Back to Journals » OncoTargets and Therapy » Volume 9
Association between proto-oncogene mutations and clinicopathologic characteristics and overall survival in colorectal cancer in East Azerbaijan, Iran
Authors Dolatkhah R, Somi MH, Asvadi Kermani I, Bonyadi M, Sepehri B, Boostani K, Azadbakht S, Fotouhi N, Farassati F , Dastgiri S
Received 4 July 2016
Accepted for publication 23 September 2016
Published 7 December 2016 Volume 2016:9 Pages 7385—7395
DOI https://doi.org/10.2147/OTT.S116373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr William C. Cho
Roya Dolatkhah,1 Mohammad Hossein Somi,2 Iraj Asvadi Kermani,1 Morteza Bonyadi,3 Bita Sepehri,2 Kamal Boostani,2 Saleh Azadbakht,2 Nikou Fotouhi,2 Faris Farassati,4 Saeed Dastgiri1,5
1Hematology and Oncology Research Center, Tabriz University of Medical Sciences, Tabriz, Iran; 2Liver and Gastrointestinal Diseases Research Center, Tabriz University of Medical Sciences, Tabriz, Iran; 3Center of Excellence for Biodiversity, Faculty of Natural Sciences, University of Tabriz, Tabriz, Iran; 4Molecular Medicine Laboratory, University of Kansas Medical School, Kansas City, KS, USA; 5Tabriz Health Services Research Center, Tabriz University of Medical Sciences, Tabriz, Iran
Background: Colorectal cancer (CRC) is the third-most common cancer in Iran. The increasing incidence of CRC in the past three decades has made it a major public health burden in the country. This study aimed to determine any relationship of specific mutations in CRCs with clinicopathologic aspects and outcome of patients.
Materials and methods: This study was conducted on 100 CRC patients by the case-only method. Polymerase chain-reaction products were analyzed by Sanger sequencing, and sequence results were compared with the significant KRAS and BRAF gene mutations in the My Cancer Genome database. Logistic regression models were used to detect associations of clinicopathologic characteristics with each of the mutations. Kaplan–Meier and Cox regression models were constructed to estimate overall survival in patients.
Results: A total of 26 subjects (26%) had heterozygote-mutant KRAS, and mutations were not detected in the amplified exon of BRAF in both tumor and normal tissues of the 100 CRCs. Rectal tumors had 1.53-fold higher likelihood of KRAS mutations than colon tumors, and men had 1.37-fold higher odds than women. The presence of metastasis increased the likelihood of KRAS mutations 2.36-fold over those with nonmetastatic CRCs. Compared to patients with KRAS wild-type cancers, those with KRAS mutations had significantly higher mortality (hazard ratio 3.74, 95% confidence interval 1.44–9.68; log-rank P=0.003).
Conclusion: Better understanding of the causality of CRC can be established by combining epidemiology and research on molecular mechanisms of the disease.
Keywords: proto-oncogene, sequence analysis, regression, colorectal cancer, survival
Introduction
The annual report of the Iranian National Cancer Registry Program (2008) showed that colorectal cancer (CRC) is the fourth-most common cancer in men in Iran, after stomach, bladder, and prostate cancer. It is however the second-most common among women, after breast cancer.1–4 A study using 5-year data obtained from the records of patients with CRC showed that although the incidence of CRC in Iran was lower than in Western countries, particularly in older populations, it was higher than reported in previous studies.1 The latest data from the cancer patients’ registry program showed that the age-standardized incidence rate had risen from 2.8 to 5.5 in 2009 and reached 9.2 in 2012 per 100,000 persons.2,3 According to the latest data from the northwest of the country in 2014, annual age-standardized incidence rates were estimated to be 11.2 and 8.93 in men and women, respectively. The crude rates for men and women were reported as 11.5 and 9.22, respectively, in the same study.4
Several epidemiological studies in Iran have shown that the proportion of CRC occurring among the younger population is considerably higher than in Western populations. Early CRC (at age less than 40 years) accounts for almost one-fifth of all cases of CRC in Iran, whereas in high-risk countries rates range from 2% to 8%.1,5–13 Studies have shown that genetic factors probably play a role in the development of CRC among this younger population in Iran as well. This suggests that different factors, both environmental and genetic, may be implicated in CRC in Iran.14,15
The pathogenesis of CRC is complex and diverse, with different molecular pathways, leading to different phenotypes. Mutations in the KRAS and BRAF genes are considered important primary incidents in the development of CRC.16 KRAS- and BRAF-gene mutations in CRC are caused by point mutations, resulting in single amino acid substitutions. KRAS mutations are present in 17%–25% of all cancers and in more than 35%–45% of CRC. In more than 90% of cases, these mutations occur in codons 12 and 13 of exon 2. Recent reports have shown that mutation in codon 12 is observed at frequencies of 12%–30% and 35%–50% among CRC patients in Asian and Western countries, respectively.17
BRAF mutations are observed in 15.7% of sporadic cases of CRC and in approximately 50% of colon cancers with microsatellite instabilities; point mutations occur in exons 12, 15, and 21, and >90% mutations in codons (599 of 600) of the BRAF gene. Although the role of KRAS and BRAF mutations has been widely accepted for predicting responses to anti-EGFR treatments, particularly in patients with advanced CRC, there is no consensus on the role of KRAS mutations in the prognosis and survival of patients with CRC. While the negative prognostic role of KRAS-gene mutation in patients with CRC has been reported, the presence of BRAF mutations is associated with poor prognosis and lack of response to anti-EGFR treatment.18–21
The increasing incidence of CRC in the past three decades in most Asian countries has been reported by Asia-Pacific consensus, especially in young adults.22,23 A better understanding of the causality of CRC can be established by combining epidemiology and research on molecular mechanisms. The present study was performed in the East Azerbaijan province of Iran. This study aimed to investigate the frequency of KRAS and BRAF mutations in patients with CRC, and to determine any relationship with clinicopathological aspects of the condition.
