Back to Journals » Cancer Management and Research » Volume 12
Association Between Intermediate-Acting Neuromuscular-Blocking Agents and Short-Term Postoperative Outcomes in Patients with Gastric Cancer
Authors Niu L, Yao C, Wang Y, Sun Y, Xu J , Lin Y , Yao S
Received 23 April 2020
Accepted for publication 2 October 2020
Published 6 November 2020 Volume 2020:12 Pages 11391—11402
DOI https://doi.org/10.2147/CMAR.S258016
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
Lingxia Niu,1,* Chunlin Yao,1,* Yu Wang,1 Yan Sun,1 Juan Xu,2 Yun Lin,1 Shanglong Yao1
1Department of Anesthesiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan 430022, Hubei, People’s Republic of China; 2School of Medicine and Health Management, Tongji Medical College of Huazhong University of Science and Technology, Wuhan 430022, Hubei, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Yun Lin; Shanglong Yao
Department of Anesthesiology, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, 1277# Jiefang Avenue, Wuhan 430022, Hubei, People’s Republic of China
Tel +86 13986288403; +86 13886128437
Fax +86 27 85726970
Email [email protected]; [email protected]
Purpose: This study examined whether different neuromuscular-blocking agents (NMBAs) work differently on the short-term outcomes of gastric cancer patients in terms of laboratory test results and severity of postoperative illness, and whether the effect is dose-related.
Patients and Methods: Data of 1643 adult patients receiving gastric cancer surgery were analyzed by employing generalized linear models (GLMs), to explore the effects of different NMBAs on neutrophil–lymphocyte ratio (NLR), platelet–lymphocyte ratio (PLR), lymphocyte–monocyte ratio (LMR) at postoperative day 1 (POD1), POD3, POD7, and return to intended oncologic therapy (RIOT), among others. We adjusted multiple covariants, including patient-, anesthesia-, and surgical complexity-related risk factors.
Results: Without adjusting dosage of NMBAs, POD1NLR, POD1PLR (P < 0.05), POD3NLR, POD7NLR, POD3 lymphocytes, POD7LMR (P < 0.01) in gastric cancer patients administered with benzylisoquinoline NMBAs worsened, and the administration of aminosteroidal NMBAs was associated with less risk of transfer to ICU (P < 0.01); without adjusting the types of NMBAs, the highest dose of NMBAs postponed the RIOT (P < 0.05) and was negatively associated with POD3NLR, POD7NLR and POD7LMR (P < 0.01), and increased risk of postoperative transfer to ICU (P < 0.01). When patients given benzylisoquinolines were re-divided in terms of five equal quintiles, from low to high dose, RIOT was delayed and POD7LMR decreased significantly in the fourth and fifth quintile groups as compared to the first quintile group. A higher risk for postoperative transfer to ICU was found in the fifth quintile group as compared to the first quintile group.
Conclusion: Patients with gastric cancer given benzylisoquinoline NMBAs had more unfavorable short-term outcomes, such as more severe inflammation and increased risk of transfer to ICU than their counterparts administered aminosteroidal NMBAs, and the effect of benzylisoquinolines was dose-related. The effect of aminosteroids on short-term outcomes was not dose-related in the dosage range we used.
Keywords: neuromuscular-blocking agents, benzylisoquinoline, aminosteroid, short-term postoperative outcomes, gastric cancer surgery
Introduction
Currently, cancer remains one of leading causes of death worldwide, and gastric cancer represents the second most common cause of cancer-related deaths around the globe, and, clinically, surgical resection is one of the principal treatment alternatives.1,2 It has long been generally believed that the surgery can lead to cell-mediated immunosuppression,3 and inhibit anti-tumor immunity.4 Moreover, multiple perioperative factors, especially anesthesia management, affect the survival of patients.5,6 Until now, researchers have failed to achieve consensus about the impact of anesthesia on postoperative recurrence and metastasis of gastric cancer.
