Back to Journals » Journal of Pain Research » Volume 15
Association Between Serum 25-Hydroxyvitamin D Concentrations, CDX2 Polymorphism in Promoter Region of Vitamin D Receptor Gene, and Chronic Pain in Rural Japanese Residents
Authors Suzuki K , Tsujiguchi H, Hara A, Pham OK, Miyagi S , Nguyen TTT, Nakamura H, Suzuki F , Kasahara T , Shimizu Y, Yamada Y, Kambayashi Y, Tsuboi H, Sato T , Kannon T , Hosomichi K, Tajima A , Takamura T , Nakamura H
Received 12 January 2022
Accepted for publication 17 May 2022
Published 23 May 2022 Volume 2022:15 Pages 1475—1485
DOI https://doi.org/10.2147/JPR.S356630
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Qi Fang
Keita Suzuki,1 Hiromasa Tsujiguchi,1– 3 Akinori Hara,1– 3 Oanh Kim Pham,1 Sakae Miyagi,3,4 Thao Thi Thu Nguyen,5 Haruki Nakamura,1 Fumihiko Suzuki,2,6 Tomoko Kasahara,2 Yukari Shimizu,7 Yohei Yamada,2 Yasuhiro Kambayashi,8 Hirohito Tsuboi,9 Takehiro Sato,3,10 Takayuki Kannon,3,10 Kazuyoshi Hosomichi,3,10 Atsushi Tajima,3,10 Toshinari Takamura,11 Hiroyuki Nakamura1– 3
1Department of Hygiene and Public Health, Faculty of Medicine, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, Kanazawa, Ishikawa, Japan; 2Department of Public Health, Graduate School of Advanced Preventive Medical Sciences, Kanazawa University, Kanazawa, Ishikawa, Japan; 3Kanazawa University Advanced Preventive Medical Sciences Research Center, Kanazawa, Ishikawa, Japan; 4Innovative Clinical Research Center, Kanazawa University, Kanazawa, Ishikawa, Japan; 5Department of Epidemiology, Faculty of Public Health, Haiphong University of Medicine and Pharmacy, Hai Phong, Vietnam; 6Community Medicine Support Dentistry, Ohu University Hospital, Koriyama, Fukushima, Japan; 7Department of Nursing, Faculty of Health Sciences, Komatsu University, Komatsu, Ishikawa, Japan; 8Department of Public Health, Faculty of Veterinary Medicine, Okayama University of Science, Imabari, Ehime, Japan; 9Institute of Medical, Pharmaceutical, and Health Sciences, Kanazawa University, Kanazawa, Ishikawa, Japan; 10Department of Bioinformatics and Genomics, Graduate School of Advanced Preventive Medical Sciences, Kanazawa University, Kanazawa, Ishikawa, Japan; 11Department of Endocrinology and Metabolism, Kanazawa University Graduate School of Medical Sciences, Kanazawa, Ishikawa, Japan
Correspondence: Keita Suzuki, Department of Hygiene and Public Health, Faculty of Medicine, Institute of Medical, Pharmaceutical and Health Sciences, Kanazawa University, 13-1, Takara-machi, Kanazawa, Ishikawa, 920-8640, Japan, Tel +81 76 265 2218, Fax +81 76 234 4233, Email [email protected]
Background: Previous studies examined the association between chronic pain (CP) and serum 25-hydroxyvitamin D (25(OH)D) concentrations; however, the findings obtained were inconsistent. Single nucleotide polymorphisms (SNP) associated with the transcriptional activity of the vitamin D receptor (VDR) gene may influence the association of 25(OH)D levels with CP. We aimed to clarify the association between CP, serum 25(OH)D concentration, and SNPs.
Methods: In the Shika study, we performed a cross-sectional analysis of 551 participants older than 40 years who were asked whether they had been having persistent pain lasting for at least 3 months in any part of the body on a self-administered questionnaire. Serum 25(OH)D concentrations were assessed as a biomarker of the vitamin D status using a radioimmunoassay. rs731236, rs7975232, rs1544410, rs2228570, and rs11568820 were identified using peripheral blood samples, and participants were assigned to those with or without the minor allele for each SNP.