Materials and methods
Samples
To perform molecular tests, fresh tissue samples, including neoplastic cells and normal or CRC-unaffected tissue samples from the colon and rectal areas of the 100 patients with suspected CRC, were obtained using biopsy during colonoscopy (tissue size 2–4 mm). These biopsy samples were sent to a molecular laboratory under standard conditions and were coded and stored at −80°C. All samples were evaluated and subjected to histological diagnosis by expert pathologists. One hundred patients with confirmed CRC were included in this study.
KRAS- and BRAF-mutation analysis
Genomic DNA extracted from the cancer and normal tissue samples according to DNA-extraction kit (CinnaGen, Tehran, Iran) guidelines and used as a template in polymerase chain reactions (PCRs) using specific KRAS and BRAF primers encompassing KRAS exon 2 (codons 12 and 13) and BRAF exon 25 (codon 600)24 (Table 1).
  | Table 1 Sequence primers used for study of mutation of KRAS and BRAF genes |
PCR was conducted in 50 μL final volume containing 2.5 μL PCR buffer, 1 μL MgCl2, 1 μL from each primer, 6–7 μL genomic DNA, 0.5 μL deoxynucleotide triphosphate, 0.18 μL of BioTaq polymerase (CinnaGen), and 17 μL PCR-grade water. KRAS and BRAF were individually amplified using a 2720 Thermal Cycler (Thermo Fisher Scientific, Waltham, MA, USA).
KRAS PCR
Program conditions were 5 minutes at 95°C, followed by 35 cycles of 30 seconds at 95°C, 30 seconds at 59°C, 30 seconds at 72°C, and 8 minutes at 72°C.
BRAF PCR
Program conditions were 3 minutes at 94°C, followed by 30 cycles of 20 seconds at 94°C, 150 seconds at 61°C, 20 seconds at 72°C, and 3 minutes at 72°C. PCR products were analyzed by 1.5% agarose-gel electrophoresis and visualized by ethidium bromide staining, using UVItec (Cambridge, UK) equipment.
Sanger sequencing was performed after purification of PCR products with a PCR product-purification kit (MBST, Tehran, Iran) as per the manufacturer’s protocol. The forward primers of PCR amplification of KRAS and BRAF genes were used for sequencing, using a genetic analyzer (ABI 4-capillary 3130; Thermo Fisher Scientific).
Sequencing electropherograms were analyzed using Chroma 233, FinchTV, and DNAman software. Mutation status and probable nucleotide changes in the specific exon were determined using “Database Nucleotide Collection Blast” in NCBI/BLAST. All sequence results (normal and tumor) were compared with the significant KRAS- and BRAF-gene mutations in the My Cancer Genome database of CRC, to identify mutations and the presence of new mutations.
Descriptive data
Histopathological criteria were obtained including histological subtype, grade of cell differentiation, pathological TNM (tumor, node, metastasis) stage, and tumor location. We used the World Health Organization (WHO) histological classification of tumors of the colon and rectum, including adenocarcinoma, mucinous adenocarcinoma, signet ring-cell carcinoma, small-cell carcinoma, squamous cell carcinoma, adenosquamous carcinoma, medullary carcinoma, and undifferentiated carcinoma. The stage of tumor was specified using the TNM classification of tumors of the colon and rectum (primary tumor, regional involved lymph nodes, and distant metastasis) according to pathology reports and the anatomic extent of disease according to the clinical and radiological extent of the disease. Colorectal tumors were graded on the basis of the extension of the glandular appearance, and classified into four groups: well, moderately, poorly, and undifferentiated. Well and moderately differentiated adenocarcinomas were considered low-grade and poorly differentiated adenocarcinomas and undifferentiated carcinomas high-grade tumors.25 According to the colonoscopy and surgery reports, the anatomic subsites of the colorectal tumors were determined according to the WHO’s International Classification of Diseases (ICD-0–3).26 The splenic flexure of the colon was used to delineate the left and right sides of the colon. The right or proximal colon included the cecum, appendix, ascending colon, hepatic flexure, transverse colon, and splenic flexure. The left or distal colon included the descending and sigmoid colon. The rectum, anus, and anal canal regions were considered rectal.