Some factors, including anesthesia techniques and anesthetic agents, may alter the behaviors of residual tumor cells after surgery, thereby promoting cancer recurrence and/or metastasis.7 It was reported that several perioperative factors could directly stimulate cancer cells and elicit cell-mediated immunity, thereby causing the spread of tumors.4,5,8 A number of studies indicated that the use of neuromuscular-blocking agents (NMBAs) was associated, dose-dependently, with elevated risk of postoperative respiratory complications,9 and with increased risk of 30-day readmission after abdominal surgery.10 Reports were scanty concerning whether NMBAs exert any effect on the outcomes of cancer patients, and whether this effect is dose-related. At present, most research was conducted in cells and animals and the results varied. Some studies exhibited that benzylisoquinolines might be able to ameliorate inflammation and immunosuppression, possess tumor-suppressing effect, inhibit proliferation and migration of cancer cells, and induce apoptosis.11,12 However, other studies showed that aminosteroids promoted tumor metastasis and recurrence.13,14
It was reported that a higher level of postoperative inflammatory cells might be associated with more unfavorable outcomes.15 Increased neutrophil–lymphocyte ratio (NLR) is indicative of severity of inflammation and is linked with the more adverse events of cancer. Gastric cancer patients with high postoperative NLR after surgery had significantly worse prognosis,16 and low postoperative NLR was significantly correlated with longer survival.17 When the NLR at postoperative day 3 (POD3) was greater than 7.7, the post-gastrectomy risk for 5-year cancer recurrence was 4.2 times higher.18 Thrombocytopenia was found to be positively associated with decreased postoperative complications in patients receiving colorectal surgery.19 Platelet–lymphocyte ratio (PLR) is considered to be an independent prognostic factor for a variety of solid tumors, including colorectal cancer, non-small cell lung cancer, pancreatic cancer and gastric cancer.20 Meanwhile, decreased lymphocyte count could lead to lowered resistance to tumor.21 These parameters can serve as prognostic indicators for post-gastrectomy patients.
The main objective of this retrospective study was to explore whether the types or dosage of NMBAs are associated with the short-term outcomes of patients with gastric cancer, including laboratory tests and severity of postoperative illness.
Patients and Methods
Patient Population
The study was approved by the Ethical Committee of Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China (2019-S891), and was conducted in strict accordance with the Declaration of Helsinki. As the study was of retrospective nature and only aggregated non-identifiable data were used, consent from individuals was not required. We state that patient data were confidential. The research has been registered at the China Clinical Trial Registration Center (ChiCTR1900023305).
We performed an observational analysis by using data on adult patients undergoing gastric cancer surgery at Wuhan Union Hospital from January 2014 to December 2018. Data came from the electronic medical record system and anesthesia information management system, which included baseline information, concomitant diseases, oncological features, anesthesia and operative procedures, and laboratory test results.
Inclusion and Exclusion Criteria
We included patients aged from 18 to 75 years who underwent gastric cancer surgical procedures, and received intermediate-acting NMBAs. Exclusion criteria included: (1) American Society of Anesthesiologists (ASA) > 3; (2) having other cancers; (3) having received re-operation for the gastric cancer; (4) preoperative hemoglobin (Hb) < 90 g/L; (5) having distant metastasis; (6) having received intraoperative hyperthermic intraperitoneal chemotherapy or preoperative chemotherapy, palliative operation; (7) in-hospital death.22,23
Clinical Parameters
The intraoperative dosage of NMBAs (adjusted to body weight24) was defined by the multiples of ED9525,26 (the median effective dose at which a 95% reduction can be achieved in the maximal twitch response from baseline) for intraoperatively given NMBAs.9 Based on multiples of NMBA ED95, from low to high dose, the data were subdivided into five equal quintile groups.9 It is worth noting that in this observational study, these quintuples did not match 1-, 2-, 3-, 4-, and 5-fold of ED95. The first quintile group consisted of patients receiving the lowest NMBA equivalent dose, whereas the fifth quintile group comprised patients administered the highest equivalent dose.
