Back to Journals » International Journal of General Medicine » Volume 15
Association Between Plasma Lipoprotein Levels and Aortic Valve Calcification Among Patients with Aortic Valve Replacement Surgery: A Retrospective Study
Authors Tao T, Zheng J, Han Y, Yang Q, Ni Y, Ma L
Received 26 February 2022
Accepted for publication 2 May 2022
Published 4 May 2022 Volume 2022:15 Pages 4665—4673
DOI https://doi.org/10.2147/IJGM.S363989
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Tingting Tao, Junnan Zheng, Yu Han, Qiqi Yang, Yiming Ni, Liang Ma
Department of Cardiovascular Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, Zhejiang, People’s Republic of China
Correspondence: Liang Ma, Department of Cardiovascular Surgery, The First Affiliated Hospital, Zhejiang University School of Medicine, 79#, Qingchun Road, Hangzhou, Zhejiang, 310003, People’s Republic of China, Tel +86 571 87236841, Fax +86 571 87236843, Email [email protected]
Objective: Calcific aortic valve disease (CAVD) is a prevalent type of valvular heart disease, its association with dyslipidemia remains controversial.
Methods: Of 449 CAVD patients who underwent aortic valve replacement, 228 formed the aortic valve calcification (AVC) group, and 221 were the non-calcification group. We retrospectively reviewed the preoperative and one-year postoperative plasma lipoprotein levels of both and performed a logistic regression to evaluate the factors associated with AVC.
Results: Preoperatively, AVC patients had significantly higher coronary heart disease (43.0% vs 24.9%, p< 0.001), peripheral vascular disease (41.7% vs 26.2%, p< 0.001), and heart failure rates (63.6% vs 47.1%, p< 0.001), and a higher level of total cholesterol (4.1± 0.9 vs 3.9± 0.8 mmol/L, p=0.032) and very low-density cholesterol (0.6 (0.4– 0.7) vs 0.5 (0.3– 0.7) mmol/L, p=0.054). Echocardiography revealed a significant difference of aortic stenosis in both AVC and non-AVC groups (p< 0.05), and also identified aortic regurgitation (AR) with a significant difference between these two groups (p=0.003). The peak transaortic jet velocity, peak transaortic gradient, and mean transaortic gradient were significantly higher in the calcification group (all p< 0.001), but the aortic valve area (0.7 (0.5– 1.0) vs 4 (0.9– 4.5) cm2; p< 0.001) was smaller. Age (OR=1.023), total cholesterol (OR=1.272), and mean transaortic gradient (OR=1.182) were AVC risk factors. A larger aortic valve area (OR=0.010) were protective factors. The one-year mortality and perivalvular leakage rates were significantly higher in the calcification group.
Conclusion: Total cholesterol was significantly higher in AVC patients and may be an AVC risk factor along with age and mean transaortic gradient. AVC patients had a relatively poorer outcome within one year.
Keywords: aortic valve calcification, total cholesterol, plasma lipoprotein levels, low-density lipoprotein, aortic valve replacement
Introduction
Calcific aortic valve disease (CAVD) is a prevalent type of valvular heart disease, which can ultimately result in chronic heart failure. Different types of cardiovascular diseases (CVDs) share similar etiological pathways,1,2 and the process of calcific aortic valve stenosis (CAVS) is highly akin to that of atherosclerosis.3–5 Evidence has shown that dyslipidemia may contribute to atherosclerotic-related diseases.6,7 However, recent findings about the association between plasma lipoprotein levels and aortic valve calcification (AVC) have demonstrated inconsistent results,2,5,8,9 and no convincing randomized controlled trials (RCTs) have confirmed the effect of statin therapy on the progression of aortic stenosis (AS).10–12 Despite some disappointing results from prospective treatment trials, the retrospective analyses continue to suggest that there may be a positive association between lipid-lowering therapy and AVC,13,14 some lipid-lowering therapies may influence the progression of CAVD.7,14
In this clinical retrospective study, we aimed to analyze the association between plasma lipoprotein levels and AVC severity among patients who underwent aortic valve replacement (AVR) surgery. We explored factors associated with AVC and hope the present study will contribute to the therapy for CAVD patients.