Results: The prevalence of CP was 37.2%. We observed a tendency for lower 25(OH)D levels in participants with CP than in those without CP in the hetero/minor group of rs11568820, which is a polymorphism within the CDX2-binding site in the 1e promoter region of the VDR gene. Furthermore, a logistic regression analysis revealed that lower serum 25(OH)D concentrations were significantly associated with CP in the hetero/minor group, but not in the major group.
Conclusion: These results suggest that sufficient serum 25(OH)D concentration reduces the risk of CP in individuals with the minor allele of the CDX2 polymorphism.
Keywords: chronic pain, 25-hydroxyvitamin D, vitamin D receptor gene, promoter, polymorphism, epidemiology
Introduction
Chronic pain (CP) is defined by the International Association for the Study of Pain as pain without an apparent biological cause that persists for longer than 3 months.1 The prevalence of CP was previously reported to range between 35.0 and 51.3% in the United Kingdom,2 and another review revealed that 25−76% of community-dwelling older individuals had CP.3 CP negatively affects health-related quality of life and work productivity, and increases the risk of developing depression and anxiety.4 Furthermore, the national cost of pain in the United States in 2008 was estimated to be between 560 and 635 billion dollars.5 Therefore, the development of effective and reasonable treatments is urgently needed.
Previous studies examined the therapeutic effects of vitamin D supplementation as a treatment for CP; however, the findings obtained were inconsistent.6,7 The association between CP and serum 25-hydroxyvitamin D [25(OH)D] concentrations, a biomarker of the vitamin D status, has also been investigated. Although low vitamin D concentrations were found to correlate with the CP status,8,9 recent cohort studies did not observe an association between 25(OH)D levels and CP.10 This discrepancy in the association between the vitamin D status and CP may be because confounding factors that critically influence the association between serum 25(OH)D concentrations and CP were not considered.
Vitamin D is metabolized into 25(OH)D in the liver and then into 1,25-dihydroxyvitamin D (1,25(OH)2D), which is the active form of vitamin D, in the kidneys.11 1.25(OH)2D in the blood then enters cells and binds to the vitamin D receptor (VDR) to exert its biological effects.12 Therefore, VDR may alter the biological functions of vitamin D in pain management. rs731236, rs7975232, rs1544410, rs2228570, and rs11568820 have been reported to be involved in VDR expression levels.13 VDR polymorphism in the binding site for the intestinal specific transcription factor CDX2 in the promoter region (rs11568820) has been shown to affect the expression levels of the VDR gene.13,14 The transcriptional activity of the VDR promoter with the G allele of the CDX2-binding site was previously reported to be 70% of that with the A allele.14 Therefore, the CDX2 polymorphism in the VDR promoter seems to affect the association between CP and serum 25(OH)D levels.
Although previous genome-wide association studies reported the association of several genes and single nucleotide polymorphisms (SNP) with CP,15,16 VDR-related SNPs did not show a significant association in those studies. This may be because these studies did not include vitamin D status as a covariate. Therefore, we performed a cross-sectional analysis to investigate whether VDR-related SNPs modify the association between the prevalence of CP and serum 25(OH)D concentration in the Shika study.
Materials and Methods
Study Design and Subjects
Cross-sectional data gathered between 2011 and 2015 in the Shika study were utilized. The Shika study is a longitudinal community-based observational study that has been conducted since 2011 among the residents of Shika town, which is located on the Noto Peninsula in Ishikawa prefecture, Japan.17 There are approximately 20,000 residents and the climate is humid subtropical. In the present study, we recruited participants from two model districts in the town. An invitation letter to this study was distributed to all adults older than 40 years (n = 2314) in the districts. Trained interviewers who were explained the outline of the Shika study and how to fill in the questionnaire delivered the questionnaire to all participants in person. In total, 2199 subjects (95%) responded to the questionnaire, and 972 subjects (42%) participated in the comprehensive health examination.