The outcome measures included lymphocyte count, NLR, PLR, lymphocyte–monocyte ratio (LMR) at POD1, POD3, POD7, and postoperative transfer to ICU, complications occurring within one month after surgery, the length of hospital stay, postoperative hospital stay, and the return to intended oncologic therapy (RIOT). The decision to transfer to ICU after surgery was made by two anesthetists with more than 5 years’ work experience. Complications included fever, pulmonary infection, pleural effusion, anastomotic leakage, pancreatic fistula, surgical incision infection, new-onset obstruction, hemorrhage, venous thrombosis or pulmonary embolism, wound rupture, and acute renal failure. RIOT was defined as the time between surgery and inception or resumption of non-surgical oncological therapies (eg chemotherapy, radiotherapy or endocrine treatments).27
Statistical Analysis
Data analyses were performed using SPSS, version 28.0 (IBM SPSS, Chicago, IL). Continuous variables were expressed as mean ± standard deviation (SD), and categorical variables as frequency (percentage).
We established generalized linear models (GLM) to examine the association between the type and dose of NMBAs (independent variables) and laboratory test results and prognostic outcomes. Each GLM was adjusted to patients’ features (age, sex, body weight, ASA physical status, comorbidity), oncological data (tumor stage, differentiation status, vascular or nerve invasion, positive margin), surgery-related variables (open or laparoscopic surgery, surgical techniques, duration of surgery), anesthesia methods, intraoperative blood transfusion. The variable categorization is shown in Table 1.
 |
Table 1 Characteristics of Study Population |
When the result of postoperative blood test, as a dependent variable, was included in the GLM, the corresponding preoperative examination data were also adjusted. Because our study was of retrospective nature and some patients did not receive the corresponding examinations when we postoperatively collected the blood test results, some data were absent. In spite of this, the corresponding GLM covered more than 70% of the data, and with some cases, the coverage was over 90%. Data on RIOT were difficult to obtain because the patients were geographically diverse and did not receive follow-up oncological therapy in a single center. The corresponding GLM included 42% of the data.
Results of GLM were presented as equation coefficient (β) or odds ratio (OR) with 95% confidence intervals (CI) and P-values. A two-tailed P-value less than 0.05 was considered statistically significant.
Results
During the study period, 2249 patients underwent gastric cancer surgery. Upon the evaluation against the aforementioned inclusion and exclusion criteria, 1643 patients were eventually included (Figure 1). In those patients, the NMBAs used in the benzylisoquinoline group included cisatracurium and atracurium, while in the aminosteroid group only rocuronium was given. The gastric cancer patients in our series did not use NMT monitors, and there was no difference in reversal agents of NMBAs because neostigmine was administered in all cases. Intraoperative muscle relaxants were given in a single intravenous injection by anesthesiologists with more than 5 years of work experience. In our preliminary analysis, normality test and distribution curve showed that the obtained data essentially follow the pattern of normal distribution.
 |
Figure 1 Flow diagram of subject selection. |
Table 1 shows the features of the study population. Intraoperatively, 58 patients underwent laparotomy instead of endoscopic operation, and they were counted as subjects receiving laparotomy. In patients receiving other non-specified surgical techniques, there were no significant differences in the proportion of patients receiving gastrectomy plus splenectomy among five dosage groups and among three groups treated with different NMBAs. The ED95 dose equivalents of administered NMBAs, from the first to the fifth quintiles, were 2.01–5.95 (the 1st quintile), 5.96–8.10 (the 2nd quintile), 8.12–11.21 (the 3rd quintile), 11.22–15.54 (the 4th quintile) and >15.54 (the 5th quintile), respectively.
