Back to Journals » Journal of Pain Research » Volume 15
Association Between Experimental Pain Thresholds and Trajectories of Postoperative Recovery Measures After Benign Hysterectomy
Authors Lukas P, Gerdle B , Nilsson L , Wodlin NB , Fredrikson M , Arendt-Nielsen L, Kjølhede P
Received 31 July 2022
Accepted for publication 16 November 2022
Published 23 November 2022 Volume 2022:15 Pages 3657—3674
DOI https://doi.org/10.2147/JPR.S383795
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jinlei Li
Peter Lukas,1 Björn Gerdle,2 Lena Nilsson,3 Ninnie Borendal Wodlin,1 Mats Fredrikson,4,5 Lars Arendt-Nielsen,6,7 Preben Kjølhede1
1Department of Obstetrics and Gynecology in Linköping, and Department of Biomedical and Clinical Sciences, Faculty of Medicine Health Sciences, Linköping University, Linköping, Sweden; 2Pain and Rehabilitation Centre, and Department of Health, Medicine and Caring Sciences, Faculty of Medicine Health Sciences, Linköping University, Linköping, Sweden; 3Department of Anesthesiology and Intensive Care in Linköping, and Department of Biomedical and Clinical Sciences, Faculty of Medicine Health Sciences, Linköping University, Linköping, Sweden; 4Forum Östergötland, Faculty of Medicine and Health Sciences, Linköping University, Linköping, Sweden; 5Occupational and Environmental Medicine, Department of Experimental and Clinical Medicine, Faculty of Medicine Health Sciences, Linköping University, Linköping, Sweden; 6Center for Neuroplasticity and Pain (CNAP), Department of Health Science and Technology, Faculty of Medicine, Aalborg University, Aalborg, Denmark; 7Department of Medical Gastroenterology, Mech-Sense, Aalborg University Hospital, Aalborg, Denmark
Correspondence: Preben Kjølhede, Department of Obstetrics and Gynecology, University Hospital, Linköping, 581 85, Sweden, Tel +46 705688201, Email [email protected]
Purpose: Quantitative sensory testing (QST) can be applied to quantify the sensitivity to different painful stimuli. This study aims to evaluate the association between preoperative pressure and thermal pain thresholds and trajectories of measurements of postoperative recovery (patient-reported daily maximum and average pain intensity, sum score of symptoms, and analgesic consumption) after benign hysterectomy.
Patients and Methods: A prospective, longitudinal single-blinded, observational multicenter study was conducted in five hospitals in the southeast of Sweden between 2011 and 2017. A total of 406 women scheduled for abdominal or vaginal hysterectomy for benign conditions were enrolled in the study. QST measuring pressure (PPT), heat (HPT), and cold pain thresholds (CPT) were performed preoperatively. The cut-off levels for dichotomizing the pain thresholds (low/high) were set at the 25-percentile for PPT and HPT and the 75-percentile for CPT. The Swedish Postoperative Symptom Questionnaire was used to measure postoperative pain and other symptoms of discomfort (symptom sum score) on 13 occasions for six weeks postoperatively. Daily analgesic consumption of opioids and non-opioids was registered.
Results: A CPT above the 75-percentile was associated with high postoperative maximum pain intensity (p = 0.04), high symptom sum score (p = 0.03) and greater consumption of non-opioids (p = 0.03). A HPT below the 25-percentile was only associated with greater consumption of non-opioids (p = 0.02). PPT was not associated with any of the outcome measures.
Conclusion: CPT seemed to be predictive for postoperative pain and symptoms of discomfort after benign hysterectomy. Preoperative QST may be used to individualize the management of postoperative recovery for low pain threshold individuals.
Keywords: hysterectomy, quantitative sensory testing, postoperative symptoms, postoperative recovery, pressure and thermal pain thresholds
Corrigendum for this paper has been published.
Introduction
Hysterectomy for benign gynecological conditions is one of the most common major gynecological operations.1 The main goal of the surgery is to improve the health-related quality of life. The recovery after surgery may be affected by many factors. Postoperative symptoms such as pain, nausea, fatigue, and gastrointestinal paralysis are commonly reported, and may affect hospital stay and recovery.2 Besides postoperative complications, other factors may affect the recovery for instance, patient-related factors (eg, psychosocial characteristics and comorbidities), health care-related factors (eg, medical and local treatment traditions), and society factors for example, the social security system.
Enhanced recovery after surgery (ERAS) programs were introduced to facilitate faster recovery to normal daily living without compromising medical safety.3 ERAS programs consist of a series of evidence-based multimodal treatments covering the perioperative period and are aimed at preparing the patient physically and psychologically for the surgery, reducing surgical stress and postoperative symptoms, maintaining physiological functions and providing early ambulation.4,5
Postoperative pain and adequate analgesia are important issues that concern patients preoperatively.6 The individual pain experience differs among patients despite optimal pain management regimes. A patient’s vulnerability to anxiety and their ability to cope with stressful situations affect the postoperative recovery to a high degree. Hence, several studies have shown that patients that are prone to anxiety and depression or have low stress coping ability have a slower recovery after hysterectomy, longer hospital stay and longer sick leave.7–9 Even though studies have identified risk factors (eg, preoperative pain conditions) for developing severe acute postoperative10 and chronic postoperative pain11 after benign hysterectomy it is a challenge to predict individuals who are at risk and to prepare targeted management. Hence, the mechanisms leading to chronic pain in some but not in others remain to be discovered. The pain experience seems to be affected by the preoperative sensitivity to pain (ie, pain thresholds) and coping factors such as catastrophizing.12 Experimental pain sensitivity preoperatively, eg, using pressure and thermal stimuli has been shown to be predictors for postoperative pain13 and chronic pain.14,15 Quantitative sensory testing (QST) can be applied to quantify the sensitivity to different painful stimulus modalities16 using static stimuli such as pressure and temperature.17
In the current study, we hypothesize that deviant pain thresholds for thermal (heat and cold) and mechanical stimuli (pressure) can predict the trajectories of postoperative pain intensity, analgesic consumption, and overall postoperative discomfort (indicated as the sum score of eight commonly occurring postoperative symptoms).