Statistical analysis
The main aim of this study was to determine distinct clinicopathological characteristics associated with KRAS and BRAF mutation in 100 CRC patients. Descriptive data were analyzed statistically using SPSS software (version 19.0; IBM, Armonk, NY, USA). Simple and multiple logistic regression analyses were performed for computing unadjusted and adjusted odds ratios (ORs) with 95% confidence intervals (CIs). In the univariate analysis, each variable – sex, age, anatomic subsite, histological type, family history of CRC, family history of other cancers, disease stage, tumor grade, presence of metastasis, tobacco smoking, and alcohol consumption – was analyzed separately, and the presence of KRAS and/or BRAF mutations was considered the dependent variable. In the multivariate analysis, a backward-elimination multiple logistic regression was performed to find the set of best predictors of mutant genes. KRAS and BRAF mutants were compared with wild-type (WT) genes.
For survival analysis, the outcome of interest was overall survival (OS), defined as the time from CRC diagnosis to date of death or date of last follow-up. OS was estimated using the Kaplan–Meier method. Statistical significance was assessed using the log-rank test. The Cox proportional-hazard model was used to assess the effect of mutation on OS.
Ethics statement
This study was reviewed and approved by the ethics committee of Tabriz University of Medical Sciences. All patients enrolled in the study provided written informed consent forms for the use of tumor and normal tissue samples.
Results
Mutational distribution of KRAS and BRAF genes
This study was performed on 100 patients with colon and rectal cancers (72 with colon and 28 with rectal cancer). Primary samples from tumor and normal tissues were analyzed for KRAS- and BRAF-mutation status. PCR products were analyzed using Sanger sequencing, and their sequences were investigated using DNAman, Chromas 233, and FinchTV software to identify mutations in the mutant and WT KRAS and BRAF genes. Codon numbers of the mutant genes and their changed amino acid sequences were determined by referring to the NCBI/BLAST website. Representative electropherograms of KRAS mutant (cancer 135), and WT are shown in Figure 1. All mutations found had previously been described and were found in the My Cancer Genome database.
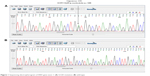  | Figure 1 Sequencing electropherogram of KRAS gene exon 1: (A) G12D mutation; (B) wild-type. |
Of the 100 patients, 26 (26%) had heterozygote-mutant KRAS and 74 (74%) had WT KRAS in tumor tissues. KRAS mutations were not detected in normal tissues. Moreover, mutations were not detected in the amplified exon of BRAF in either tumor or normal tissues of the 100 patients with CRC. Table 2 shows the status and type of mutations in KRAS detected in patients included in the study and the change in nucleotide sequences and corresponding amino acid sequences.
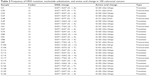  | Table 2 Frequency of KRAS mutation, nucleotide substitution, and amino acid change in 100 colorectal cancers |
Clinical information and descriptive analysis
Of the 100 patients, 65 were male and 35 female; the male:female ratio was 1.86. The mean age was 61.94 (±15.34) years, and ranged from 23 to 90 years. Twenty (20%) were aged below 50 years. The peak incidence was in the fifth decade of life (26%). The mean body mass index (BMI) of patients was 23.88 (±3.63), and ranged from 15.24 to 33.31.
A total of 26 patients (26%) had a positive family history of CRC in at least one of their first-degree relatives. Positive family history was 33% more common in younger CRCs (<50 years; OR 0.33, 95% CI 0.12–0.93). None of these patients had hereditary nonpolyposis CRC. Thirty-four (34%) patients had a positive family history of other cancers, and only four patients with CRC had a family history of polyps. Forty-nine (49%) tumors were located in the proximal (or right) colon, 23 tumors in the distal (or left) colon, and 28 tumors were in the rectum area.
The most common histological type of CRC (determined by pathological analysis) was adenocarcinoma, which was detected in 81% of patients. The stage of the disease was determined using the TNM system. Staging could only be performed in 66 patients, of whom 15 (22.7%) had stage I cancer, 22 (33.3%) had stage II cancer, nine (13.6%) had stage III cancer, and 20 (30.3%) had stage IV cancer. Differentiation of cancer cells (determined by pathological analysis) according to the WHO criteria25 was performed in 84% of CRC tumors. Of the 100 patients, 22 had well-differentiated, 22 moderately differentiated, three poorly differentiated, and 26 undifferentiated cancer cells.