Table 2 shows that POD1NLR, POD1PLR, POD3NLR, POD7NLR were lower in gastric cancer patients given aminosteroidal NMBAs than in those administered benzylisoquinolines while POD3 lymphocytes, POD7LMR were higher in the aminosteroid group than the benzylisoquinoline group. Moreover, the use of aminosteroids was associated with reduced risk of postoperative transfer to ICU (OR, 0.38; 95% CI, 0.21 to 0.68; P = 0.001). In patients given mixed NMBAs, POD3NLR, POD7NLR were lower but POD3 lymphocytes and POD7LMR were higher than in those administered benzylisoquinolines, and the application of mixed NMBAs was associated with lowered risk of postoperative transfer to ICU (OR, 0.50; 95% CI, 0.26 to 0.98; P = 0.044). The types of NMBAs had no significant effect on remaining prognostic indicators.
 |
Table 2 Association Between the Type of NMBAs and Short-Term Postoperative Outcomes |
GLM analysis showed that, in patients given the highest dose (the 5th quintile group) of NMBAs, RIOT (β, 6.20; 95% CI, 2.32 to 10.07; P = 0.002), POD3NLR (β, 2.00; 95% CI, 0.92 to 3.07; P < 0.01), POD7NLR (β, 1.22; 95% CI, 0.68 to 1.77; P < 0.01), and their risk for postoperative transfer to ICU (OR, 5.38; 95% CI, 2.29 to 12.62; P < 0.01) were higher than their counterparts receiving the lowest dose (the 1st quintile group). On the other hand, POD7LMR was lower in patients given the highest dose of NMBAs than in those administered the lowest dose (β, −0.35; 95% CI, −0.53 to −0.17; P < 0.01) (Table 3). The dosage of NMBAs exerted no significant effect on remaining prognostic indicators.
 |
Table 3 Association Between the Dose of NMBAs and Short-Term Postoperative Outcomes |
In the aforementioned experiments, because the doses were not evenly distributed among the types of NMBAs (Table 4), we did not adjust the dosage when analyzing the relationship between the types of NMBAs and the prognostic outcomes. Similarly, in the analyses of the relationship between the dosage of NMBAs and prognostic outcomes, the types of NMBAs were not adjusted. The above results might be ascribed to the differences in types or doses. The effects of the types on the prognosis were compared within appropriate dosage range of NMBAs and we selected doses of the second and third quintiles groups. The benzylisoquinoline group and the aminosteroid group were re-grouped into five equally sized groups, respectively, in terms of the multiples of NMBA ED95 from low to high dose, and the effects of the doses on the short-term outcomes were compared within the same NMBA. Due to the small number of patients in the mixed NMBAs group, we did not conduct a separate analysis.
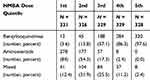 |
Table 4 The Distribution of Administered Doses |
In the second quintile group, only POD3 lymphocytes in the mixed NMBAs group were higher than in the benzylisoquinolines group, and there were no significant differences in other indicators between the aminosteroid group and the benzylisoquinolines group, and, similarly between the mixed NMBAs group and the benzylisoquinolines group. In the third quintile group, the POD1NLR and POD1PLR were lower in the aminosteroid group than in the benzylisoquinolines group (Table 5).
 |
Table 5 Effect of Two Types of NMBAs on Prognosis at the Second and Third Quintile |
For benzylisoquinolines, RIOT in the fourth and fifth quintile groups were significantly higher than in the first quintile group, and POD7LMR was significantly lower. The risk for postoperative transfer to ICU was higher in the fifth quintile group than in the first quintile group (OR, 2.50; 95% CI, 1.02 to 6.17; P = 0.046). Although there existed no significant differences in POD3NLR and POD7NLR between the high-quintile group and the low-quintile group, the P-value for the trend was <0.05, indicating that the effect of benzylisoquinoline NMBAs on these two indicators was dose-related (Table 6). For aminosteroids, there were no statistically significant association between dose and all short-term prognostic indicators (Table 7).
 |
Table 6 Relationship Between Dosage and Prognosis in Benzylisoquinolines |
 |
Table 7 Relationship Between Dose and Prognosis in Aminosteroids |
Discussion
In this retrospective study, we found that patients with gastric cancer operation intraoperatively administered benzylisoquinoline NMBAs had worse short-term outcomes than their counterparts given aminosteroids, and some postoperative inflammatory indicators and the risk for postoperative transfer to ICU were related to the dosage of benzylisoquinolines. The effect of aminosteroids on the short-term outcomes was not dose-related in the dose range we used.