The primary aim of the study was to determine if associations exist between preoperative pain thresholds for pressure (PPT), heat (HPT) and cold (CPT) and trajectories of postoperative pain intensity in subjects who underwent hysterectomy on benign indications. Secondary aims were to evaluate the corresponding associations with analgesics consumption and postoperative discomfort.
Materials and Methods
This was a prospective single-blinded longitudinal observational multicenter study of pain thresholds for heat, cold, and pressure in women undergoing abdominal or vaginal hysterectomy for benign gynecological diseases at the departments of Obstetrics and Gynecology in five hospitals in the southeast health region of Sweden.
The women who participated in a randomized controlled multicenter study, the POSTHYSTREC trial, which aimed to determine the effect of various models of follow-up contact on postoperative recovery after benign hysterectomy18 were given verbal and written information about the pain threshold study simultaneously with the information about the POSTHYSTREC trial and were asked to participate in the study at the time of admittance, approximately one week prior to surgery. The research person could refrain from participating in the pain threshold study. Written informed consent was obtained from all participants before inclusion. The study was approved by the Regional Ethical Board in Linköping (Dnr. 2011/106-31; date of approval May 23; 2011) and complies with the Declaration of Helsinki. The trial was registered in ClinicalTrial.gov (NCT01526668).
Women eligible for the study were 18–60 years of age, were scheduled for abdominal (subtotal or total) or vaginal hysterectomy on benign indication between October 2011 and March 2017. At least one ovary had to be left behind after the operation. Proficiency in Swedish was a request. Exclusion criteria were scheduled prolapse surgery, suspected gynecological malignancy with the exception of cervical dysplasia, former or planned bilateral salpingo-oophorectomy, surgery expected to include more than the hysterectomy with or without adnexal surgery or appendectomy, being physically disabled, severe psychiatric or mental disorder, and ongoing drug or alcohol abuse.
Demographic data, medical history and clinical information were assessed at the time of inclusion. Demographic data comprised age (years), body mass index (BMI; kg/m2), smoking (yes/no), gainfully employed (yes/no), and physical workload categorized as sedentary, medium or heavy workload. Medical data comprised parity, comorbidity of cardiovascular diseases, mental illness or chronic pain disorder, categorization according to the American Society of Anesthesiology Physical Status Classification System, and previous laparotomy. The clinical data included indication of hysterectomy, mode of hysterectomy and anesthesia, occurrence of postoperative complications and re-operations, and Clavien-Dindo classification of postoperative complications.19
Quantitative Sensory Testing (QST)
QST was used to assess pain thresholds for thermal (heat and cold) and pressure stimuli. The PPT, HPT and CPT were measured preoperatively on one occasion, either directly after inclusion or at the latest before premedication on the day of surgery. Pain thresholds were measured on four locations on the body, three locations assumed to be referred pain areas of lower abdominal and pelvic conditions, on the back just below the fifth lumbar vertebra L5, and on the anterior abdominal wall 7 cm lateral to the umbilicus on both sides. As a non-pain referral control area, the front side, four cm distal of the tuberositas tibiae of the dominant leg, was selected.
HPT and CPT were conducted according to the standardized protocol recommended by the German Research Network on Neuropathic Pain20 with a Medoc TSA II NeuroSensory Analyzer (Medoc Ltd. 1 Ha’dekel St. Ramat Yishai 30095 Israel). The baseline temperature of the thermode with a surface area of 3×3 cm2 was set to 32°C, which corresponded to the normal skin temperature of the body. The temperature was raised or decreased with the computer-controlled thermode to a maximum of 50°C or a minimum of 0°C with a preset rate of 1.5°C/s. When the participants perceived the first painful stimulus, they were instructed to press the stop button, which was connected to the computer to end the stimulus. The temperature of the thermode was registered in the computer as the threshold for heat or cold. After returning, the thermode to the baseline temperature of 32°C the procedure was repeated three times on each location with an interval of 10 seconds. The average temperature of the three measurements for each location was used as the measure of the pain threshold on each location.
The PPT measurements were conducted with a handheld electronic algometer (Somedic SensetLab AB, Sösdala, Sweden) with a probe area of 1 cm2. The threshold was measured in a standardized manner with a constant pressure rate of approximately 40 kPa/s. The participants were instructed to say “stop” when they perceived the first sensation of pain. The same repetitive cycle as for measurements of heat and cold was performed to detect pressure thresholds. The average of three consecutive measurements on each location was defined as the pressure pain threshold.