Of the 100 patients, 28 (28%) had a history of tobacco smoking, of whom 10% had smoked more than one packet of cigarettes per day for more than 1 year. Only 19 (19%) patients had a history of moderate-to-low alcohol consumption. None had a history of high alcohol consumption. Male patients were more likely to smoke (male 44.6% vs female 8.6%) and drink (male 29.2% vs female 0%) than females. Table 3 shows the clinicopathologic characteristics of study participants.
  | Table 3 Summary statistics of clinicopathologic characteristics of 100 CRC patients |
Association of clinicopathologic characteristics by KRAS and BRAF mutation
Simple logistic regression was used to determine statistical significance between KRAS-mutated carcinoma vs WT carcinoma for sex, age, marital status, anatomic subsite, histological type, family history of CRC, family history of any other cancers, disease stage, tumor grade, metastasis, tobacco smoking, alcohol consumption, BMI, CEA, and CA19-9.
Males had 37% higher odds of KRAS-gene mutation than females (OR 1.37, 95% CI 0.53–3.56), and the odds of mutation were 1.29 times higher in younger CRC patients (<50 years; OR 1.29, 95% CI 0.44–3.79). A 1-year increase in age raised the odds of KRAS-gene mutation by 0.1% (OR 1.001, 95% CI 0.97–1.03). KRAS mutation was observed 2.32 times more frequently in patients with a negative family history of CRC than those positive for CRC (29.7% vs 15.4%) (OR 2.323, 95% CI 0.72–7.54). The odds of KRAS mutation were significantly higher in patients who had a positive family history of any other cancers (OR 0.40, 95% CI 0.16–0.99). Right-side colon cancers had lower odds for KRAS mutation, while left-side colon and rectal tumors had respectively 22% and 64% higher odds of KRAS-gene mutation than right-side tumors (OR 1.22, 95% CI 0.39–3.84 and OR 1.64, 95% CI 0.58–4.63, respectively). Low-stage tumors (stage I and II) had a slightly higher frequency of KRAS mutation than the high-stage tumors (24.3% vs 24.1%). Low-stage tumors had only 1% greater odds of KRAS mutation than high-stage tumors. The odds of KRAS mutation were 2.31 times higher in low-grade tumors (well or moderately differentiated) than high-grade tumors (poorly or undifferentiated tumors), and metastatic CRC had 85% greater odds of KRAS mutation than those in nonmetastatic patients. Despite adenocarcinoma being more common, the other histological CRC types had 2% higher odds of KRAS-gene mutation. The odds of KRAS mutation increased 3% with one unit increase in BMI (kg/m2), but this was not significant (OR 0.97, 95% CI 0.86–1.10). In patients who had higher serum levels of CEA and CA19-9 (5–200 ng/mL), the odds of mutation were 1.63 and 2.31 times the odds of KRAS-gene mutation, respectively.
The results of the present study showed that smokers had 1.17 times the odds of KRAS mutations of nonsmokers (OR 1.17, 95% CI 0.46–3.02). Alcohol consumption increased the odds of KRAS-gene mutation by 90% (OR 1.90, 95% CI 0.66–5.52). However, no statistically meaningful difference was observed between alcohol consumption and the presence of KRAS mutation (Table 4).
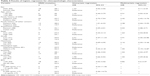  | Table 4 Results of logistic regression for clinicopathologic characteristics |
The results of multivariate logistic regression showed that after adjustment of all of the aforementioned variables, a positive family history of CRC in at least one first-degree relative interestingly reduced the odds of KRAS-gene mutation by 2.42 times (OR 2.42, 95% CI 0.42–13.76), while a positive family history of any other malignancy was associated with 2.38 times higher odds of mutation (OR 2.38, 95% CI 0.44–13.02). Low-stage tumors had 64% higher odds than high-stage tumors (OR 1.64, 95% CI 0.34–8.04), and high-grade tumors had 2.91 times lower odds than low-grade tumors (OR 2.91, 95% CI 0.36–23.86) of KRAS-gene mutation. Presence of metastasis (OR 2.16, 95% CI 0.35–13.46), and alcohol consumption (OR 1.55, 95% CI 0.29–8.26) also increased the odds of KRAS mutation. In addition, backward-elimination multiple logistic regression in our study showed that a negative family history of CRC (OR 2.74, 95% CI 0.55–13.64) and positive family history of any other cancers (OR 2.76, 95% CI 0.71–10.7) are the best predictors of a mutant KRAS gene.
Survival analysis
The mortality rate was 43.3% in WT KRAS tumors and 35% in mutated KRAS tumors at the end of follow-up. The mean OS for patients with tumors harboring KRAS mutation was 74 days (range 20–813) compared to 250 days (range 10–162) for patients with WT KRAS genes. The median OS was significantly shorter among KRAS-mutated patients (62 days, 95% CI 56.87–67.13) compared to patients with the WT gene (180 days, 95% CI 0–383.63; log-rank P=0.003). The Kaplan–Meier curve of OS by testing group and KRAS-mutation status is shown in Figure 2. The results of Cox proportional-hazard regression showed that KRAS-gene mutation in CRC was significantly associated with higher mortality (hazard ratio 3.74, 95% CI 1.44–9.68; P=0.007).