In recent years, research effort has been increasingly directed on anesthesia techniques and the effects of anesthetics on tumors. They may, in certain ways, impact the serum environment, which, in turn, works on the biological behaviors of cancer cells, thereby affecting tumor metastasis.28,29 Previous studies have shown that the intravenous anesthetics, volatile drugs, local anesthetics, and nerve-blocking agents could influence the recurrence and survival after surgery.6,30 NMBAs are widely used as adjuvants in the induction and maintenance of anesthesia for gastric cancer surgery, but their effects on cancer have not been well studied.
Currently, the commonly used intermediate-effect NMBAs are benzylisoquinolines and aminosteroids. An in-vitro cell assay showed that rocuronium could promote the growth, adhesion and invasion of breast cancer cells.13 Amann et al have shown that cisatracurium and atracurium inhibited the proliferation of human hepatoma cells (HepG2) in a concentration-dependent manner.31 Similarly, cisatracurium could upregulate p53 and its downstream genes and proteins, thereby effectively inhibiting proliferation and induce apoptosis of HCT116 cells by altering p53-dependent apoptotic pathways.11,12 Atracurium also promoted astrocyte differentiation and depleted glioblastoma stem cells, and pretreatment of GSCs expressing CHRNA1 and CHRNA9 with atracurium could significantly improve survival rate in xeno-grafted mice.32 Jiang et al pointed out that rocuronium promoted the growth, invasion and migration of gastric cancer cells SGC7901 and BGC823 in a dose-dependent fashion in an in-vitro model, while cisatracurium exerted no significant effect on gastric cancer cells at normal plasma concentrations, but enhanced the migration of SGC7901 and BGC823 cells in overdose.14 The foregoing studies demonstrated that two different NMBAs worked differently on tumor cells: cisatracurium inhibited tumor cell growth and invasion, while rocuronium promoted proliferation and migration. In contrast, our study showed that the benzylisoquinoline had an unfavorable effect on the short-term outcomes of gastric cancer patients compared with the aminosteroid.
Inflammation and immunization are believed to be implicated in the tumor development and progression. Therefore, the inflammation, assessed in terms of circulating inflammatory cells, can indirectly reflect the severity and prognosis of tumors.33 Sun et al pointed out that clinical dose of cisatracurium could ameliorate postoperative immunosuppression in patients with non-small cell lung cancer.34 A study examined the effect of cisatracurium in combination with ventilation on inflammatory factors and immune changes in septic rats and found that cisatracurium significantly reduced the levels of inflammatory factors and the ratio of peripheral blood neutrophils, thereby inhibiting the inflammatory response and regulating immune function, and eventually alleviating inflammatory damage in various organs of septic rats.35 However, our study demonstrated that the aminosteroid induced lower inflammatory responses and stronger immunity as compared with benzylisoquinoline.
Different from the aforementioned in-vitro findings, our retrospective study showed that, in patients with gastric cancer, the higher dose of the benzylisoquinoline NMBAs negatively changed postoperative POD7LMR, POD3NLR and POD7NLR, and increased the risk of postoperative admission to ICU, while the aminosteroidal NMBAs did not unfavorably change the above indicators within the dose range we used in clinical practice.
Then, we examined the possible causes of the phenomenon. On the one hand, the doses we used in clinical practice were different from the doses employed in animal cell tests. In order to satisfy the surgical requirements, we used a high NMBA dose of the benzylisoquinolines, which might lead to cancer cell migration and inflammatory changes, while the dose of aminosteroids used was low and did not reach the concentration that could effect changes in tumor cells.14 On the other hand, we only included gastric cancer patients, and mainly targeted the effects of the agents on inflammation and immunity, and did not examine the changes at cellular and molecular levels.