Materials and Methods
Study Design and Patient Data Collection
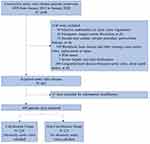
We consecutively enrolled 1608 patients who underwent isolated or concomitant AVR surgery from January 1st, 2015, to January 1st, 2020, at the Department of Cardiovascular Surgery, the First Affiliated Hospital, Zhejiang University School of Medicine. We excluded 1146 patients due to reasons shown in Figure 1. Finally, 449 patients with complete clinical data were included and were separated into two groups according to whether their aortic valve was obviously calcified.
We collected and evaluated general data from all patients, which included age, sex, medical history, comorbidities (hypertension, diabetes, coronary artery disease (CAD), peripheral artery disease (PAD), stroke, and atrial fibrillation (AF)). We analyzed the plasma lipoprotein levels of these patients using low-density lipoprotein (LDL), high-density lipoprotein (HDL), very low-density lipoprotein (VLDL), and total cholesterol levels, and also included triglyceride and C-reactive protein levels. The parameters relevant to valvular disease were all determined by two types of echocardiography: transthoracic echocardiography (TTE) and transesophageal echocardiography.
This study was reviewed and approved by the Research Ethics Committees of the First Affiliated Hospital of Zhejiang University School of Medicine, under the Reference Number IIT20210002A. The need for individual informed consent was waived by the ethics committee due to the study’s retrospective nature. All the procedures were performed under principles of the local law and the Declaration of Helsinki.
Definitions
We analyzed all consecutive patients over 18 years of age who underwent first-time selective AVR. Patients with concomitant mitral valve replacement (MVR), emergency procedures, rheumatic heart disease (RHD), infective endocarditis, active malignant tumors, or severe hepatic and renal dysfunctions were excluded.
The calcification group and the non-calcification group were separated based on the calcification condition of the aortic valve, which was confirmed by imageology data preoperatively and reconfirmed by the surgeons during the operation.
Coronary artery disease is typically confirmed according to evidence on invasive coronary angiography or computed tomography angiography.
Heart failure is defined as Classification III and IV of the New York Heart Association (NYHA). Patients with an ejection fraction (EF) lower than 30% were excluded from our study.
Medication use indicates that the medication taking condition of patients upon admission.
The stroke classification system used in this study was based on a statement issued by the American Heart Association/American Stroke Association in 2013,15 the occurrence of stroke was counted when it was specifically accompanied by overt symptoms. In addition, the presence of a perivalvular leak within one year postoperatively was defined by all degrees that can be detected by echocardiography.
Patients in hypercholesterolemia group were defined as the receipt of treatment with cholesterol-lowering medication or an elevated level of total cholesterol (>200 mg per deciliter [5.2 mmol per liter]).16
Surgical Procedure
The procedures were performed via either median sternotomy or minimally invasive incision. Isolated or concomitant AVR was carried out in all patients with the implantation of either a bioprosthetic or mechanical prosthesis. Concomitant operations in some of the patients included coronary artery bypass grafting (CABG) and tricuspid valvuloplasty (TVP). Standard general anesthesia, extracorporeal circulation, and myocardial protection methods were used for all participants. All the procedures were performed under principles of the local law and the Declaration of Helsinki.
Statistical Analysis
Continuous variables were presented as the mean ± standard deviation (SD) or the median and interquartile range (IQR) for Gaussian and non-Gaussian distributed data, respectively. Categorical variables were expressed as numbers and percentages (n%). Pearson’s χ2 test was used for descriptive, univariate statistics. Student’s unpaired t-test was carried out to compare normally distributed data. The Mann–Whitney U-test was used for the group comparison of continuous, non-Gaussian distributed variables. Regression and correlation analyses were performed using logistics regression analysis. Any horizontal pleiotropy was balanced.
All statistical analyses were performed with the Statistical Package for the Social Sciences software, version 23 (Armonk, New York). A p-value of less than 0.05 (p<0.05) was considered statistically significant for all included statistical tests.