Participants with missing data on variables were excluded from the analysis. To avoid a treatment bias, we confirmed that participants were not taking vitamin D supplements. We excluded participants with high-sensitivity C-reactive protein (hsCRP) of 0.3 mg/dl or higher to omit patients who may have inflammatory diseases such as rheumatoid arthritis. Data collected from 551 participants who completed the health examination and replied to the questionnaire were available for analyses in the present study. The details of recruitment are shown in Figure 1.
 |
Figure 1 Flow chart of participant recruitment. |
The present study was approved by the Medical Ethics Committee of Kanazawa University (approval number 1491). All participants provided written informed consent for inclusion before participation. The present study was conducted in accordance with the Declaration of Helsinki.
Pain Questions
Participants were asked “Have you been having any pain persisting for 3 months or longer?” on a self-administered questionnaire. Respondents who answered “Yes” were labeled as “with CP”, while the others were labeled as “without CP”. The definition of CP was according to that presented by the International Association for the Study of Pain.1
Serum 25(OH)D Measurement
We collected fasting blood samples from all participants in the health examinations. To avoid seasonal variations of serum 25(OH)D levels, the health examinations were held in December and January. Serum 25(OH)D concentrations were measured using a radioimmunoassay (25-hydroxyvitamin D 125I RIA Kit, DiaSorin Inc., Stillwater, Minnesota, USA). Coefficients of variation for inter-assay of high-performance liquid chromatography (HPLC), competitive protein binding, and RIA, have been reported to be 8.4%, 14%, and < 12%, respectively.18 A previous study has reported that serum 25(OH)D levels measured using the RIA were between those measured using HPLC-atmospheric pressure chemical ionization-mass spectrometry and chemiluminescent immunoassays.19
Genotyping
We extracted genomic DNA from blood samples using the QIAamp DNA Blood Maxi Kit (Qiagen, Hilden, Germany) according to manufacturer’s instructions or consigned them to a company specialized in clinical laboratory testing (SRL, Inc., Tokyo, Japan). Genome-wide SNP genotyping was performed using the Japonica Array v220 (TOSHIBA Co., Ltd., Tokyo, Japan). Details of quality control (QC) procedures for the genome-wide SNP genotype data obtained were previously described.21 Briefly, the QC filtering of SNPs and participants was based on gender identity between karyotypes and the questionnaire, call rates, the Hardy-Weinberg equilibrium test, inbreeding coefficient, cryptic relatedness, and population structure. The genotypes of rs731236, rs7975232, rs1544410, rs2228570, and rs11568820 in 551 unrelated participants (based on genome-wide 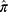 values) who passed QC were extracted from array data (only for rs7975232, n = 547). In the QC step, the call rates for SNPs ranged from 99.4% to 100%, and a departure from the Hardy–Weinberg equilibrium was not observed. According to the data obtained, participants with the minor allele were assigned to the hetero/minor homozygous group and the others to the major homozygous group for each SNP. Before combining the genotypes, the GTEx portal22 was used to confirm that the expression levels of the VDR gene changed with each additional minor allele for each SNP.
values) who passed QC were extracted from array data (only for rs7975232, n = 547). In the QC step, the call rates for SNPs ranged from 99.4% to 100%, and a departure from the Hardy–Weinberg equilibrium was not observed. According to the data obtained, participants with the minor allele were assigned to the hetero/minor homozygous group and the others to the major homozygous group for each SNP. Before combining the genotypes, the GTEx portal22 was used to confirm that the expression levels of the VDR gene changed with each additional minor allele for each SNP.
Other Variables
Age, height, weight, the osteo sono-assessment index, grip strength, and calf circumference were measured in the health examination. Body mass index (BMI) was calculated as weight in kilograms divided by the square of height in meters. The values of grip strength and calf circumference were divided by body weight to adjust for the influence of physique, and these values were used in the analysis. hsCRP and serum calcium levels were measured using blood samples taken in the health examination.