In this study, we found that the mixed group had different outcomes rather than an average one between benzylisoquinolines and aminosteroids in our study. Because two different muscle relaxants were used in the mixed group, this phenomenon might result from differences in drug delivery time or in the dosage proportion of different muscle relaxants, among others. These differences might lead to the interaction among the different muscle relaxants, which further affects the patients’ short-term results. Such intercellular effect will be further demonstrated in our future studies.
Although all our patients were administered neostigmine to reverse muscle relaxants, sugammadex, as a novel specific rocuronium antagonist, should be considered in future studies to achieve better results on the effect of muscle relaxants on cancer patients.
Compared with previous studies, our research did not target cells or animals but patients with gastric cancer, which rendered the results more clinically relevant. The sample size was adequate and representative, and the GLM was used to adjust gender and age and other variables.
Our study have some limitations. This study was retrospective in nature, and although effort was made to adjust intraoperative variables, some other confounders influencing our results were not covered. For instance, blood indicators may be affected postoperatively by such conditions as infection, inflammation and drugs used. Some patients did not have a routine blood test at POD1, POD3, POD7, and some data were not available. Therefore, further prospective studies involving more factors are warranted.
Conclusions
Our study showed that patients with gastric cancer intraoperatively treated with high doses of benzylisoquinoline NMBAs had poor postoperative outcomes. Such dose-dependence was not apparent when aminosteroidal NMBA was given. Using high-doses of benzylisoquinoline NMBAs to facilitate intraoperative conditions during gastric cancer surgery should be carefully weighed against possible unfavorable outcomes.
Abbreviations
NMBA, neuromuscular-blocking agent; GLM, generalized linear model; NLR, neutrophil–lymphocyte ratio; PLR, platelet–lymphocyte ratio; LMR, lymphocyte–monocyte ratio; POD, postoperative day; RIOT, return to intended oncologic therapy; ASA, American Society of Anesthesiologists; SD, standard deviation; OR, odds ratio; CI, confidence interval.
Data Sharing Statement
The data sets analyzed during the current study are not publicly available due to them containing information that could compromise research participant privacy, but are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The research was approved by the Ethical Committee of Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China. The committee’s reference number is 2019-S891.
Acknowledgments
We are indebted to Mr. Chaohong Yu for assistance in the professional writing of this manuscript.
Author Contributions
All authors have made substantial contributions to the conception or design of the work, or the acquisition, analysis, or interpretation of data for the work. All authors have drafted or critically revised the work, have approved the final version to be published, and have agreed on the journal in which it will be published. All authors agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. Lingxia Niu and Chunlin Yao contributed equally to this work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The author reports no conflicts of interest in this work.
References
1. Thrift AP, El-Serag HB. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18(3):534–542. doi:10.1016/j.cgh.2019.07.045
2. Sitarz R, Skierucha M, Mielko J, Offerhaus G, Maciejewski R, Polkowski WP. Gastric cancer: epidemiology, prevention, classification, and treatment. Cancer Manag Res. 2018;10:239–248. doi:10.2147/CMAR.S149619
3. Hogan BV, Peter MB, Shenoy HG, Horgan K, Hughes TA. Surgery induced immunosuppression. Surgeon. 2011;9(1):38–43. doi:10.1016/j.surge.2010.07.011
4. Tohme S, Simmons RL, Tsung A. Surgery for cancer: a trigger for metastases. Cancer Res. 2017;77(7):1548–1552. doi:10.1158/0008-5472.CAN-16-1536
