no significant difference in frequency of haplotype between control subjects and IA patients.
Conclusion: rs10811661 and rs4977574 on 9p21.3 were strongly associated with genetic susceptibility to IA in the Chinese Han population, which emphasizes a need for further investigation.
Keywords: intracranial aneurysm, CDKN2A/CDKN2B gene, Chinese Han population, SNP
Introduction
With prevalence of 1%–5% in adults, intracranial aneurysm (IA) is a complex disease with regional bulging of intracranial arteries and lesions that are usually located at the bifurcation sites.1 It is generally reported that congenital defects and environmental risk factors can both affect the occurrence of IA. Environmental risk factors include sex, age, excessive smoking or drinking, hypertension, atherosclerosis, and diabetes.2 Although a series of studies have been conducted on IA, very little information is available about its molecular pathogenesis. With regard to genetic factors, there are multiple genes associated with the etiology of IA.3
The 9p21.3 locus was first reported during a genome-wide association study of cardiovascular disease in 2007.4 That study demonstrated that the same variants of cardiovascular disease were associated with IA.5 The closest protein-coding genes are situated in a few kilobases proximal to the region of 9p21.3, which has no known protein-coding genes. These genes include CDKN2A and CDKN2B, and are located in a single cluster4 that is related to various human diseases, such as leukemia and cancer.6–9 One study demonstrated that the CDKN2BAS SNP (rs6475606) contributed to susceptibility to IA in a US population.10 Previous studies on the Chinese Han population discovered the association of rs10757278 and rs1333049 with myocardial infarction (MI), peripheral vascular disease, coronary atherothrombotic disease (CAD), and type 2 diabetes mellitus (T2DM).11–13 Asimilar study on a Japanese population revealed that rs10757272 is a new locus for susceptibility to IA while demonstrating the association between rs10757278 and IA.13 However, the relationship between SNPs and the pathophysiology of IA in the Han population requires further investigation.4
Despite these results, the mechanism of the association between 9p21 and IA remains largely unexplored. Understanding the pathophysiology of IA is the key to identify molecular markers to help identify high-risk individuals and design novel therapeutic strategies. Although the association of SNPs in 9p21 with IA has been established in the Caucasian population, these results must be corroborated in other populations. Therefore, genetic factors for IA should also be investigated in Chinese populations, owing to major differences in genetic background and environment from Caucasian cohorts.4 This study aimed to explore the relationship between SNPs (rs10811661 and rs4977574) and IA in the Han population.
Methods
Ethics Approval and Informed Consent
All procedures complied with the Declaration of Helsinki and were approved by the Institutional Review Board and Ethics Committee of Tianjin Medical University General Hospital and the collaborating hospitals’ ethics committees. All participants reported themselves to be of Han ethnicity and provided written informed consent.
Study Site and Population
To ensure the study was representative, we selected hospitals located in developed areas of China, where the patients represented a vast number of Chinese provinces. IA was confirmed by computed tomography angiography (CTA) or digital subtraction angiography (DSA). All cases underwent either surgical clipping or endovascular coiling therapy in the perioperative period to reconfirm the diagnosis of IA. Control groups were sex- and age-matched (within 5 years), diagnosed negative for IA by DSA or CTA from the same hospital of the when they were diagnosed with subarachnoid hemorrhage, had diseases that needed to be differentiated from IAs, or other diseases, including carotid stenosis, subclavian artery stenosis, cerebral infarction, and transient ischemic attack. IA and control subjects were also matched for area of residence to reduce the effect of population heterogeneity.
Exclusion Criteria
Participants were excluded if they had active inflammatory processes or autoimmune diseases, inflammatory IA with different pathophysiology from IA, had been diagnosed with mental disorders, were pregnan, or had a family history of aneurysm. Participants in the control group were screened by interview questions, with the key criterion that they not have any IA-related diseases.
Data-Collection Procedures
Each participant consented to undergo a standardized questionnaire with in-person interview to collect data involving past medical history, family history, history of chronic diseases, and lifestyle factors of smoking habits and alcohol consumption. A standard physical examination for participants included blood pressure, pupil reflex, magnetic resonance angiography, DSA, or CTA. Two radiologists performed imaging examinations on controls to exclude IA. Biochemical parameters were obtained from samples of peripheral venous blood and analyzed at the clinical laboratory of the hospital.
Data Population
We collected clinical information, including sex, age, smoking status, alcohol usage, blood pressure, and other chronic diseases. Smokers were defined as regular smokers if they smoked one or more cigarettes per day. Former and current smokers were both as defined as smokers. Drinkers were defined as regular drinkers if they drank 20 mL of alcohol per day. Hypertension (systolic pressure >140 mmHg, diastolic blood pressure >90 mmHg, or taking hypotensive drugs) was diagnosed by physical examination procedures. Chronic disease history was recorded by in-person interview.