We evaluated drinking habits, exercise habits, and the smoking status using self-administered questionnaires. To assess drinking habits, participants were asked “How often do you drink alcohol?”. We labeled participants who drink at least once a week as “drinkers”, and the others as “non-drinkers” for the analysis. We divided participants into three groups according to the frequency of walking for more than 30 min in 1 week as follows: >5 days, 1 to 4 days, and no exercise. Participants were assigned to the Non-smoker, Ex-smoker, and Current smoker groups according to their answer about smoking habits. The analgesic administration status was also assessed in the self-administered questionnaire.
Daily dietary intakes of protein, lipids, carbohydrates, vitamin D, calcium, and total energy per day were assessed using a brief-type self-administered diet history questionnaire (BDHQ) that asked participants about the consumption frequency of 58 food and beverage items during the previous month. These items are mainly from the food list used in the National Health and Nutrition Survey of Japan and commonly consumed in Japan. Previous studies demonstrated the validity of BDHQ, and it is considered to have a satisfactory ranking ability for many nutrients in Japanese subjects.23,24 All participants who reported a total energy intake of less than 600 kcal/day (half of the energy intake required for the lowest physical activity category) or more than 4000 kcal/day (1.5-fold the energy intake required for the moderate physical activity category) were excluded from the analysis of nutrient data to avoid under-/overestimations of nutrient intake. Nutrient data were adjusted for daily energy intake using the density method.
Statistical Analysis
Continuous variables were summarized as means and standard deviation (SD), and categorical variables as numbers (N) and percentages (%). The Student’s t-test and chi-squared test were used to compare differences in the mean levels of continuous variables and categorical variables between participants with and without CP. A one-way analysis of covariance (ANCOVA) was used to compare serum 25(OH)D levels between participants with and without CP in each genotype group with adjustment for age, sex, and BMI. A multiple logistic regression analysis was performed to examine the association between CP and serum 25(OH)D concentrations according to the genotypes with adjustment for the following independent factors: sex, age, BMI, drinking habits, exercise habits, smoking status, and grip strength/body weight.
We performed all statistical analyses using the Japanese version of IBM SPSS Statistics version 27.0 (IBM Japan, Tokyo, Japan). A two-sided p-value <0.05 was considered to be significant.
Results
Participant Characteristics
The characteristics of participants according to the CP status are shown in Table 1. A total of 551 subjects (mean age and SD, 63.7 ± 9.9 years) participated in the present study. A total of 205 participants had CP and its prevalence was 37.2%. Serum 25(OH)D concentrations in the groups with and without CP were 24.50 ± 8.48 and 23.47 ± 7.48 ng/mL, respectively. No SNP showed a significant difference in the proportion of genotypes between the groups with and without CP.
 |
Table 1 Participant Characteristics |
Comparison of Serum 25(OH)D Concentrations Between Participants with and without CP According to SNP
To identify the SNPs that modify the association of 25(OH)D levels with CP, we performed the one-way ANCOVA to compare serum 25(OH)D concentrations between participants with and without CP according to the genotypes for each SNP. Table 2 shows the results of the one-way ANCOVA with adjustment for age, sex, and BMI. Significantly low 25(OH)D levels in the CP group were observed only in the GA/AA group of rs11568820, which is a VDR polymorphism in the binding site of the intestinal-specific transcription factor CDX2 in the promoter region.
 |
Table 2 Comparison of Serum 25(OH)D Concentrations Between Participants with and without CP According to SNP |
Comparison of Characteristics of Participants with and without CP According to the CDX2 Polymorphism
Table 3 shows a comparison of the characteristics of participants with and without CP according to the CDX2 polymorphism. No significant difference between participants with and without CP was found in 25(OH)D levels in the GG group (p = 0.992), whereas participants with CP showed a tendency of lower concentrations of serum 25(OH)D than those without CP in the GA/AA group (p = 0.062). Furthermore, the effect size of CP on the 25(OH)D levels in the GG and GA/AA groups were 0.001 and 0.209, respectively. Although participants with CP had a significantly lower vitamin D intake than those without CP in the GG group (p = 0.020), no significant difference was observed in the GA/AA group (p = 0.533). Significant differences were noted in grip strength/body weight (p =0.043), and protein intake (p = 0.003) between participants with and without CP in the GG group. Participants with CP had a significantly higher total energy intake (p = 0.040) and less frequent exercise habits (p = 0.026) than those without CP in the GA/AA group.