The QST measurements were performed by the research nurses in each center. All were educated and trained by an experienced research nurse in QST at the University of Linköping prior to the start of the study to ensure that all measurements were performed according to the standard operating procedure established in the study. The participants were blinded to the outcome of the pain threshold measurements.
The Swedish Postoperative Symptom Questionnaire (SPSQ)
The validated SPSQ form was used to measure symptoms reported by patients postoperatively.2,21 The patient was requested to describe how, at the time of completing the SPSQ, she experienced eight commonly reported postoperative symptoms (nausea, retching, headache, abdominal pain, tiredness, drowsiness, blurred vision, and itching). The intensity of each of these symptoms was rated on a four-point scale as “none” (1), “yes, a little” (2), “yes, somewhat” (3), and “yes, a lot” (4). The sum score of these eight symptoms, which constitutes a measure of the overall discomfort, ranged between 8 and 32. The higher the sum score, the more discomfort was experienced. The patient also reported the maximum experienced intensity of the pain, ie, when the pain was at its worst, and how the pain was felt on average on the particular day. The maximum and average pain intensity was rated on a seven-point scale as: “none” (0), “very mild” (1), “mild” (2), “moderate” (3), “bad” (4), “severe” (5), and “very severe” (6). The SPSQ questionnaire was completed in total on 13 occasions, starting in the evening after surgery and then once daily during the first postoperative week and at the same time of the day. Thereafter, it was completed once a week for five weeks until the planned clinical follow-up visit with the research nurse six weeks after surgery.
Perioperative Care
All five participating centers practiced ERAS principles to ensure that all patients received the same preoperative information regarding the surgical procedure, postoperative symptoms, and treatments thereof, expected time of discharge from the hospital and time to full recovery. Fasting was ordered for six hours for solid food, and two hours for clear liquids prior to anesthesia. Preemptive antiemetic medication, antibiotic and analgesics were administered on commencing the surgery.
The mode of surgery (abdominal or vaginal hysterectomy) and anesthesia was decided after discussion between the patient, gynecologist, and anesthesiologist. Regional anesthesia, preferably intrathecal with morphine, and local analgesics were to be used when possible and opioids were to be kept to a minimum. Indwelling urinary catheter was to be removed the morning after surgery to promote early mobilization.
The standardized postoperative analgesics were oral paracetamol 665 mg 2 x 3, diclofenac 50 mg 1×3 and oxycodone 10–20 mg 1×2 during hospital stay. When necessary, rescue analgesics were intermittently given with morphine or ketobemidone 0.5–1 mg intravenously. At discharge, the patient received prescriptions of oral non-opioids (paracetamol and diclofenac), and if necessary weak opioid-containing analgesics (codeine or tramadol). Oxycodone 10–20 mg x 2 was continued after discharge for a few days, if adequate pain relief was not achieved by the non-opioid and weak opioid analgesics.
During the hospital stay, the analgesic consumption was registered and after discharge, the patients registered the daily analgesic consumption in a diary until the clinical follow up after six weeks. The dose of opioid-containing analgesics was converted into an intravenously morphine equivalent dose. The dose of non-opioid analgesic was converted into the World Health Organization’s defined daily dose (https://www.who.int/medicines/regulation/medicines-safety/toolkit_ddd/en/).22
Primary Outcome Measure
Maximum and average postoperative pain intensity.
Secondary Outcome Measures
Non-opioid and opioid analgesics consumption, and postoperative discomfort measured as the symptom sum score of the SPSQ.
Patient and Public Involvement
Patients or the public were not involved in the planning, development of the research plan, the analyses, or the reporting.
Statistics
The statistical analyses were performed using the TIBCO StatisticaTM software, Version 13.5 (TIBCO Software Inc, Palo Alto, CA 94303, USA) and the factor analysis was run using the IBM SPSS® Statistics software, Version 25 (IBM Corporation, Armonk, NY, USA).
Continuous data are described as mean and one standard deviation (SD) and nominal data as number and percent. Comparison between groups of continuous data were performed using one-way analysis of variance (ANOVA) tests and Mann–Whitney U-tests, and nominal data were analyzed by means of Pearson’s chi-squared tests. The significance level was set at p < 0.05 in two-tailed tests.
To assess the outcome of continuous data, measured on multiple occasions, a repeated measures ANOVA was used. Adjustments were made for known or potential confounders. The results are presented as crude and adjusted p-values. To ensure that the assumptions of the repeated measures ANOVA were met, assessment of normal distribution was performed, and the homogeneity of variance was assessed using the Mauchly sphericity test. If the sphericity was violated and epsilon <0.75, adjustments of the within-subject factor were made with the Greenhouse-Geisser correction method. If a variable was not normally distributed, a logarithmic transformation of the variable was used in the analysis.
A principal component analysis with Varimax rotation and Kaiser normalization fixed to three dimensions was conducted to determine whether the three pain threshold modalities were associated and to evaluate whether the pain thresholds on the four measure point locations could be gathered as the average value of the pain thresholds of the four locations to reduce the dimensionality. Factor loadings ≥0.4 were regarded as significant. The internal consistency reliability was estimated by means of Cronbach’s alpha.