DNA Extraction and Genotyping
Candidate SNPs were selected on the basis of studies of epidemiological risk factors for IA. Our primary analyses examined 30 SNPs associated with established IA risk factors, including hypertension, atherosclerotic diseases, MI, hypercholesterolemia, T2DM, and autosomal-dominant polycystic kidney disease, and SNPs were correlated with IA,with P<5×10–8 from a published genome-wide association study. Peripheral blood (2 mL) was collected in tubes and stored at –80°C until analyzed. DNA was extracted from blood by salt fractionation. PCR was performed on an ABI Veriti 384-cell PCR thermal cycler. Genotypes were analyzed with Typer 4.0 software (MassArray compact system, Sequenom). The genotyping-success rate of all SNPs was >99%. Two SNPs on 9p21.3 were studied. The primers were designed by Primer 5, and sequences were:
rs10811661: ACGTTGGATGATAAGCGTTCTTGCCCTGTC (second PCRP), ACGTTGGATGAGATCAGGAGGGTAATAGAC (first PCRP).
rs4977574: ACGTTGGATGGTTTGCTTTCAGGGTACATC (second PCRP), ACGTTGGATGGTTGGTGTTCCAAACAGGAC (first PCRP).
Statistical Analysis
Continuous variables were analyzed using nonparametric Mann–Whitney tests. Hardy–Weinberg equilibrium for each SNP was tested with Pearson’s χ2 tests. These tests was used to examine any differences in allelic and genotypic frequencies between patients and controls. The 95% CIs and ORs for the impact of heterozygous and homozygous genotypes on the risk of IA were analyzed with multivariate logistic regression analysis. Two-sided P-values were applied, with P<0.05 considered statistically significant. All statistical analyses were performed by SPSS 17.0. Haplotypes and haplotype frequencies between patients and controls for the two SNPs in 9p21 were also compared. Linkage disequilibrium (LD) haplotypes of SNPs was examined by Haploview software. Adjusted ORs of haplotype were calculated by logistic regression analysis via Plink.
Results
Patient Characteristics
The study included a total of 1,195 subjects between July 2014 and December 2018 in China (Table 1). IA patients were diagnosed in the neurosurgery departments of the hospitals participating. Of the 595 IA patients enrolled, 251 were men (42.2%), 1.4 times the number of female patients. Sex distribution was consistent with previous studies. All participants were aged 18–80 years. A total of 676 IAs were detected, including 530 single IAs and 65 multiple IAs. More details are shown in Table 1.
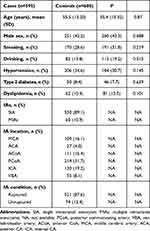 |
Table 1 Characteristics of Sporadic and Control Cases |
SNP Analysis
Minor-allele frequency for both SNPs was >0.05, and we hypothesized that they were prime targets for the HapMap project. As shown in Table 2,neither IA patients nor control subjects showed significant deviation from the Hardy–Weinberg equilibrium for either SNP. Associations between the two SNPs and IAs and differences among the seven genotype sites and allele frequencywere assessed withχ2 or Fisher’s exact tests (Table 3).There were significant differences for genotype frequency of rs10811661 between the IA cases and controls (χ2=7.584, P=0.005). Genotype distribution of the two polymorphisms and ORs, 95% CIs, and P-values of the SNPs were estimated using logistic regression analysis between IA cases and controls (Table 4). Risk-allele frequencies of rs10811661 were significantly different between IA patients and controls (OR 1.26, 95% CI 1.07–1.49).
 |
Table 2 Hardy–Weinberg Equilibrium SNP Analysis |
 |
Table 3 χ2 Results |
 |
Table 4 Genotype Analysis |
Haplotype Analysis
SNPs rs3217992 and rs1063192 were in obvious LD (D’=98, r2=22) within block 1 and rs2285489 and rs2301612 were in LD (D’=97, r2=73) within block 2 (Figure 1). No significant differences were observed in haplotype frequency between IA patients and control. Frequencies, ORs, and P-values for haplotypes are in Table 5.
 |
Table 5 Haplotype Analysis |
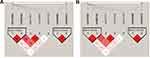 |
Figure 1 LD heat map (Haploview). Notes: White to red represents the degree of linkage from low to high, and deep red indicates full chain (r2=100). (A) D values; (B) R2 values. |
Discussion
In this study, the CDKN2A/CDKN2B genetic relationship with IAs of a Chinese Han population was investigated. We observed that the SNPs rs10811661 and rs4977574 were related to IAs and the most frequently mutated sites of the CDKN2A/CDKN2B gene locus. Genotype and allele frequencies were statistically significant. The T allele of rs10811661 is a risk factor of IA in the Han population.