 |
Table 3 Comparison of Characteristics Between Participants with and without CP According to the CDX2 Polymorphism |
Logistic Regression Analysis of CP According to the CDX2 Polymorphism
Table 4 shows the results of a logistic regression analysis of CP as a dependent variable with serum 25(OH)D concentrations in each group assigned with or without the minor allele in the CDX2 polymorphism. The results showed that lower serum 25(OH)D levels were associated with the prevalence of CP in the GA/AA group after adjustment for sex, age, BMI, drinking habits, smoking status, exercise habits, and grip strength/body weight (OR: 0.961; 95% CI: 0.929–0.994; p = 0.020), but not in the GG group (OR: 0.990; 95% CI: 0.952–1.030; p = 0.627).
 |
Table 4 Logistic Analysis of Chronic Pain as a Dependent Variable with 25(OH)D Concentrations as an Independent Variable |
Discussion
The results obtained in this cross-sectional study demonstrated that lower serum 25(OH)D levels were associated with the prevalence of CP in the GA/AA group even after adjustment for covariates, whereas this association was not observed in the GG group.
The results need to be corrected for multiple testing because we included five SNPs and divided the participants into two groups according to their genotypes in the present study. In this case, the p-value should be 0.005 based on the Bonferroni correction. However, none of the results showed a p-value less than 0.005. This may be attributed to the insufficient number of participants in this study. As shown in Table 2, the partial eta squared value in the GA/AA group of rs11568820 was 0.015. Assuming a p-value of 0.005, power of 80%, and partial eta squared of 0.015, the required sample size was estimated as 879. Therefore, if the recruited sample size of participants with the minor allele was approximately 900, a significant result could have been obtained after Bonferroni correction. Although the results were not significant after Bonferroni correction, we believe that these results may be valuable because our findings suggest that the CDX2 polymorphism of the VDR promoter modifies the association between serum 25(OH)D levels and CP.
The prevalence of CP was 37.2% in the present study. The prevalence of CP reported in previous studies in Japan ranges 15.4–39.3%.25,26 The difference in prevalence seems to be attributed to the definition of CP in each study. One defined CP as pain lasting more than 6 months, corresponding to a visual analog scale of at least five. Another study, which presented a prevalence of 39.3%, defined CP as pain lasting more than three months, which is similar to the present study. Therefore, the prevalence in the present study was considered reasonable.
Previous studies reported an association between serum 25(OH)D concentrations and CP, with low 25(OH)D levels being associated with CP, and a systematic review published in 2018 implicated low 25(OH)D concentrations in pain development.27 In contrast, no relationship was observed between 25(OH)D levels and the CP status in large population-based studies.10,28 One of the reasons for this may be that participants were not stratified by factors that influence the relationship between CP and 25(OH)D, such as the CDX2 polymorphism of the VDR promoter. To the best of our knowledge, the present study is the first to investigate the association between serum 25(OH)D levels and CP according to the CDX2 polymorphism.
An association between low concentrations of 25(OH)D and CP was observed in the GA/AA group in the present study. Transcriptional activity was higher with than without the A allele in the CDX2 polymorphism of the VDR promoter.14 A-allele carriers may benefit more from vitamin D than those without the A allele. A randomized controlled trial that examined the interventional effects of vitamin D intake on central obesity indicators detected improvements in these indicators in participants with A-allele homozygotes only.29 This finding suggests that the effects of changes in serum 25(OH)D levels are more pronounced in A-allele carriers of the CDX2 polymorphism, which appears to be supported by the present results.