Determination of Cut-Off Levels for Normal versus Deviant Pain Threshold Levels
No clinical cut-off levels have been established to categorize normal from deviant pain thresholds. We used an empirical cut-off level based on the lower quartile of the PPT and HPT (368 kPa and 46.4°C, respectively), and the upper quartile for CPT (5.7°C). These limits coincided closely with the mean pain threshold levels of the patients with chronic pain disorder in our study population (pressure 444 kPa, heat 46.8°C, and cold 5.7°C). Consequently, PPT and HPT were dichotomized into ≤ or > the lower quartile pain thresholds, and CPT was dichotomized into ≥ or < the upper quartile. To assess the impact of various pain threshold levels on the outcome measures the 10-, 20-, 30- and the 40-percentile for PPT and HPT, and 60-, 70-, 80- and 90-percentiles for CPT, were evaluated.
Results
The flow chart of the study population is presented in Figure 1. Hence, four-hundred and six women who underwent hysterectomy for benign conditions were assessed preoperatively with QST and were followed-up clinically after six weeks. The demographic and clinical data are presented in Table 1. The mean age of the women was 46.6 years (SD 5.4 years) and the mean BMI was 26.7 kg/m2 (SD 4.5 kg/m2). The daily analgesic consumptions of opioids and non-opioids from the day of surgery are listed in Table 2.
 |
Table 1 Demographic and Clinical Data of 406 Women Undergoing Benign Hysterectomy |
 |
Table 2 Consumption of Opioids (Equivalent i.v. Morphine Dose (mg)) and Non-Opioids (in DDD) Day-by-Day in 406 Women Undergoing Benign Hysterectomy |
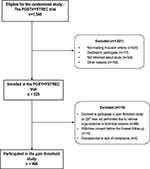 |
Figure 1 Flow chart of participants in the POSTHYSTREC trial and the pain threshold study. |
Pain Thresholds
The average PPT, HPT and CPT from the four measure points and the 25-percentile value for PPT and HPT, and the 75-percentile value for CPT are shown in Table 3. The results of the principal component analysis, presented in Table 4, revealed that the pain thresholds were gathered into three groups, a group for each of the three pain threshold modalities, and the pain thresholds on the four locations for each modality had strong internal consistency reliability with Cronbach’s alpha values between 0.85 and 0.90. Thus, the average pain threshold of the four measure points was eventually used as a measure of the pain threshold for each of the three modalities.
 |
Table 4 Results of the Principal Component Analysis of Pain Threshold Measurements. Varimax Rotation with Kaiser Normalization. Only Factor Loadings ≥0.4 are Presented |
The demographic and descriptive data were subdivided into two groups according to the cut-off level, the 25-percentile for PPT and HPT, and the 75-percentile for CPT (Table 5), to determine potential confounders. The groups were compared and the variables that differed statistically significantly in univariate analysis in all or at least in two of the three pain threshold modalities, ie, chronic pain disorder and mental illness, were used as confounders in the multivariate repeated measures ANOVA models. In addition, mode of hysterectomy was also entered as a confounder into the models due to its potentially strong impact on the outcome measures.
 |
Table 5 Demographic and Clinical Factors in Relation to Pain Thresholds for Pressure (PPT) and Heat (HPT) Categorized After the 25th-Percentile, and Cold (CPT) After the 75th-Percentile |
Pain Thresholds in Relation to the Trajectories of Maximum and Average Pain Intensity, Opioid- and Non-Opioid Consumption, and Symptom Sum Score
The results of the repeated measures ANOVA analyses of the trajectories of the five outcome measures are presented in Table 6. The adjusted models disclosed significant outcomes between groups (effect between groups) in CPT and HPT, but none in PPT. The four significant group associations in the adjusted models for CPT and HPT are illustrated in Figure 2A–D. A high CPT preoperatively was significantly associated with higher maximum postoperative pain intensity (p = 0.04), higher symptom sum score indicating more overall discomfort (p = 0.03), and greater consumption of non-opioids (p = 0.03). Likewise, a low HPT was associated with greater consumption of non-opioids (p = 0.02). Overall, the five outcomes decreased significantly over time (effect over time) in all three pain threshold modalities, according to both the crude and the adjusted models; the only exception was the non-significant adjusted model of opioids. Additionally, no interaction effects were observed between groups in any of the outcomes for either of the pain threshold modalities, demonstrating that the development of the outcome over time was similar in the two pain threshold groups.
 |
Figure 2 Continued. |
Sensitivity Analysis of Impact of Pain Threshold Cut-Off Levels on Outcome Measures
To evaluate a clinically relevant cut-off level in PPT, HPT and CPT, we analyzed the outcome of the outcome measures in different categories based on percentiles. As shown in Table 7, the p-values of the outcomes varied with different cut-off levels. However, to obtain the numerically largest number of outcomes with significant results, the cut-off level for CPTs seemed to be at the 80-percentile, and for HPTs the cut-off seemed to be at the 25-percentile. For PPT, no cut-off levels were found to distinguish the trajectories of recovery outcomes between high and low pain threshold.
Discussion
Major Results
This study showed that preoperative CPT and HPT predicted the development (trajectories) of some of the postoperative recovery outcomes. Hence, CPT was significantly associated with the trajectories of maximum postoperative pain intensity, symptom sum score, and consumption of non-opioid analgesics. HPT was significantly associated with the trajectory of non-opioid analgesic consumption. In contrast, PPT was not associated with any of the outcome measures. This differentiated prediction value of different pain testing modalities may pave the way for exploring fundamental aspects of which management procedures may be most suitable for interacting with mechanisms important for patients’ vulnerability.