Genome-wide association studies on Japanese IA patients, including 419 sporadic IA cases and 408 controls confirmed a positive site of rs1333040.14 Bilguvar et al15 illustrated that rs1333040 (9p21), rs10958409 (8q11), and rs700651 (2q33) were associated with IA. However, we observed the variants rs10811661 and rs4977574 were associated only with sporadic IAs. It has been hypothesized that genetic heterogeneity among diverse ethnic populations can lead to such paradoxical results and may be relevant to variations among different populations with a positive family history.14 Genetic transmission suggests that a haplotype is shared by the four SNPs, namely rs3217992, rs1063192, rs2285489, and rs2301612. However, rs10811661 and rs4977574 did not demonstrate either an association or LD with them. An independent publication revealed the possibility of a small LD block between introns 7 and 15 of CDKN2BAS.16 The 9p21 locus of CDKN2A/CDKN2B has not only been associated with T2DM but also with atherosclerosis, and its SNPs play a crucial role in the formation of IAs.
In this study, two genetic variants, rs10811661 and rs4977574 in 9p21, were identified as independent risk elements for the onset of IAs in Chinese population. The results emphasize the need to investigate genetic associations in diverse ethnic populations and evaluate the disease-related genes due to genetic heterogeneity. It is difficult to discover the exact loci associated with IA because of the long sequences of the CDKN2A and CDKN2B genes. The interaction of genes and racial differences both equally affect the formation process of IA, which is a complex genetic disease.17 Although lncRNAs, CDKN2A or CDKN2B plays a critical role in regulating the expression of gene loci in their vicinity in disease conditions.14 Bai et al reported that there are major differences in several lncRNA genes between aneurysm and normal cerebrovascular tissue, and showed that CDKN2A and CDKN2B affect atherosclerosis progress by regulating the expression of CARD8.18–20 Although the SNPs associated with IA in overseas groups (rs10811661 and rs4977574) had the highest mutation rates and associations of rs10811661-T with IA in two independent populations are firmly suggestive of a substantial association with IAs in our study population, there is a hypothesis that different populationsare more susceptible to IA due to CDKN2A/CDKN2B.
The underlying mechanism of CDKN2A/CDKN2B variants affecting IA risk remains to be elucidated. Functional studies have demonstrated 9p21 leads to CAD via atherosclerosis induced by variants located near CDKN2A and CDKN2B, which are involved in regulating vascular smooth-muscle cell (VSMC) proliferation, aging, senescence, and apoptosis, or by modulating the inflammatory pathway involved in the atherosclerosis process. Some studies have identified the ANRIL gene, which encodes a an ncRNA, as a genetic susceptibility locus associated with IA. Also, a mouse model confirmed a key role of ANRIL in regulation of CDKN2A/CDKN2B expression via a cis-acting mechanism and its effect on proliferation and senescence.13 ET1 and its receptors, ETA and ETB, have been known to play an important role in the physiopathology of IA. ETA is located predominantly on VSMCs of the cerebrovascular system and mediates vasoconstriction and proliferation. A study performed functional analysis with CDKN2BAS genetic variants on ETA. The variant affected the expression of ETA and subsequently contributed to IA susceptibility.13 A series of studies on Chinese populations have suggested the association of haplotypes at 9p21 with metabolic syndrome, CAD, peripheral artery disease, and T2DM.12,21 In this study, four SNPs — rs3217992 and rs1063192, rs2285489, and rs2301612 — also demonstrated strong LD in the Chinese Han population. These results indicate that the risk loci belonging to 9p21 region may confer susceptibility to IA.
Several limitations should be noted. This was not a cohort study and may not be adequate for assessing the effect of these SNPs on the formation of IAs. Molecular biology experiments and a reliable animal model are required to give a more accurate assessment of the relationship between these SNPs and the progression of IAs and enable a better understanding of the biological mechanism of IA formation.
Conclusion
This study implicates rs10811661 and rs4977574 as independent genetic risk factors of the formation of IA in the Chinese Han population and emphasizes the critical role of 9p21 in the disease.
Acknowledgments
We thank BioMiao Biotechnology (Beijing) Co Ltd and the Chinese Multicenter Cerebral Aneurysm Database (CMAD) for helping with the bioinformatic analysis and clinical data and blood samples. We thank the support of the National Natural Science Foundation of China (81571144), Natural Science Foundation of Tianjin, China (20JCZDJC00300, 18JCZDJC45400), and the Tianjin Medical University Clinical Research Program (2018kylc008).
Funding
This study was supported by the National Natural Science Foundation of China (81571144), Natural Science Foundation of Tianjin, China (20JCZDJC00300 and 18JCZDJC45400), and Tianjin Medical University Clinical Research Program (2018kylc008).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vlak M, Algra A, Brandenburg R, Rinkel G. Prevalence of unruptured intracranial aneurysms, with emphasis on sex, age, comorbidity, country, and time period: a systematic review and meta-analysis. The Lancet Neurology. 2011;10(7):626–636.