Results
Demographics, Preoperative Plasma Lipid Levels and AVC
Baseline clinical characteristics are shown in Table 1. Of the 449 patients we reviewed, calcification was detected in 50.8% (228/449) of the patients (calcification group); the remaining participants were placed in the non-calcification group. The median age (63 vs 62 years old) and percentages of males (69.7% vs 67.9%), current smokers (27.2% vs 26.7%), hypertension (44.3% vs 53.4%), diabetes (6.6% vs 5%), stroke (3.9% vs 5%), and AF (7.5% vs 7.2%) were similar in the calcification and non-calcification groups, respectively. Patients with AVC had significantly higher rates of coronary heart disease (43.0% vs 24.9%, p<0.001), peripheral vascular disease (41.7% vs 26.2%, p<0.001), and heart failure (63.6% vs 47.1%, p<0.001). They also had higher total cholesterol (4.1±0.9 vs 3.9±0.8 mmol/L, p=0.032) and VLDL cholesterol (0.6 (0.4–0.7) vs 0.5 (0.3–0.7) mmol/L, p=0.054) levels. Other results of lipid profile components were similar (Table 1).
 |
Table 1 Baseline Clinical Characteristics |
Echocardiographic Data
The pre-operative echocardiographic findings are shown in Table 2. AS was detected in 86% (196/228) patients with AVC and in only 24% (53/221) of participants without AVC (p<0.001). Echocardiography identified 210 (92.1%) aortic regurgitation (AR) patients with AVC and 217 (98.2%) in patients without AVC, with a significant difference between the two groups (p=0.003). The peak transaortic jet velocity, peak transaortic gradient, and mean transaortic gradient were significantly higher in patients with AVC (p<0.001 for all), while the aortic valve area was significantly smaller in this group of patients (0.7 (0.5–1.0) vs 4 (3.9–4.5) cm2, p<0.001).
 |
Table 2 Pre-Operative Echocardiographic Findings |
Logistic Regression Analysis of Lipids Associated with AVC
The final results of our multivariate logistic regression analysis are shown in Table 3. Age, total cholesterol, and mean transaortic gradient were significant risk factors for AVC in these AVR patients, with an odds ratio (OR) of 1.023 (95% CI: 1.005–1.041, p=0.011), 1.272 (95% CI: 1.021–1.585, p=0.032), and 1.182 (95% CI: 1.116–1.252, p<0.001), respectively. The analysis revealed a protective effect of larger aortic valve area (OR=0.010, 95% CI: 0.000–0.265, p=0.006) on AVC. The factor of HDL cholesterol may be associated with AVC, but statistically insignificant (OR=0.017, 95% CI: 0.000–1.316, p=0.066).
 |
Table 3 Multivariate Logistic Regression Analysis of Lipids Associated with Aortic Valve Calcification (r2=0.594) |
One-Year Outcomes
The one-year mortality was significantly higher in the calcification group (1.8%) compared to 0% in the non-calcification group (p=0.048). AVC patients also had significantly higher rates of perivalvular leakage (10.5% vs 4.5%, p=0.016). Other aspects of one-year outcomes, including the incidence of valvular vegetation, revision cardiac surgery, new onset stroke, new onset heart failure, re-entering the intensive care unit (ICU), or new AF after surgery were roughly the same in this study (p>0.05; Table 4).
 |
Table 4 Outcomes Within 1 Year |
Patients with High Cholesterol
Since patients with AVC had a higher level of total cholesterol, we regrouped them based on an elevated level of total cholesterol (>200 mg per deciliter [5.2 mmol per liter]), the results are shown in Table 5. Patients in hypercholesterolemia group (n=41) also had higher rate with AVC (70.7%, p=0.005) comparing to the rest patients in non-hypercholesterolemia group (n=408), and they significantly tended to suffer AS (70.7% vs 53.9%, p=0.027). The prognosis of patients with higher cholesterol was relatively poor as far as the significantly high rate of new AF after surgery (9.8% vs 1.5%, p=0.008).
 |
Table 5 Patients with High Cholesterol |
Discussion
CAVD is a relatively prevalent valvular heart disease in western societies.3,4,8 With the recent rise in life expectancy and decline in RHD, age-related diseases like CAVD have also increased in China.17 CAVD has been considered as an age-related benign degenerative condition for a long time.9 Many recent studies have shown a link between the process of this disease and lipidic accumulation,18 calcium deposition,4 and chronic inflammation.19 However, the exact causal factors that lead to AVC remain unknown.8 Therefore, there is currently limited medical treatment to prevent the progression of CAVD.20,21 When the disease progresses to a severe stage, the only available option is surgical,22 including open-heart AVR or transcatheter aortic valve implantation.5 Approximately 50% of these patients will die within the next 12–18 months19 without surgery or interventional treatment.