Interpretation of Results
The discussion will focus upon pain sensitivity assessments related to surgery; the relevance for musculoskeletal conditions and their developments have recently been reviewed.23,24
Preoperative pain sensitivity has been associated with postoperative acute and chronic pain and analgesic consumption in conditions associated with acute and chronic pain after different surgical procedures.25 In a systematic review, 17 of 25 surgical studies demonstrated associations between preoperative QST and chronic postoperative pain.26 The systematic review by Petersen et al26 and a review of systematic reviews27 concluded consistently that temporal summation of pain and conditioned pain modulation were most frequently associated with acute and/or chronic postoperative pain and/or analgesic effects.
To the best of our knowledge, the association between pain sensitivity and the trajectories of short-term recovery after hysterectomy besides postoperative pain has not previously been described. Only a very few studies have investigated the association between pain threshold and a broader panel of clinical outcomes after benign hysterectomy.28 Hsu et al29 investigated the associations between PPT and tolerance and postoperative pain intensity and morphine consumption at 24 hours postoperatively; only pressure pain tolerance was associated with pain intensity postoperatively. Ahmad et al30 analyzed the relationships between CPT and HPT preoperatively and 24-hour morphine consumption after hysterectomy and reported significant associations. With special reference to pain threshold assessments in association with benign hysterectomy, no clear picture with respect to outcomes emerges from the present study and these studies taken together. Several methodological aspects differ, which may explain lack of consistent results. The present study (n = 406) is considerably larger than the studies by Ahmad et al30 (n = 124) and Hsu et al (n = 40).29 Our study focused upon the trajectories (over several days) of outcomes, while Hsu et a29 and Ahmad et al30 focused upon 24 hours postoperatively. It is well established that patients with chronic pain have lower pain thresholds than subjects without pain. For instance, both vaginal and abdominal PPTs were lower preoperatively in those with pelvic pain.31 Pain is frequent before10,31 and constitutes a risk factor for chronic pain four months after hysterectomy.11 We found 24% of women with chronic pain preoperatively (Table 1), which was adjusted for in the final analysis. The prevalence was not reported or adjusted for in the studies by Ahmad et al30 and Hsu et al.29 In chronic pain patients, thresholds measurements not only include the acute stimuli response but may also include central alterations with habitually increased sensitivity. In contrast to our study, none of the two studies by Hsu and Ahmad was conducted in an ERAS framework which specifically recommends the use of regional analgesia to reduce opioid consumption. In the present study, 60% of the patients received regional analgesia. The opioid consumption in the first 24 hours was therefore less than half of that reported in the above-mentioned studies, where patient-controlled analgesia with intravenous morphine was used. Regional anesthesia combined with single dose intrathecal morphine has been shown to reduce postoperative morphine consumption significantly compared with general anesthesia followed by on-demand opioid administration.32 At discharge, the present participants received prescription of non-opioids, usually both paracetamol and NSAID, to achieve sufficient pain relief. This approach may explain why the non-opioid trajectories were significantly associated with CPT and HPT whereas the opioid consumption was not.
Modality-Specific Prediction of Postoperative Outcome
Our results indicate that CPT is the most promising pain threshold for predicting trajectories of important clinical variables postoperatively. Hence, besides non-opioid consumption, CPT was significantly associated with trajectories of maximum postoperative pain intensity, and symptom sum score. As seen in Figure 2 and Table 5, the two subgroups of CPT had very similar patterns over time for the three outcomes, even though they differed significantly in overall levels. Hence, the interaction effects did not differ between the two subgroups of CPT. Also, other studies have indicated that especially CPT is a predictor of poor outcomes, eg, pain and disability.33
Recovery after surgery is multifactorial and is influenced, not only by the physical disability caused by the surgery, but even by several psychological factors. The psychological factors also influence the intensity of the discomfort the patient perceives after surgery. The outcome measure “symptom sum score” is a summary of this discomfort. Psychological factors covariate with pain threshold measures.34 Wallin et al also found that the three pain thresholds differed with respect to their associations with pain intensity and psychological factors.35 The association between CPT and the symptom sum score may support the assumption of a multifactorial etiology and the option to use specific sensory testing modalities of preoperative pain threshold to discriminate persons at risk of presenting more disabling postoperative symptoms after hysterectomy. Thus, our results support the need to consider that a blend of factors influences the pain thresholds, and emphasize the need for a biopsychosocial model when interpreting QST variables.