  | Figure 2 Kaplan–Meier survival estimate: KRAS mutant versus KRAS wild-type colorectal cancers. |
Discussion
According to the Iranian National Cancer Registry Report, the incidence of CRC has increased during the last 25 years, showing that the distribution of CRC has shifted toward lower age-groups in the country. In addition, Iran still faces challenges regarding access to anticancer medicines, especially costly anti-EGFR-targeted cancer-therapy protocols. As such, despite the high cost of molecular techniques, it is obvious that screening patients with CRC who are candidates for these therapies by detecting mutations in KRAS and BRAF genes is recommended. In general, it is now clear that routine molecular analysis of CRC, including KRAS and BRAF testing, is cost-saving.27
The overall reported frequency for KRAS mutations is in the range of 20%–50% worldwide. Several studies have indicated that KRAS mutations in codons 12 and 13 are present in >90% of cases. Mutations in codons 61 and 146 are observed in fewer patients. The most common mutations are transitions of nucleotides G>A, G>T, and G>A.18–21
In the present study, PCR amplification of KRAS exon 2 (codons 12 and 13) and BRAF exon 25 (codon V600E) and subsequent Sanger sequencing were performed to identify the mutation types and location in these genes. In this study, KRAS mutations were detected in 26 patients (26%), of whom 16 showed mutations in codon 12, nine in codon 13, and one patient showed mutations in codon 10. KRAS mutation was more common in our patients than those in Sobhani et al, in which 20.3% of patients had mutant KRAS, with most of these mutations involving a same-nucleotide change.28 In another study from Iran, KRAS mutations were reported in 12.5% of patients, with 85% of these patients showing an amino acid change of Gly12Asp.29 These results are comparable to those of a recent study in Albania, where the incidence of KRAS mutations was almost equivalent to that reported in our study (KRAS mutations in 18% patients and BRAF mutations in 6%). Moreover, no statistically meaningful differences were observed between the presence of KRAS mutations and differences in clinical and pathological appearances of the tumor.30
Few studies have been performed in Iran on determining KRAS- and BRAF-gene mutations by using a sequencing method as a gold standard. Some studies that were performed using restriction fragment-length polymorphism (RFLP) of codons 12 and 13 of KRAS showed that mutations in codon 12 were present in 65% of patients,31 while those in codon 13 were present in 18.5% of patients.32 These percentages are much higher than in previous studies, including studies performed in high-risk countries, which was unexpected. This may be because of a lack of sensitivity and specificity of the RFLP method for determining mutations in KRAS and BRAF, although the use of PCR-RFLP reduces the delay in reporting results, and is significantly more cost-effective.33 A study by Asl et al34 using RFLP showed that BRAFV660E mutations were present in approximately 46% of patients. This percentage was not consistent with previous reports on BRAF mutations in high-risk countries. Although next-generation sequencing has recently been designated as a superior technique in terms of sensitivity and specificity, Sanger sequencing has 100% specificity and acceptable sensitivity. In other terms, Sanger sequencing has a better track record and is more cost-effective for routine KRAS- and BRAF-mutation analysis.35
Several studies have shown that BRAF mutations are present in 5%–10% of patients with sporadic CRC.24 Controversial data are available on the prevalence of this mutation in different countries. The highest frequency is reported in the US and Australia, with 9.5%–12.5% of patients with CRC showing this mutation.36 Furthermore, 4.2%–4.4% of patients carry mutant genes.37 A study by Brim et al showed that the frequency of this mutation was very low in Eastern countries.38 Results in Iran have been consistent with this study, and showed that BRAF mutations were present in 0%–3.7% of patients with CRC.17,36
In our study, all the 100 patients with CRC were examined for the presence of mutations in exon 15 of BRAF using the Sanger sequencing method. The results of sequencing showed no mutation in the V600E region of BRAF. This finding was similar to that reported in several studies in Iran and some Asian countries.17,37
There are controversial results about the clinical significance of the KRAS mutation in CRC patients,39 and there is no consensus on the role of KRAS mutations in the prognosis and survival of patients with CRC. In the RASCAL study, which included CRC-patient data from 22 research groups, multivariate analysis showed a significant association between the presence of KRAS mutation and poorer prognosis, but other studies reported an independent association.18–21 In this study, low-stage tumors had slightly higher odds of KRAS mutation than high-stage tumors, and the odds of KRAS mutation were 2.31 times higher in low-grade than high-grade tumors. Moreover, multivariate logistic regression showed that the presence of metastasis increased the odds of KRAS mutations by 2.16 times compared with nonmetastatic CRC. Also, the results of Cox proportional-hazard regression showed that KRAS-gene mutation in CRC was significantly associated with higher mortality (hazard ratio 3.74, 95% CI 1.44–9.68; P=0.007) and shorter OS (log-rank P=0.003).