Studies on CVDs have disclosed that different types CVDs can share similar aetiological pathways1,2 and substantial evidence show that elevated lipoprotein levels contribute to CVD and CAVS.12 At the early stage of AVC, valvular endothelial cells are dysfunctional, leading to amplified mechanical stress and diminished shear stress, which inevitably resulted in adhesion, deposition, proliferation, a transition of microcalcification.21 This series of process is highly akin to atherosclerosis, like CAD and PAD,3 and 50% of CAVS patients have comorbid CAD,4 which is consistent with our findings that a significant number of patients had a history of CAD or PAD. However, although lipid-lowering therapies have been widely applied to CAD and PAD patients, the findings about the association between plasma lipoprotein levels and AVC are still inconsistent.2,5,8 In addition, no convincing RCTs have confirmed the effect of statin therapy on the progression of AS.10–12 Recently, several researchers have turned their attention to lipoprotein(a) lowering therapy.23,24
AVC assessment presents quite a challenge. Doppler echocardiography, the commonly preferred method, relies on the combined results of the valve area and mean transvalvular gradients, which can be unclear, particularly in patients with a poor EF.25,26 Studies on computed tomography (CT) calcium scoring suggest that this modality can aid in the diagnosis and also demonstrates close correlations between AVC and CAVS severity.18,25 The recent electron beam computed tomography (EBCT) and multidetector row computed tomography (MDCT) technologies have validated for the quantification and assessment of AVC load.26,27 In this study, cardiac surgeons reconfirmed the AVC severity based on the grading of AVC that Warren BA and Yong JL proposed in 1997,28 which is in addition to the TTE preoperative assessment.
AVC is considered a possible source of embolism for CAVD patients. Even after undergoing TAVR, they risk having a shorter long-term survival rate,29 which emphasizes the need to prevent or reduce to the progress of calcification and therefore improve the prognosis of CAVD patients.30
In this retrospective study, we screened patients who underwent isolated or concomitant AVR surgery at our institution from January 2015 to January 2020. After we analyzed the association between plasma lipoprotein levels and AVC severity, we found that patients with AVC had a significantly higher total cholesterol level, and the later logistics regression analysis revealed this valve to be a risk factor associated with AVC, along with age and mean transaortic gradient. AVC patients also showed a high level of VLDL cholesterol (0.6 (0.4–0.7) vs 0.5 (0.3–0.7) mmol/L, p=0.054), but other lipid profile components were rather similar in this study, which may have been caused by the limitations of this investigation as a single center study. The total cholesterol findings support the notion that higher triglycerides and remnant cholesterol may be factors that facilitate the evolution of AS.20 In addition, our logistic regression analysis also suggested a large aortic valve area may have a protective effect on AVC. We also found the one-year mortality and the incidence of perivalvular leakage to be significantly higher in the calcification group, which implied patients with AVC might experience a poorer outcome.
The limitations of this research include it being a single-center study and having a relatively small sample size, a short study length, and a short follow-up period. The mortality rate may be underestimated, since only hospital-reported mortality was recorded. In addition, the criterion of calcification is a qualitative indicator that mainly relies on imageology data and cardiac surgeons’ intraoperative reconfirmation. As a result, the lack of quantitative indicators may add bias to our findings. We hope to contribute evidence in support of lipid-lowering therapy to aid in the treatment of CAVD at the early initiation phase.22 A larger sample analysis and future experimental studies will be required to further explore the mechanism of action of cholesterol on the progression of AVC.
Conclusion
In summary, we concluded that the association between plasma lipoprotein levels and AVC with the total cholesterol level was significant in patients with AVC, and it could be one important risk factor in the progress of CAVD.