2. Feigin V, Rinkel G, Lawes C, et al. Risk factors for subarachnoid hemorrhage: an updated systematic review of epidemiological studies. Stroke. 2005;36(12):2773–2780.
3. Zhang L, Wei F, Zhao Y, et al. Intracranial aneurysm risk factor genes: relationship with intracranial aneurysm risk in a Chinese Han population. Genetics Mol Res. 2015;14(2):6865–6878.
4. Wei Y, Xiong J, Zuo S, et al. Association of polymorphisms on chromosome 9p21.3 region with increased susceptibility of abdominal aortic aneurysm in a Chinese Han population. J Vasc Surg. 2014;59(4):879–885.
5. Helgadottir A, Thorleifsson G, Magnusson K, et al. The same sequence variant on 9p21 associates with myocardial infarction, abdominal aortic aneurysm and intracranial aneurysm. Nat Genet. 2008;40(2):217–224.
6. Broadbent H, Peden J, Lorkowski S, et al. Susceptibility to coronary artery disease and diabetes is encoded by distinct, tightly linked SNPs in the ANRIL locus on chromosome 9p. Hum Mol Genet. 2008;17(6):806–814.
7. Pasmant E, Sabbagh A, Vidaud M, Bièche I. ANRIL, a long, noncoding RNA, is an unexpected major hotspot in GWAS. FASEB J. 2011;25(2):444–448.
8. Bochenek G, Häsler R, Ne EM, et al. The large non-coding RNA ANRIL, which is associated with atherosclerosis, periodontitis and several forms of cancer, regulates ADIPOR1, VAMP3 and C11ORF10. Hum Mol Genet. 2013;22(22):4516–4527.
9. Nie F, Sun M, Yang J, et al. Long noncoding RNA ANRIL promotes non-small cell lung cancer cell proliferation and inhibits apoptosis by silencing KLF2 and P21 expression. Mol Cancer Ther. 2015;14(1):268–277.
10. Salvi E, Kutalik Z, Glorioso N, et al. Genomewide association study using a high-density single nucleotide polymorphism array and case-control design identifies a novel essential hypertension susceptibility locus in the promoter region of endothelial NO synthase. Hypertension. 2012;59(2):248–255.
11. Wang W, Peng W, Zhang X, et al. Chromosome 9p21.3 polymorphism in a Chinese Han population is associated with angiographic coronary plaque progression in non-diabetic but not in type 2 diabetic patients. Cardiovasc Diabetol. 2010;9:33.
12. Tian L, Fang H, Gao L, et al. 9p21 polymorphisms increase the risk of peripheral artery disease in the Han Chinese population. J Int Med Res. 2013;41(1):106–114.
13. Low S, Takahashi A, Cha P, et al. Genome-wide association study for intracranial aneurysm in the Japanese population identifies three candidate susceptible loci and a functional genetic variant at EDNRA. Hum Mol Genet. 2012;21(9):2102–2110.
14. Chen Y, Li G, Fan H, et al. CDKN2BAS gene polymorphisms and the risk of intracranial aneurysm in the Chinese population. BMC Neurol. 2017;17(1):214.
15. Bilguvar K, Yasuno K, Niemelä M, et al. Susceptibility loci for intracranial aneurysm in European and Japanese populations. Nat Genet. 2008;40(12):1472–1477.
16. Hashikata H, Liu W, Inoue K, et al. Confirmation of an association of single-nucleotide polymorphism rs1333040 on 9p21 with familial and sporadic intracranial aneurysms in Japanese patients. Stroke. 2010;41(6):1138–1144.
17. Bruno G, Todor R, Lewis I, Chyatte D. Vascular extracellular matrix remodeling in cerebral aneurysms. J Neurosurg. 1998;89(3):431–440.
18. Wu S, Kallin E, Zhang Y. Role of H3K27 methylation in the regulation of lncRNA expression. Cell Res. 2010;20(10):1109–1116.
19. Bai Y, Nie S, Jiang G, et al. Regulation of CARD8 expression by ANRIL and association of CARD8 single nucleotide polymorphism rs2043211 (p.C10X) with ischemic stroke. Stroke. 2014;45(2):383–388.
20. Pan W, Liu L, Wei J, et al. A functional lncRNA HOTAIR genetic variant contributes to gastric cancer susceptibility. Mol Carcinog. 2016;55(1):90–96.
21. Cheng X, Shi L, Nie S, et al. The same chromosome 9p21.3 locus is associated with type 2 diabetes and coronary artery disease in a Chinese Han population. Diabetes. 2011;60(2):680–684.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