There have been conflicting results about the relationship between overall KRAS mutations and sex. A cohort study from Brazil reported an association between sex and a mutant KRAS gene: KRAS mutations affected more women compared to men according to this survey (34.8% vs 32.5%, respectively; P=0.03).40 According to other research, there was no significant relationship between overall KRAS-gene mutations and sex.41 Some lifestyle factors, such as tobacco smoking and alcohol consumption, to which males and females are differentially exposed, as well as hormonal differences and dietary patterns, may influence the chance of KRAS mutations. However, we found that the odds of KRAS mutation were 1.37 times higher in males than females, but there was no significant association between sex and KRAS mutation.
A significant relationship between KRAS mutations and anatomical subsite was reported by Elnatan et al: rates of mutations were significantly higher in right-side tumors (right colon 43%, left colon 23%; P=0.0025),42 but this hypothesis was not confirmed by others. Another study from the US showed a significant difference in KRAS mutations in proximal tumors (36%) and distal tumors (26.8%).41 However, based on the results of the present study, rectal cancers had the highest odds of KRAS mutation (increased by 53%), and rates of KRAS mutations were 22.4% in right-side and 26.1% in left-side tumors vs 32.1% in rectal cancers.
Several studies in Western countries have shown that CRCs, like other solid tumors, occur in individuals aged >60 years. Adults aged 4–50 years in Western countries account for only 2%–8% of CRC.1 Various epidemiological studies in Iran have shown higher rates of CRC at an earlier age, with approximately a fifth of patients with CRC aged below 40 years.1 In the present study of 100 patients, 20 (20%) were aged below 50 years. This number was slightly lower than that reported in a previous study from Iran, but was almost equal to the number reported in studies performed in Western communities and in high-risk countries.43 Moreover, according to our results, the most commonly affected age-group was the fifth decade of life (26% of patients with CRC), and the odds of KRAS mutation were 29% higher in younger patients with CRC.
Westernized lifestyle and diet, specifically high consumption of red and processed meat, obesity, and low physical activity, have long been established as the main causes of increased risk of CRC in most Asian countries. Recent studies have shown that early onset CRC may be associated with worse-differentiated and higher-stage disease, which should be taken into consideration. However, in our study there was no correlation among age, stage, and grade of the tumors. The mechanism by which a Westernized diet increases the risk of CRC remains a subject of debate, as it may be related to both genetic and epigenetic polymorphisms.22,23 These findings, along with the results obtained previously, highlight the need for screening programs to prevent CRC in younger individuals in Iran.1,43
Small sample size and low sample enrichment were limitations in this study. The small biopsy sizes (2–4 mm) were because of difficulties in collecting fresh tumor samples during colonoscopy from patients who were newly diagnosed with CRC. The association of KRAS mutation with clinical and pathological features and patient survival were based on the small number of cases and the low frequency of KRAS mutation, which may have led to the lack of statistical power. Further studies involving larger sample sizes should be performed to validate the relationship between KRAS and BRAF mutations and such factors as ethnicity, geographical area, nutrition factors, and lifestyle. This has been the subject of one of our ongoing studies, and the results will be published soon.
Conclusion
Over the past two decades, molecular studies have led to a tremendous increase in our knowledge of genetic changes that affect malignancy in CRC. This has enabled us better to characterize tumors individually and classify them according to certain molecular or genetic features. However, mutation-detection methods with high sensitivity will increase the possibility of individualized therapy, and reduce the risk of metastasis in low-stage diseases.
Mutations in proto-oncogenes KRAS and BRAF are among the key findings of CRC. Several studies have been performed on KRAS and BRAF in many oncology centers worldwide for determining CRC treatment. Limited data are available in Iran on the prevalence of mutations in these genes. Moreover, the significant familial distribution of CRC, particularly among the young population, necessitates more studies on the underlying molecular mechanisms of the disease in Iran and other geographical regions. Wider age distribution of CRC, particularly among young adults in Iran, highlights the need for screening programs to prevent this disease among younger age-groups.
Clinical practice points
Mutations in the KRAS and BRAF genes are considered important primary incidents in the development of CRC. Although the role of KRAS and BRAF mutations has been widely accepted for predicting responses to anti-EGFR treatments, particularly in patients with advanced CRC, there is no consensus on the role of KRAS mutations in prognosis and survival. However, the presence of BRAF mutations is associated with poor prognosis and lack of response to anti-EGFR treatment.
Acknowledgments
The authors would like to thank the Hematology and Oncology Research Center of Tabriz University of Medical Sciences for supporting this project (grant 5/4/9366), which was a part of the PhD thesis of RD (5/74/1392). The study protocol was approved by the ethics committee of Tabriz University of Medical Sciences (permit 5.74.1235). We thank all of the patients who took part in this research. The authors would also like to thank Susan Mirnajd Gherami for her help, and all staff of the Liver and Gastrointestinal Diseases Research Center of Tabriz University of Medical Sciences.
Disclosure
The authors report no conflicts of interest in this work.