The effects of vitamin D on skeletal muscle function, inflammation, and the peripheral and central nervous systems have been reported as the physiological mechanisms linking serum concentrations of 25(OH)D and pain in basic and clinical research.30 The contribution of the central and peripheral nervous systems to pain was unknown in the present study because we did not evaluate these indicators. On the other hand, no significant differences were observed in grip strength normalized by body weight, an index of muscle strength, or hsCRP between participants with and without CP in the GA/AA group. Therefore, the effects of vitamin D on skeletal muscle function and inflammation may not explain the association between the serum concentrations of 25(OH)D and CP observed in the present study. Vitamin D plays an important role in calcium homeostasis by enhancing calcium absorption in the small intestine and reabsorption in the kidneys. Vitamin D deficiency contributes to the development of osteoporosis.31 Increased bone resorption, which is a pathology of osteoporosis, has been reported to induce an acidic microenvironment and the production of inflammatory cytokines, such as IL-1, IL-6, and TNF-α and these factors may be involved in the development of pain through their activation of sensory nerve fibers or nociceptive receptors.32,33 In the present study, serum calcium concentrations and the osteo sono-assessment index, an index of bone mass, did not significantly differ between participants with and without CP in the GA/AA group. Therefore, osteoporosis-related pain does not explain the association observed between 25(OH)D levels and CP, assuming that the underlying mechanisms for this association remain unclear. 1.25(OH)2D, the active form of vitamin D, has been suggested to directly/indirectly influence the expression of 200–2000 genes through binding to the VDR.34 In other words, the physiological mechanisms of action of vitamin D in the body, which are regulated by complex pathways, have not yet been elucidated in detail. Therefore, further studies are needed to clarify the mechanisms contributing to the association between serum concentrations of 25(OH)D and CP.
There are several limitations that need to be addressed. The causality between serum 25(OH)D concentrations and CP remains unclear because of the nature of a cross-sectional study. In addition, because CP was defined based on a single question in a self-administered questionnaire in this study, many types of CP with different pathologies were included. Information from medical records or evaluations with other questionnaires on pain is required to distinguish its pathology. Furthermore, the generalizability of the present results may have been limited due to selection bias. The ratio of health-conscious individuals was likely to be high in the present study because we only performed a health examination on voluntary participants. Moreover, information on the mental health of participants, which is regarded as a confounder, was not included in the analysis because the number of participants with complete data on this variable was too small. Finally, the lack of Bonferroni correction and replication analysis using publicly available genetic databases prevented the present study from providing robust suggestions. Further studies with a larger sample size and replication analysis are needed to clarify the effect of the CDX2 polymorphism of the VDR promoter on the association of serum 25(OH)D concentrations with CP.
Conclusions
In conclusion, lower serum 25(OH)D levels were associated with CP in participants with the minor allele in the CDX2 polymorphism of the VDR promoter. The high transcriptional activity of the VDR gene in minor allele carriers appeared to enhance the effects of changes in serum 25(OH)D concentrations. These results suggest that sufficient 25(OH)D levels are important for preventing the development of CP in participants with the A allele in the CDX2 polymorphism of the VDR promoter.
Abbreviations
ANCOVA, analysis of covariance; BDHQ, brief-type self-administered diet history questionnaire; BMI, body mass index; CI, confidence interval CP, chronic pain; HPLC, high-performance liquid chromatography; hsCRP, high sensitivity C-reactive protein; OR, odds ratio; QC, quality control; RIA, radioimmunoassay; SD, standard deviation; SNP, single nucleotide polymorphism; VDR, vitamin D receptor; 25(OH)D, 25-hydroxyvitamin D; 1,25(OH)2D, 1,25-dihydroxyvitamin D.
Acknowledgments
The authors wish to thank all the field workers and laboratory personnel of the Shika Study for their efforts.
Funding
The present study was funded by a Grant-in-Aid for Scientific Research (B) (number: JP19H03882) and a Grant-in-Aid for Research Activity Start-up (number: JP21K21251) by the Japan Society for the Promotion of Science.
Disclosure
There are no conflicts of interest.