5. Tavare AN, Perry NJ, Benzonana LL, Takata M, Ma D. Cancer recurrence after surgery: direct and indirect effects of anesthetic agents. Int J Cancer. 2012;130(6):1237–1250. doi:10.1002/ijc.26448
6. Wigmore TJ, Mohammed K, Jhanji S. Long-term survival for patients undergoing volatile versus IV anesthesia for cancer surgery: a retrospective analysis. Anesthesiology. 2016;124(1):69–79. doi:10.1097/ALN.0000000000000936
7. Snyder GL, Greenberg S. Effect of anaesthetic technique and other perioperative factors on cancer recurrence. Br J Anaesth. 2010;105(2):106–115. doi:10.1093/bja/aeq164
8. Kavanagh T, Buggy DJ. Can anaesthetic technique effect postoperative outcome? Curr Opin Anaesthesiol. 2012;25(2):185–198. doi:10.1097/ACO.0b013e32834f6c4c
9. McLean DJ, Diaz-Gil D, Farhan HN, Ladha KS, Kurth T, Eikermann M. Dose-dependent association between intermediate-acting neuromuscular-blocking agents and postoperative respiratory complications. Anesthesiology. 2015;122(6):1201–1213. doi:10.1097/ALN.0000000000000674
10. Thevathasan T, Shih SL, Safavi KC, et al. Association between intraoperative non-depolarising neuromuscular blocking agent dose and 30-day readmission after abdominal surgery. Br J Anaesth. 2017;119(4):595–605. doi:10.1093/bja/aex240
11. Yabasin IB, Lu Z, J C Y, Wen Q. Cisatracurium-induced proliferation impairment and death of colorectal cancer cells, HCT116 is mediated by p53 dependent intrinsic apoptotic pathway in vitro. Biomed Pharmacother. 2017;91:320–329. doi:10.1016/j.biopha.2017.04.044
12. Yabasin IB, Sanches J, Ibrahim MM, et al. Cisatracurium retards cell migration and invasion upon upregulation of p53 and inhibits the aggressiveness of colorectal cancer. Front Physiol. 2018;9:941. doi:10.3389/fphys.2018.00941
13. Jiang A, Zhao H, Cai J, Jiang WG. Possible effect of muscle-relaxant anaesthetics on invasion, adhesion and migration of breast cancer cells. Anticancer Res. 2016;36(3):1259–1265.
14. Jiang A, Zhao H, Liu X, Yu M, Chen J, Jiang WG. Comparison of different muscle-relaxant anesthetics on growth, migration and invasion of gastric cancer cells. Anticancer Res. 2017;37(8):4371–4378.
15. Pang S, Zhou Z, Yu X, et al. The predictive value of integrated inflammation scores in the survival of patients with resected hepatocellular carcinoma: a Retrospective Cohort Study. Int J Surg. 2017;42:170–177. doi:10.1016/j.ijsu.2017.04.018
16. Miyatani K, Saito H, Kono Y, et al. Combined analysis of the pre- and postoperative neutrophil-lymphocyte ratio predicts the outcomes of patients with gastric cancer. Surg Today. 2018;48(3):300–307. doi:10.1007/s00595-017-1587-6
17. Turner N, Tran B, Tran PV, et al. Primary tumor resection in patients with metastatic colorectal cancer is associated with reversal of systemic inflammation and improved survival. Clin Colorectal Cancer. 2015;14(3):185–191. doi:10.1016/j.clcc.2015.02.004
18. Kim YH, Choi WJ. The effectiveness of postoperative neutrophils to lymphocytes ratio in predicting long-term recurrence after stomach cancer surgery. J Korean Surg Soc. 2012;83(6):352–359. doi:10.4174/jkss.2012.83.6.352
19. Mohamud M, Osborne L, Jones HG, et al. Thrombocytosis as a marker for postoperative complications in colorectal surgery. Gastroenterol Res Pract. 2018;2018:1978639. doi:10.1155/2018/1978639
20. Lian L, Xia YY, Zhou C, et al. Application of platelet/lymphocyte and neutrophil/lymphocyte ratios in early diagnosis and prognostic prediction in patients with resectable gastric cancer. Cancer Biomark. 2015;15(6):899–907. doi:10.3233/CBM-150534
21. Liu S, Lachapelle J, Leung S, Gao D, Foulkes WD, Nielsen TO. CD8+ lymphocyte infiltration is an independent favorable prognostic indicator in basal-like breast cancer. Breast Cancer Res. 2012;14(2):R48. doi:10.1186/bcr3148
22. Dixon M, Mahar AL, Helyer LK, Vasilevska-Ristovska J, Law C, Coburn NG. Prognostic factors in metastatic gastric cancer: results of a population-based, retrospective cohort study in Ontario. Gastric Cancer. 2016;19(1):150–159. doi:10.1007/s10120-014-0442-3
23. Zare A, Mahmoodi M, Mohammad K, Zeraati H, Hosseini M, Holakouie NK. Factors affecting the survival of patients with gastric cancer undergone surgery at Iran Cancer Institute: univariate and multivariate analyses. Iran J Public Health. 2014;43(6):800–808.