Our study indicates that PPT may not be valuable for predicting pain from visceral structures or uncomfortable symptoms after surgery. This agrees with the review by Sangesland et al from 2017 who found in several studies a significant association between PPT and pain intensity after musculoskeletal surgery but no significant association after visceral surgery.28 Partly in contrast, Petersen et al in 2021, investigating chronic postoperative pain, concluded that joint-related surgeries and abdominal and gynecological surgeries showed the strongest associations with QST.26 The different conclusions in the two reviews may be due to whether the patient cohorts have preoperative pain or not. The diverging results for the three pain thresholds in our study may be explained by various neuropathic pathways for the stimuli and the involvement of different tissues (all three stimuli affect the skin while PPT also includes underlying tissues such as muscles).36 Different channels on the nociceptors are also involved for the three pain modalities.37–44
Cut-Off Levels for Pain Thresholds
Clinical cut-off levels for deviant thresholds of PPT, HPT and CPT have not yet been established. Ahmad et al in 2014 used the median value as the cut-off level but did not explain the rationale behind this.30 This is not meaningful from a clinical point of view as it suggests that half of the population has low pain thresholds. This is also supported by our sensitivity analysis (Table 7). The median CPT in our population was 0°C and the median HPT was 48.1°C. These figures differ substantial from the 10.7°C and the 44.8°C for the median CPT and HPT in the Ahmad study. Thresholds differ between locations and since we used several and different locations unlike the other studies it is difficult to compare the data and no normative data are available from any of the locations we used. The sensitivity analysis revealed that a cut-off level at the 25- and 80-percentiles for HPT and CPT, respectively, would increase the number of significant postoperative recovery outcomes. However, from a clinical perspective it might be reasonable to use the 25- and 75-percentile levels as cut-offs.
Strengths and Limitations
The strengths were the large number of women included, the principal component analysis and validation of using the mean value of the four measuring points, and the sensitivity analysis of cut-off levels for clinical characterization of high and low pain thresholds for pressure, heat and cold. However, the factor analysis revealed a high internal consistency for the three thresholds, indicating that the measurements are reliable, and the results thus may be generalizable.
Conclusion
Deviant CPT was significantly associated with an adverse postoperative recovery including a high rating of postoperative pain intensity, a high sum score of symptoms, and high consumption of non-opioid analgesics. CPT may be useful in preoperative assessment of patients undergoing benign hysterectomy as has been shown recently to apply to other surgical procedures. This may give a new hint to plan individualized postoperative treatment according to the pathways associated with this specific pain modality. However, this needs to be confirmed in other studies, which should include a broader set of QST variables including temporal summation of pain and conditioned pain modulation.
Data Sharing Statement
Data are available upon reasonable request made to the corresponding author, and in agreement with Swedish legislation.
Acknowledgments
The authors thank all women who participated in this trial. We are deeply grateful for the committed work conducted by all in the POSTHYSTREC study group, in particular by the research nurses for their meticulous work and Eva-Britt Lind, RN, for teaching the research nurses in the pain threshold measuring.
The POSTHYSTREC Study Group consisted of members from five hospitals in the Southeast region of Sweden.
Linköping University Hospital: Preben Kjølhede, MD, PhD, Gunilla Sydsjö, PhD, Ninnie Borendal Wodlin, MD, PhD, Lena Nilsson, MD, PhD, Gulnara Kassymova, MD, Peter Lukas, MD, Björn Gerdle, MD, PhD, Lars Arendt-Nielsen, PhD, Dr.Med, Petra Langström, RN, Pernilla Nilsson, RN, Linda Shosholli, RN, Sofia Bergström, RN, and Åsa Rydmark Kersley, RN, MSc.
Vrinnevi Hospital, Norrköping: Leif Hidmark, MD, Anders Bolling, MD, Kristina Ekman, RNM, and Karin Granberg-Karlsson, RNM.
Ryhov Hospital, Jönköping: Laila Falknäs, MD, Maria Häggström, MD, Ewa Hermansson RNM.
Eksjö Highland Hospital: Tomaz Stypa, MD, PhD, Linda Myllimäki, MD, Iréne Johannesson, RNM, and Martina Ekeroth Wikander, RNM.
Värnamo Hospital: Christina Gunnervik, MD, Fatima Johansson, MD, Magnus Trofast, MD, Mari-Ann Andersson, RNM, and Carita Jacobsson, RN.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
The study was supported by grants from the Medical Research Council of Southeast Sweden (grant numbers FORSS-228581; FORSS-308471; FORSS-387681, and FORSS-482051) and from Region Östergötland Council (ALF grants RÖ-200641, RÖ-276871, RÖ-35651, RÖ-448391, RÖ-540551, RÖ-607891, RÖ-699021, RÖ-794531, RÖ-931528, RÖ-936208, and RÖ-968764). In addition, Linköping University contributed with unrestricted grants. The Danish National Research Foundation (DNRF121) supported the Center for Neuroplasticity and Pain (CNAP). The funders have not been involved in the study design, data collection, data analysis, and manuscript preparation or publication decisions.
Disclosure
Prof. Dr. Björn Gerdle reports grants from Vetenskapsrådet (Swedish research council), outside the submitted work. The authors report no conflicts of interest in this work.