Abbreviations
AF, atrial fibrillation; ALT, alanine transaminase; AR, aortic regurgitation; AS, aortic stenosis; AST, aspartate aminotransferase; AVC, aortic valve calcification; AVR, aortic valve replacement; BUN, blood urea nitrogen; CABG, coronary artery bypass grafting; CAVD, calcific aortic valve disease; CAVS, calcific aortic valve stenosis; CCB, calcium channel blocker; CT, computed tomography; EBCT, electron beam computed tomography; EF, ejection fraction; HDL, high density lipoprotein; ICU, intensive care unit; IQR, interquartile range; LDL, low-density lipoprotein; MDCT, multidetector row computed tomography; MVR, mitral valve replacement; NYHA, New York Heart Association; OR, odds ratio; RCT, randomized controlled trial; RHD, rheumatic heart disease; SD, standard deviation; TEE, transesophageal echocardiography; TTE, transthoracic echocardiography; TVP, tricuspid valvuloplasty; UA, uric acid; VHD, valvular heart disease; VLDL, very low-density lipoprotein.
Funding
There is no funding to report.
Disclosure
The authors have no conflicts of interest to declare in this work.
References
1. Nazarzadeh M, Pinho-Gomes AC, Smith Byrne K, et al. Systolic blood pressure and risk of valvular heart disease: a Mendelian Randomization Study. JAMA Cardiol. 2019;4(8):788–795. doi:10.1001/jamacardio.2019.2202
2. Nazarzadeh M, Pinho-Gomes AC, Bidel Z, et al. Plasma lipids and risk of aortic valve stenosis: a Mendelian Randomization Study. Eur Heart J. 2020;41(40):3913–3920. doi:10.1093/eurheartj/ehaa070
3. Côté C, Pibarot P, Després JP, et al. Association between circulating oxidised low-density lipoprotein and fibrocalcific remodelling of the aortic valve in aortic stenosis. Heart. 2008;94(9):1175–1180. doi:10.1136/hrt.2007.125740
4. de Oliveira Sá MPB, Cavalcanti LRP, Perazzo ÁM, et al. Calcific aortic valve stenosis and atherosclerotic calcification. Curr Atheroscler Rep. 2020;22(2):2. doi:10.1007/s11883-020-0821-7
5. Perrot N, Valerio V, Moschetta D, et al. Genetic and in vitro inhibition of PCSK9 and calcific aortic valve stenosis. JACC Basic Transl Sci. 2020;5(7):649–661. doi:10.1016/j.jacbts.2020.05.004
6. Duran EK, Aday AW, Cook NR, et al. Triglyceride-rich lipoprotein cholesterol, small dense LDL cholesterol, and incident cardiovascular disease. J Am Coll Cardiol. 2020;75(17):2122–2135. doi:10.1016/j.jacc.2020.02.059
7. Greve AM, Bang CN, Boman K, et al. Relation of lipid-lowering therapy to need for aortic valve replacement in patients with asymptomatic mild to moderate aortic stenosis. Am J Cardiol. 2019;124(11):1736–1740. doi:10.1016/j.amjcard.2019.08.037
8. Smith JG, Luk K, Schulz CA, et al. Association of low-density lipoprotein cholesterol-related genetic variants with aortic valve calcium and incident aortic stenosis. JAMA. 2014;312(17):1764–1771. doi:10.1001/jama.2014.13959
9. Cao J, Steffen BT, Budoff M, et al. Lipoprotein(a) levels are associated with subclinical calcific aortic valve disease in white and black individuals: the Multi-Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2016;36(5):1003–1009. doi:10.1161/ATVBAHA.115.306683
10. Chan KL, Teo K, Dumesnil JG, et al. Effect of Lipid lowering with rosuvastatin on progression of aortic stenosis: results of the aortic stenosis progression observation: measuring effects of rosuvastatin (ASTRONOMER) trial. Circulation. 2010;121(2):306–314. doi:10.1161/CIRCULATIONAHA.109.900027
11. Rossebø AB, Pedersen TR, Boman K, et al. Intensive lipid lowering with simvastatin and ezetimibe in aortic stenosis. N Engl J Med. 2008;359(13):1343–1356. doi:10.1056/NEJMoa0804602
12. Tsimikas S. A test in context: lipoprotein(a): diagnosis, prognosis, controversies, and emerging therapies. J Am Coll Cardiol. 2017;69(6):692–711. doi:10.1016/j.jacc.2016.11.042