24. van Kralingen S, van de Garde EM, Knibbe CA, et al. Comparative evaluation of atracurium dosed on ideal body weight vs. total body weight in morbidly obese patients. Br J Clin Pharmacol. 2011;71(1):34–40. doi:10.1111/j.1365-2125.2010.03803.x
25. Belmont MR, C A L, Quessy S, et al. The clinical neuromuscular pharmacology of 51W89 in patients receiving nitrous oxide/opioid/barbiturate anesthesia. Anesthesiology. 1995;82(5):1139–1145. doi:10.1097/00000542-199505000-00008
26. Naguib M, Samarkandi AH, Bakhamees HS, Magboul MA, El-Bakry AK. Comparative potency of steroidal neuromuscular blocking drugs and isobolographic analysis of the interaction between rocuronium and other aminosteroids. Br J Anaesth. 1995;75(1):37–42. doi:10.1093/bja/75.1.37
27. E A N, Burns D, Riedel B, Sessler DI, Buggy DJ. The effect of anaesthetic technique during primary breast cancer surgery on neutrophil-lymphocyte ratio, platelet-lymphocyte ratio and return to intended oncological therapy. Anaesthesia. 2018;73(5):603–611. doi:10.1111/anae.14207
28. Y J X, S Y L, Cheng Q, et al. Effects of anaesthesia on proliferation, invasion and apoptosis of LoVo colon cancer cells in vitro. Anaesthesia. 2016;71(2):147–154. doi:10.1111/anae.13331
29. Looney M, Doran P, Buggy DJ. Effect of anesthetic technique on serum vascular endothelial growth factor C and transforming growth factor beta in women undergoing anesthesia and surgery for breast cancer. Anesthesiology. 2010;113(5):1118–1125. doi:10.1097/ALN.0b013e3181f79a69
30. Buckley A, McQuaid S, Johnson P, Buggy DJ. Effect of anaesthetic technique on the natural killer cell anti-tumour activity of serum from women undergoing breast cancer surgery: a pilot study. Br J Anaesth. 2014;113(Suppl 1):i56–i62. doi:10.1093/bja/aeu200
31. Amann A, Rieder J, Fleischer M, et al. The influence of atracurium, cisatracurium, and mivacurium on the proliferation of two human cell lines in vitro. Anesth Analg. 2001;93(3):690–696. doi:10.1097/00000539-200109000-00031
32. Spina R, Voss DM, Asnaghi L, Sloan A, Bar EE. Atracurium besylate and other neuromuscular blocking agents promote astroglial differentiation and deplete glioblastoma stem cells. Oncotarget. 2016;7(1):459–472. doi:10.18632/oncotarget.6314
33. Pasini FS, Zilberstein B, Snitcovsky I, et al. A gene expression profile related to immune dampening in the tumor microenvironment is associated with poor prognosis in gastric adenocarcinoma. J Gastroenterol. 2014;49(11):1453–1466. doi:10.1007/s00535-013-0904-0
34. Sun WQ, Pan DB, Zhou AG, Mo H. Does cisatracurium at a clinical dose attenuate the immunosuppression after surgery in smoking patients with non-small cell lung cancer? Middle East J Anaesthesiol. 2015;23(3):375–377.
35. He T, Tao J, Wang X, Wang X. Effects of cisatracurium in combination with ventilation on inflammatory factors and immune variations in sepsis rats. Exp Ther Med. 2018;15(5):4414–4418.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