References
1. Aarts JWN, Nieboer T, Johnson N, et al. Surgical approach to hysterectomy for benign gynaecological disease. Cochrane Database Syst Rev. 2015;8:CD003677. doi:10.1002/14651858.CD003677.pub5
2. Borendal Wodlin N, Nilsson L, Årestedt K, Kjølhede P. Mode of anesthesia and postoperative symptoms following abdominal hysterectomy in a fast-track setting. Acta Obstet Gynecol Scand. 2011;90(4):369–379. doi:10.1111/j.1600-0412.2010.01059.x
3. Kehlet H, Wilmore DW. Multimodal strategies to improve surgical outcome. Am J Surg. 2002;183(6):630–641. doi:10.1016/s0002-9610(02)00866-8
4. Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) society recommendations – part I. Gynecol Oncol. 2016;140:313–322. doi:10.1016/j.ygyno.2015.11.015
5. Nelson G, Altman AD, Nick A, et al. Guidelines for pre- and intra-operative care in gynecologic/oncology surgery: Enhanced Recovery After Surgery (ERAS®) society recommendations – part II. Gynecol Oncol. 2016;140:323–332. doi:10.1016/j.ygyno.2015.12.019
6. Björkström LM, Wodlin NB, Nilsson L, Kjølhede P. The impact of preoperative assessment and planning on the outcome of benign hysterectomy - a systematic review. Geburtshilfe Frauenheilkd. 2021;81(2):200–213. doi:10.1055/a-1263-0811
7. Oates M, Gath D. Psychological aspects of gynaecological surgery. Baillieres Clin Obstet Gynaecol. 1989;3(4):729–749. doi:10.1016/s0950-3552(89)80062-8
8. Persson P, Brynhildsen J, Kjølhede P. Short-term recovery after subtotal and total hysterectomy – a randomised clinical trial. BJOG. 2010;117(4):469–478. doi:10.1111/j.1471-0528.2009.02468.x
9. Kjølhede P, Borendal WN, Nilsson L, Wijma K. Impact of stress coping capacity on recovery from abdominal hysterectomy in a fast track program. BJOG. 2012;119(8):998–1007. doi:10.1111/j.1471-0528.2012.03342.x
10. Brandsborg B, Nikolajsen L, Hansen CT, Kehlet H, Jensen TS. Risk factors for chronic pain after hysterectomy: a nationwide questionnaire and database study. Anesthesiology. 2007;2106(5):1003–1012. doi:10.1097/01.anes.0000265161.39932.e8
11. Brandsborg B, Dueholm M, Nikolajsen L, Kehlet H, Jensen TS. A prospective study of risk factors for pain persisting 4 months after hysterectomy. Clin J Pain. 2009;25(4):263–268. doi:10.1097/AJP.0b013e31819655ca
12. Pavlin DJ, Sullivan MJL, Freund PR, Roesen K. Catastrophizing: a risk factor for postsurgical pain. Clin J Pain. 2005;21(1):83–90. doi:10.1097/00002508-200501000-00010
13. Aasvang EK, Brandsborg B, Christensen B, Jensen TS, Kehlet H. Neurophysiological characterization of postherniotomy pain. Pain. 2008;137(1):173–181. doi:10.1016/j.pain.2007.09.026
14. Aasvang EK, Gmaehle E, Hansen JB, et al. Predictive risk factors for persistent postherniotomy pain. Anesthesiology. 2010;112(4):957–969. doi:10.1097/ALN.0b013e3181d31ff8
15. Aasvang EK, Brandsborg B, Jensen TS, Kehlet H. Heterogeneous sensory processing in persistent postherniotomy pain. Pain. 2010;150(2):237–242. doi:10.1016/j.pain.2010.03.025
16. Fruhstorfer H, Lindblom U, Schmidt W. Method for quantitative estimation of thermal thresholds in patients. J Neurol Neurosurg Psychiatry. 1976;39(11):1071–1075. doi:10.1136/jnnp.39.11.1071
17. Arendt-Nielsen L, Yarnitsky D. Experimental and clinical applications of quantitative sensory testing applied to skin, muscles and viscera. J Pain. 2009;10(6):556–572. doi:10.1016/j.jpain.2009.02.002
18. Kassymova G, Sydsjö G, Borendal Wodlin N, Nilsson L, Kjølhede P. The effect of follow-up contact on recovery after benign hysterectomy: a randomized, single-blinded, four-arm, controlled multicenter trial. J Womens Health. 2020;30(6):872–881. doi:10.1089/jwh.2020.8752
19. Dindo D, Demartines N, Clavien PA. Classification of surgical complications. A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213. doi:10.1097/01.sla.0000133083.54934.ae
20. Rolke R, Baron R, Maier C, et al. Quantitative sensory testing in the German research network on neuropathic pain (DFNS): standardized protocol and reference values. Pain. 2006;123(3):231–243. doi:10.1016/j.pain.2006.01.041
21. Alkaissi A, Gunnarsson H, Johnsson V, Evertsson K, Ofenbartl L, Kalman S. Disturbing post-operative symptoms are not reduced by prophylactic antiemetic treatment in patients at high risk of post-operative nausea and vomiting. Acta Anaesthesiol Scand. 2004;48(6):761–771. doi:10.1111/j.0001-5172.2004.00403.x
22. World Health Organization. Defined Daily Doses (DDD). Available from: https://www.who.int/medicines/regulation/medicines-safety/toolkit_ddd/en/.