13. Gerdts E, Rossebø AB, Pedersen TR, et al. Impact of baseline severity of aortic valve stenosis on effect of intensive lipid lowering therapy (from the SEAS study). Am J Cardiol. 2010;106(11):1634–1639. doi:10.1016/j.amjcard.2010.07.042
14. Kostyunin AE, Yuzhalin AE, Ovcharenko EA, et al. Development of calcific aortic valve disease: do we know enough for new clinical trials? J Mol Cell Cardiol. 2019;132:189–209. doi:10.1016/j.yjmcc.2019.05.016
15. Sacco RL, Kasner SE, Broderick JP, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44(7):2064–2089. doi:10.1161/STR.0b013e318296aeca
16. Puymirat E, Cayla G, Simon T, et al. Multivessel PCI guided by FFR or angiography for myocardial infarction. N Engl J Med. 2021;385(4):297–308. doi:10.1056/NEJMoa2104650
17. Arora S, Ramm CJ, Bahekar AA, et al. Evaluating health of emerging economies through the eyes of heart valve disease in the transcatheter era. Glob Heart. 2017;12(4):301–304. doi:10.1016/j.gheart.2017.01.016
18. Thaden JJ, Nkomo VT, Suri RM, et al. Sex-related differences in calcific aortic stenosis: correlating clinical and echocardiographic characteristics and computed tomography aortic valve calcium score to excised aortic valve weight. Eur Heart J. 2016;37(8):693–699. doi:10.1093/eurheartj/ehv560
19. Hofmanis J, Hofmane D, Svirskis S, et al. HDL-C role in acquired aortic valve stenosis patients and its relationship with oxidative stress. Medicina. 2019;55(8):416. doi:10.3390/medicina55080416
20. Kaltoft M, Langsted A, Nordestgaard BG. Triglycerides and remnant cholesterol associated with risk of aortic valve stenosis: Mendelian randomization in the Copenhagen General Population Study. Eur Heart J. 2020;41(24):2288–2299. doi:10.1093/eurheartj/ehaa172
21. Pedriali G, Morciano G, Patergnani S, et al. Aortic valve stenosis and mitochondrial dysfunctions: clinical and molecular perspectives. Int J Mol Sci. 2020;21(14):4899. doi:10.3390/ijms21144899
22. Pawade TA, Newby DE, Dweck MR. Calcification in aortic stenosis: the skeleton key. J Am Coll Cardiol. 2015;66(5):561–577. doi:10.1016/j.jacc.2015.05.066
23. Hung MY, Tsimikas S. What is the ultimate test that lowering lipoprotein(a) is beneficial for cardiovascular disease and aortic stenosis? Curr Opin Lipidol. 2014;25(6):423–430. doi:10.1097/MOL.0000000000000131
24. Capoulade R, Chan KL, Yeang C, et al. Oxidized phospholipids, lipoprotein(a), and progression of calcific aortic valve stenosis. J Am Coll Cardiol. 2015;66(11):1236–1246. doi:10.1016/j.jacc.2015.07.020
25. Pawade T, Sheth T, Guzzetti E, et al. Why and how to measure aortic valve calcification in patients with aortic stenosis. JACC Cardiovasc Imaging. 2019;12(9):1835–1848. doi:10.1016/j.jcmg.2019.01.045
26. Boulif J, Gerber B, Slimani A, et al. Assessment of aortic valve calcium load by multidetector computed tomography. Anatomical validation, impact of scanner settings and incremental diagnostic value. J Cardiovasc Comput Tomogr. 2017;11(5):360–366. doi:10.1016/j.jcct.2017.07.004
27. Shavelle DM, Budoff MJ, Buljubasic N, et al. Usefulness of aortic valve calcium scores by electron beam computed tomography as a marker for aortic stenosis. Am J Cardiol. 2003;92(3):349–353. doi:10.1016/S0002-9149(03)00646-5
28. Warren BA, Yong JL. Calcification of the aortic valve: its progression and grading. Pathology. 1997;29(4):360–368. doi:10.1080/00313029700169315
29. Pollari F, Hitzl W, Vogt F, et al. Aortic valve calcification as a risk factor for major complications and reduced survival after transcatheter replacement. J Cardiovasc Comput Tomogr. 2020;14(4):307–313. doi:10.1016/j.jcct.2019.12.001
30. Peeters FECM, Meex SJR, Dweck MR, et al. Calcific aortic valve stenosis: hard disease in the heart: a biomolecular approach towards diagnosis and treatment. Eur Heart J. 2018;39(28):2618–2624. doi:10.1093/eurheartj/ehx653
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.