References
1. Treede R-D, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP Classification of Chronic Pain for the International Classification of Diseases (ICD-11). Pain. 2019;160(1):19–27. doi:10.1097/j.pain.0000000000001384
2. Fayaz A, Croft P, Langford RM, Donaldson LJ, Jones GT. Prevalence of chronic pain in the UK: a systematic review and meta-analysis of population studies. BMJ Open. 2016;6(6):e010364. doi:10.1136/bmjopen-2015-010364
3. Abdulla A, Adams N, Bone M, et al. Guidance on the management of pain in older people. Age Ageing. 2013;42(Suppl 1):i1–57. doi:10.1093/ageing/afs200
4. Kawai K, Kawai AT, Wollan P, Yawn BP. Adverse impacts of chronic pain on health-related quality of life, work productivity, depression and anxiety in a community-based study. Fam Pract. 2017;34(6):656–661. doi:10.1093/fampra/cmx034
5. Gaskin DJ, Richard P. The economic costs of pain in the United States. J Pain. 2012;13(8):715–724. doi:10.1016/j.jpain.2012.03.009
6. Straube S, Derry S, Straube C, Moore RA. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;2015(5):CD007771. doi:10.1002/14651858.CD007771.pub3
7. Wu Z, Malihi Z, Stewart AW, Lawes CM, Scragg R. Effect of Vitamin D Supplementation on Pain: a Systematic Review and Meta-analysis. Pain Physician. 2016;19(7):415–427.
8. Macfarlane GJ, Palmer B, Roy D, Afzal C, Silman AJ, O’Neill T. An excess of widespread pain among South Asians: are low levels of vitamin D implicated? Ann Rheum Dis. 2005;64(8):1217–1219. doi:10.1136/ard.2004.032656
9. Heidari B, Shirvani JS, Firouzjahi A, Heidari P, Hajian-Tilaki KO. Association between nonspecific skeletal pain and vitamin D deficiency. Int J Rheum Dis. 2010;13(4):340–346. doi:10.1111/j.1756-185X.2010.01561.x
10. Wu Z, Camargo CAJ, Sluyter JD, et al. Association between serum 25-hydroxyvitamin D levels and self-reported chronic pain in older adults: a cross-sectional analysis from the ViDA study. J Steroid Biochem Mol Biol. 2019;188:17–22. doi:10.1016/j.jsbmb.2018.11.018
11. Berridge MJ. Vitamin D cell signalling in health and disease. Biochem Biophys Res Commun. 2015;460(1):53–71. doi:10.1016/j.bbrc.2015.01.008
12. Wimalawansa SJ. Non-musculoskeletal benefits of vitamin D. J Steroid Biochem Mol Biol. 2018;175:60–81. doi:10.1016/j.jsbmb.2016.09.016
13. Uitterlinden AG, Fang Y, Van Meurs JBJ, Pols HAP, Van Leeuwen JPTM. Genetics and biology of vitamin D receptor polymorphisms. Gene. 2004;338(2):143–156. doi:10.1016/j.gene.2004.05.014
14. Arai H, Miyamoto KI, Yoshida M, et al. The polymorphism in the caudal-related homeodomain protein Cdx-2 binding element in the human vitamin D receptor gene. J Bone Miner Res. 2001;16(7):1256–1264. doi:10.1359/jbmr.2001.16.7.1256
15. Nishizawa D, Iseki M, Arita H, et al. Genome-wide association study identifies candidate loci associated with chronic pain and postherpetic neuralgia. Mol Pain. 2021;17:1744806921999924. doi:10.1177/1744806921999924
16. Johnston KJA, Adams MJ, Nicholl BI, et al. Genome-wide association study of multisite chronic pain in UK Biobank. PLoS Genet. 2019;15(6):e1008164. doi:10.1371/journal.pgen.1008164
17. Nguyen TTT, Tsujiguchi H, Kambayashi Y, et al. Relationship between Vitamin Intake and Depressive Symptoms in Elderly Japanese Individuals: differences with Gender and Body Mass Index. Nutrients. 2017;9:12. doi:10.3390/nu9121319
18. Zerwekh JE. The measurement of vitamin D: analytical aspects. Ann Clin Biochem. 2004;41(Pt 4):272–281. doi:10.1258/0004563041201464
19. Snellman G, Melhus H, Gedeborg R, et al. Determining vitamin D status: a comparison between commercially available assays. PLoS One. 2010;5(7):e11555. doi:10.1371/journal.pone.0011555
20. Kawai Y, Mimori T, Kojima K, et al. Japonica array: improved genotype imputation by designing a population-specific SNP array with 1070 Japanese individuals. J Hum Genet. 2015;60(10):581–587. doi:10.1038/jhg.2015.68
21. Nomura A, Sato T, Tada H, et al. Polygenic risk scores for low-density lipoprotein cholesterol and familial hypercholesterolemia. J Hum Genet. 2021. doi:10.1038/s10038-021-00929-7