23. Uddin Z, MacDermid JC. Quantitative sensory testing in chronic musculoskeletal pain. Pain Med. 2015;17(9):1694–1703. doi:10.1093/pm/pnv105
24. Georgopoulos V, Akin-Akinyosoye K, Zhang W, McWilliams DF, Hendrick P, Walsh DA. Quantitative sensory testing and predicting outcomes for musculoskeletal pain, disability, and negative affect: a systematic review and meta-analysis. Pain. 2019;160(9):1920–1932. doi:10.1097/j.pain.0000000000001590
25. van Helmond N, Aarts HM, Timmerman H, et al. Is preoperative quantitative sensory testing related to persistent postsurgical pain? A systematic literature review. Anesth Analg. 2020;131(4):1146–1155. doi:10.1213/ANE.0000000000004871
26. Petersen KK, Vaegter HB, Stubhaug A, et al. The predictive value of quantitative sensory testing: a systematic review on chronic postoperative pain and the analgesic effect of pharmacological therapies in patients with chronic pain. Pain. 2021;62(1):31–44. doi:10.1097/j.pain.0000000000002019
27. Braun M, Bello C, Riva T, et al. Quantitative sensory testing to predict postoperative pain. Curr Pain Headache Rep. 2021;25(1):3. doi:10.1007/s11916-020-00920-5
28. Sangesland A, Støren C, Vaegter HB. Are preoperative experimental pain assessment correlated with pain outcome after surgery? A systematic review. Scand J Pain. 2017;15:44–52. doi:10.1016/j.sjpain.2016.12.002
29. Hsu YW, Somma J, Hung YC, Tsai PS, Yang CH, Chen CC. Predicting postoperative pain by preoperative pressure pain assessment. Anesthesiology. 2005;103(3):613–618. doi:10.1097/00000542-200509000-00026
30. Ahmad S, De Oliveira GS Jr, Bialek JM, McCarthy RJ. Thermal quantitative sensory testing to predict postoperative pain outcomes following gynecologic surgery. Pain Med. 2014;5(5):857–864. doi:10.1111/pme.12374
31. Brandsborg B, Dueholm M, Kehlet H, Jensen TS, Nikolajsen L. Mechanosensitivity before and after hysterectomy: a prospective study on the prediction of acute and chronic postoperative pain. Br J Anaesth. 2011;107(6):940–947. doi:10.1093/bja/aer264
32. Borendal Wodlin N, Nilsson L, Kjølhede P. Fast track abdominal hysterectomy. The impact of mode of anaesthesia on postoperative recovery – a randomised clinical trial. BJOG. 2011;118(3):299–308. doi:10.1111/j.1471-0528.2010.02697.x
33. Müller M, Bütikofer L, Andersen OK, et al. Cold pain hypersensitivity predicts trajectories of pain and disability after low back surgery: a prospective cohort study. Pain. 2021;162(1):184–194. doi:10.1097/j.pain.0000000000002006
34. Grundström H, Larsson B, Arendt-Nielsen L, Gerdle B, Kjølhede P. Pain catastrophizing is associated with pain thresholds for heat, cold and pressure in women with chronic pelvic pain. Scand J Pain. 2020;20(3):635–646. doi:10.1515/sjpain-2020-0015
35. Wallin M, Liedberg G, Börsbo B, Gerdle B. Thermal detection and pain thresholds but not pressure pain thresholds are correlated with psychological factors in women with chronic whiplash-associated pain. Clin J Pain. 2012;28(3):211–221. doi:10.1097/AJP.0b013e318226c3fd
36. Almeida TF, Roizenblatt S, Tufik S. Afferent pain pathways: a neuroanatomical review. Brain Res. 2004;1000(1–2):40–56. doi:10.1016/j.brainres.2003.10.073
37. Vandewauw I, De Clercq K, Mulier M, et al. A TRP channel trio mediates acute noxious heat sensing. Nature. 2018;555(7698):662–666. Erratum: Publisher Correction: A TRP channel trio mediates acute noxious heat sensing. Nature. 559 (7713):E7. doi:10.1038/nature26137
38. Crosson T, Roversi K, Balood M, et al. Profiling of how nociceptor neurons detect danger - new and old foes. J Intern Med. 2019;286(3):268–289. doi:10.1111/joim.12957
39. Khan A, Khan S, Kim YS. Insight into pain modulation: nociceptors sensitization and therapeutic targets. Curr Drug Targets. 2019;20(7):775–788. doi:10.2174/1389450120666190131114244
40. Weyer-Menkhoff I, Lotsch J. TRPA1 sensitization produces hyperalgesia to heat but not to cold stimuli in human volunteers. Clin J Pain. 2019;35(4):321–327. doi:10.1097/AJP.0000000000000677
41. Iftinca M, Altier C. The cool things to know about TRPM8! Channels. 2020;14(1):413–420. doi:10.1080/19336950.2020.1841419
42. Bamps D, Vriens J, de Hoon J, Voets T. TRP channel cooperation for nociception: therapeutic opportunities. Annu Rev Pharmacol Toxicol. 2021;61:655–677. doi:10.1146/annurev-pharmtox-010919-023238
43. Manolache A, Babes A, Babes RM. Mini-review: the nociceptive sensory functions of the polymodal receptor Transient Receptor Potential Ankyrin Type 1 (TRPA1). Neurosci Lett. 2021;764:136286. doi:10.1016/j.neulet.2021.136286
44. Yamaki S, Chau A, Gonzales L, McKemy DD. Nociceptive afferent phenotyping reveals that transient receptor potential ankyrin 1 promotes cold pain through neurogenic inflammation upstream of the neurotrophic factor receptor GFR-α3 and the menthol receptor transient receptor potential melastatin 8. Pain. 2021;162(2):609–618. doi:10.1097/j.pain.0000000000002043
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