References
Malekzadeh R, Bishehsari F, Mahdavinia M, Ansari R. Epidemiology and molecular genetics of colorectal cancer in Iran: a review. Arch Iran Med. 2009;12(2):161–169. | ||
Pahlavan PS, Kanthan R. The epidemiology and clinical findings of colorectal cancer in Iran. J Gastrointestin Liver Dis. 2006;15(1):15–19. | ||
Mohebbi M, Nourijelyani K, Mahmoudi M, et al. Time to occurrence and age distribution of digestive cancers in northern Iran. Iran J Public Health. 2008;37(1):8–19. | ||
Somi MH, Golzari M, Farhang S, Naghashi S, Abdollahi L. Gastrointestinal cancer incidence in East Azerbaijan, Iran: update on 5 year incidence and trends. Asian Pac J Cancer Prev. 2014;15(9):3945–3949. | ||
Mahdavinia M, Bishehsari F, Ansari R, et al. Family history of colorectal cancer in Iran. BMC Cancer. 2005;5:112. | ||
Mousavi SM, Gouya MM, Ramazani R, Davanlou M, Hajsadeghi N, Seddighi Z. Cancer incidence and mortality in Iran. Ann Oncol. 2009;20(3):556–563. | ||
Sadjadi A, Malekzadeh R, Derakhshan MH, et al. Cancer occurrence in Ardabil: results of a population-based cancer registry from Iran. Int J Cancer. 2003;107(1):113–118. | ||
Sadjadi A, Nouraie M, Mohagheghi MA, Mousavi-Jarrahi A, Malekezadeh R, Parkin DM. Cancer occurrence in Iran in 2002, an international perspective. Asian Pac J Cancer Prev. 2005;6(3):359–363. | ||
Ansari R, Mahdavinia M, Sadjadi A, et al. Incidence and age distribution of colorectal cancer in Iran: results of a population-based cancer registry. Cancer Lett. 2006;240(1):143–147. | ||
Hosseini SV, Izadpanah A, Yarmohammadi H. Epidemiological changes in colorectal cancer in Shiraz, Iran: 1980–2000. ANZ J Surg. 2004;74(7):547–549. | ||
Yazdizadeh B, Jarrahi AM, Mortazavi H, Mohagheghi MA, Tahmasebi S, Nahvijo A. Time trends in the occurrence of major GI cancers in Iran. Asian Pac J Cancer Prev. 2005;6(2):130–134. | ||
Fazeli MS, Adel MG, Lebaschi AH. Colorectal carcinoma: a retrospective, descriptive study of age, gender, subsite, stage, and differentiation in Iran from 1995 to 2001 as observed in Tehran University. Dis Colon Rectum. 2007;50(7):990–995. | ||
Azadeh S, Moghimi-Dehkordi B, Fatem SR, Pourhoseingholi MA, Ghiasi S, Zali MR. Colorectal cancer in Iran: an epidemiological study. Asian Pac J Cancer Prev. 2008;9(1):123–126. | ||
Safaee A, Fatemi SR, Ashtari S, Vahedi M, Moghimi-Dehkordi B, Zali MR. Four years incidence rate of colorectal cancer in Iran: a survey of national cancer registry data – implications for screening. Asian Pac J Cancer Prev. 2012;13(6):2695–2698. | ||
Shahrier M, Ahnen DJ. Colorectal cancer survival in Europe: the Will Rogers phenomenon revisited. Gut. 2000;47(4):463–464. | ||
Mundade R, Imperiale TF, Prabhu L, Loehrer PJ, Lu T. Genetic pathways, prevention, and treatment of sporadic colorectal cancer. Oncoscience. 2014;1(6):400–406. | ||
Naghibalhossaini F, Hosseini HM, Mokarram P, Zamani M. High frequency of genes’ promoter methylation, but lack of BRAF V600E mutation among Iranian colorectal cancer patients. Pathol Oncol Res. 2011;17(4):819–825. | ||
Lewandowska MA, Jóźwicki W, Zurawski B. KRAS and BRAF mutation analysis in colorectal adenocarcinoma specimens with a low percentage of tumor cells. Mol Diagn Ther. 2013;17(3):193–203. | ||
Arrington AK, Heinrich EL, Lee W, et al. Prognostic and predictive roles of KRAS mutation in colorectal cancer. Int J Mol Sci. 2012;13(10):12153–12168. | ||
El Kader YA, Emera G, Safwat E, Kassem HA, Kassem NM. The KRAS StripAssay for detection of KRAS mutation in Egyptian patients with colorectal cancer (CRC): a pilot study. J Egypt Natl Canc Inst. 2013;25(1):37–41. | ||
Miglio U, Mezzapelle R, Paganotti A, et al. Mutation analysis of KRAS in primary colorectal cancer and matched metastases by means of highly sensitivity molecular assay. Pathol Res Pract. 2013;209(4):233–236. | ||
Yee YK, Gu Q, Hung I, et al. Trend of colorectal cancer in Hong Kong: 1983–2006. J Gastroenterol Hepatol. 2010;25(5):923–927. | ||