22. GTEx Portal. Available from: https://gtexportal.org/home/.
23. Kobayashi S, Murakami K, Sasaki S, et al. Comparison of relative validity of food group intakes estimated by comprehensive and brief-type self-administered diet history questionnaires against 16 d dietary records in Japanese adults. Public Health Nutr. 2011;14(7):1200–1211. doi:10.1017/S1368980011000504
24. Sasaki S, Yanagibori R, Amano K. Self-administered diet history questionnaire developed for health education: a relative validation of the test-version by comparison with 3-day diet record in women. J Epidemiol. 1998;8(4):203–215. doi:10.2188/jea.8.203
25. Inoue S, Kobayashi F, Nishihara M, et al. Chronic Pain in the Japanese Community–Prevalence, Characteristics and Impact on Quality of Life. PLoS One. 2015;10(6):e0129262. doi:10.1371/journal.pone.0129262
26. Nakamura M, Nishiwaki Y, Ushida T, Toyama Y. Prevalence and characteristics of chronic musculoskeletal pain in Japan. J Orthop Sci off J Japanese Orthop Assoc. 2011;16(4):424–432. doi:10.1007/s00776-011-0102-y
27. Wu Z, Malihi Z, Stewart AW, Lawes CM, Scragg R. The association between vitamin D concentration and pain: a systematic review and meta-analysis. Public Health Nutr. 2018;21(11):2022–2037. doi:10.1017/S1368980018000551
28. Hirani V, Blyth FM, Naganathan V, et al. Active vitamin D (1,25 dihydroxyvitamin D) is associated with chronic pain in older Australian men: the Concord Health and Ageing in Men Project. J Gerontol a Biol Sci Med Sci. 2015;70(3):387–395. doi:10.1093/gerona/glu126
29. Shab-Bidar S, Neyestani TR, Djazayery A. Vitamin D receptor Cdx-2-dependent response of central obesity to vitamin D intake in the subjects with type 2 diabetes: a randomised clinical trial. Br J Nutr. 2015;114(9):1375–1384. doi:10.1017/S0007114515003049
30. Helde-Frankling M, Björkhem-Bergman L. Vitamin D in Pain Management. Int J Mol Sci. 2017;18:10. doi:10.3390/ijms18102170
31. Veldurthy V, Wei R, Oz L, Dhawan P, Jeon YH, Christakos S. Vitamin D, calcium homeostasis and aging. Bone Res. 2016;4:16041. doi:10.1038/boneres.2016.41
32. Kanaya K, Iba K, Abe Y, et al. Acid-sensing ion channel 3 or P2X2/3 is involved in the pain-like behavior under a high bone turnover state in ovariectomized mice. J Orthop Res off Publ Orthop Res Soc. 2016;34(4):566–573. doi:10.1002/jor.23047
33. Abe Y, Iba K, Sasaki K, et al. Inhibitory effect of bisphosphonate on osteoclast function contributes to improved skeletal pain in ovariectomized mice. J Bone Miner Metab. 2015;33(2):125–134. doi:10.1007/s00774-014-0574-x
34. Hossein-nezhad A, Holick MF. Vitamin D for health: a global perspective. Mayo Clin Proc. 2013;88(7):720–755. doi:10.1016/j.mayocp.2013.05.011
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