Yee YK, Tan VP, Chan P, Hung IF, Pang R, Wong BC. Epidemiology of colorectal cancer in Asia. J Gastroenterol Hepatol. 2009;24(12):1810–1816. | ||
Borras E, Jurado I, Hernan I, et al. Clinical pharmacogenomic testing of KRAS, BRAF and EGFR mutations by high resolution melting analysis and ultra-deep pyrosequencing. BMC Cancer. 2011;11:406. | ||
Hamilton SR, Vogelstein B, et al. Tumors of the Colon and Rectum, Carcinoma of the colon and rectum. In: Hamilton SR, Aaltonen LA, edi World Health Organization Classification of Tumours: Pathology and Genetics of Tumours of the Digestive System. Lyon: IARC Press; 2000:103–143. | ||
Percy C, Fritz A, Jack A, et al. International Classification of Diseases for Oncology. 3rd ed. Geneva: World Health Organization; 2000. | ||
[No authors listed]. Mutation screening for KRAS and BRAF in metastatic colorectal cancer proves costly in relation to benefits. Pharmacogenomics. 2013;14(2):124. | ||
Sobhani S, Ghaffarpour M, Hosseini ZM, Kamali F, Mohammadi ZN, Houshmand M. The prevalence of common mutation frequency in K-Ras codons 12, 13 in Iranian colorectal cancer patients. Genet 3rd Millennium. 2010;8(2):2011–2018. | ||
Shemirani AI, Haghighi MM, Milanizadeh S, et al. The role of KRAS mutations and MSI status in diagnosis of colorectal cancer. Gastroenterol Hepatol Bed Bench. 2011;4(2):70–75. | ||
Martinetti D, Costanzo R, Kadare S, et al. KRAS and BRAF mutational status in colon cancer from Albanian patients. Diagn Pathol. 2014;9:187. | ||
Edalat H, Sadeghizadeh M, Zavarehei MJ. Codon 12 K-ras mutation detection in Iranian patients with colorectal cancer using PCR-RFLP method. Modares J Med Sci Pathobiol. 2007;9(4):33–38. | ||
Lary S, Ghaffarzadegan K, Afsharnezhad S, Yazdi MS, Pazooki N, Hoseinkhani S. Detection of K-ras oncogen mutation in codon 13 of CRC patients in East-North of Iran. Mod Genet. 2012;4(27):43–47. | ||
Chretien AS, Harle A, Meyer-Lefebvre M, et al. Optimization of routine KRAS mutation PCR-based testing procedure for rational individualized first-line-targeted therapy selection in metastatic colorectal cancer. Cancer Med. 2013;2(1):11–20. | ||
Asl JM, Almasi S, Tabatabaiefar MA. High frequency of BRAF proto-oncogene hot spot mutation V600E in cohort of colorectal cancer patients from Ahvaz City, Southwest Iran. Pak J Biol Sci. 2014;17(4):565–569. | ||
Altimari A, de Biase D, De Maglio G, et al. 454 Next generation-sequencing outperforms allele-specific PCR, Sanger sequencing, and pyrosequencing for routine KRAS mutation analysis of formalin-fixed, paraffin-embedded samples. Onco Targets Ther. 2013;6:1057–1064. | ||
Javadi F, Geramizadeh B, Mirzai M. BRAF gene mutation analysis in colorectal cancer in south of Iran. Ann Colorectal Res. 2014;2(2):e19917. | ||
Li L, Ma BB. Colorectal cancer in Chinese patients: current and emerging treatment options. Onco Targets Ther. 2014;7:1817–1828. | ||
Brim H, Mokarram P, Naghibalhossaini F, et al. Impact of BRAF, MLH1 on the incidence of microsatellite instability high colorectal cancer in populations based study. Mol Cancer. 2008;7:68. | ||
Li W, Qiu T, Zhi W, et al. Colorectal carcinomas with KRAS codon 12 mutation are associated with more advanced tumor stages. BMC Cancer. 2015;15:340. | ||
Ferreira CG, Aran V, Zalcberg-Renault I, et al. KRAS mutations: variable incidences in a Brazilian cohort of 8,234 metastatic colorectal cancer patients. BMC Gastroenterol. 2014;14:73. | ||
Samowitz WS, Curtin K, Schaffer D, Robertson M, Leppert M, Slattery ML. Relationship of Ki-ras mutations in colon cancers to tumor location, stage, and survival: a population-based study. Cancer Epidemiol Biomarkers Prev. 2000;9(11):1193–1197. | ||
Elnatan J, Goh HS, Smith DR. C-KI-RAS activation and the biological behaviour of proximal and distal colonic adenocarcinomas. Eur J Cancer. 1996;32A(3):491–497. | ||
Hajmanoochehri F, Asefzadeh S, Kazemifar AM, Ebtehaj M. Clinicopathological features of colon adenocarcinoma in Qazvin, Iran: a 16 year study. Asian Pac J Cancer Prev. 2014;15(2):951–955. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
