Back to Journals » International Journal of Nephrology and Renovascular Disease » Volume 16
Association Between Elevated Blood Eosinophils and Chronic Kidney Disease Progression: Analyses of a Large United States Electronic Health Records Database
Authors Kielar D, Jones AM, Wang X , Stirnadel-Farrant H, Katial RK, Bansal A, Garg M, Sharma C, Thakar S, Ye Q
Received 8 September 2023
Accepted for publication 9 December 2023
Published 21 December 2023 Volume 2023:16 Pages 269—280
DOI https://doi.org/10.2147/IJNRD.S431375
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Pravin Singhal
Danuta Kielar,1 Andrew M Jones,2 Xia Wang,3 Heide Stirnadel-Farrant,1 Rohit K Katial,4 Abhinav Bansal,5 Manu Garg,5 Chandrakant Sharma,5 Shubhankar Thakar,5 Qin Ye5
1BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK; 2Late-Stage Development, Respiratory & Immunology, BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, MD, USA; 3Data Science & AI, BioPharmaceuticals R&D, AstraZeneca, Gaithersburg, MD, USA; 4BioPharmaceuticals Medical, AstraZeneca, Gaithersburg, MD, USA; 5ZS, Philadelphia, PA, USA
Correspondence: Danuta Kielar, AstraZeneca, Academy House, 136 Hills Road, Cambridge, CB2 8PA, UK, Tel +44 7384905053, Email [email protected]
Background: Blood eosinophils can increase in response to infection, inflammation, and hypersensitivity reactions, yet their involvement in the progression of chronic kidney disease (CKD) is poorly understood. This study explores the relationship between blood eosinophils and CKD progression among patients in a real-world setting.
Methods: This retrospective study analyzed data obtained from the Optum® de-identified electronic health records dataset in the United States. Patients diagnosed with CKD stage 3 or 4 (International Classification of Diseases diagnosis code or estimated glomerular filtration rate [eGFR] < 60 mL/min) between January 2011 and March 2018 were included and followed until progression to the next CKD stage, death, or dropout. The primary objective of this study was to assess the relationship between blood eosinophil counts (bEOS) and CKD progression, adjusting for clinical and demographic features as well as known risk factors for CKD stages 3– 4. The primary outcomes were CKD progression and all-cause mortality.
Results: We found that high eosinophilic levels (bEOS ≥ 300 cells/μL) were associated with CKD progression from stage 3 to stages 4 or 5 (hazard ratio [HR] ranging from 1.30 to 1.50) and from stages 4 to 5 (HR ranging from 1.28 to 1.50). Among patients with CKD progression, those with blood eosinophils ≥ 300 cells/μL appeared to have a relatively lower eGFR, higher all-cause mortality, and reduced time to CKD progression and death than those with < 300 cells/μL. Factors including sex, race, hypertension, anemia, and treatments for cardiovascular and hematopoietic drugs were associated with CKD progression.
Conclusion: Elevated eosinophils may increase the risk for CKD progression. Larger studies are needed to assess whether the risk of mortality is increased among patients with elevated eosinophils.
Keywords: eosinophilic inflammation, renal insufficiency, glomerular filtration rate
Introduction
Eosinophils play a complex pathophysiological role in local and systemic inflammatory diseases, cancer, and thrombosis.1 Eosinophils exhibit effector functions, such as perpetuating inflammation, tissue repair, and possible antigen presentation to other immune cells.2,3 These complex interactions driven by activated eosinophils are known to play a pathogenic role in a range of inflammatory conditions, including hypersensitivity reactions, eosinophilic asthma, hypereosinophilia, eosinophilic granulomatosis with polyangiitis, and parasitic infections.4–6 Eosinophilic inflammation involves eosinophil recruitment and activation, allowing eosinophils to infiltrate into different tissues where they induce pathophysiological changes (eg, fibrosis and hypercoagulability), ultimately leading to tissue damage and impaired organ function.7,8
There is a growing interest in understanding the clinical correlation between circulating blood eosinophils and disease progression.4,9 For example, eosinophilia (>500 cells/µL) is an independent prognostic factor of long-term mortality and major adverse cardiovascular and cerebrovascular events in triple-vessel coronary artery disease.10,11 The role of eosinophilia and its association with kidney disease is poorly understood because of limited data obtained from smaller observational studies and/or specific patient cohorts (eg, cardiac and male).12–14 A study of US patients who underwent kidney biopsies previously demonstrated that peripheral eosinophilia in chronic kidney disease (CKD) patients was associated with a higher risk of progression to end-stage kidney disease (ESKD) compared with those who did not progress to end-stage disease after adjusting for a baseline estimated glomerular filtration rate, hypertension, race, and diabetes.12 In addition, patients with peripheral eosinophilia who progressed to ESKD had a higher presence of tissue eosinophil infiltration on biopsy specimens.12–14
We study the relationship between blood eosinophils and CKD progression using a large real-world US database. The primary objective of this study was to determine if elevated levels of blood eosinophils in stages 3 and 4 CKD patients contribute to CKD progression, all-cause mortality, and healthcare resource utilization (hospital admission and length of stay) compared with patients with normal blood eosinophils.
Methods
Study Design
A retrospective, observational study was conducted using electronic health records (EHR) obtained from the Pan-Therapeutic Optum® de-identified Electronic Health Record (PANTHER) database. We included adult (≥18 years) pre-dialysis patients with CKD stage 3 or 4 enrolled between January 1, 2011, and March 31, 2018. Criteria for CKD diagnosis consisted of either two International Classification of Diseases, 9th (ICD-9-CM) or 10th (ICD-10-CM) Revision diagnosis codes; two laboratory values for estimated glomerular filtration rate (eGFR) levels <60 mL/min captured at baseline; or a combination of one ICD code and one eGFR test value at least 90 days apart and up to 730 days apart. Patients were also required to have at least two eosinophil blood tests during each CKD episode and at least 1 year of continuous enrollment in the database’s prior index date. Full inclusion and exclusion criteria are provided in Table S1. The final analytical sample and details on sample size are shown in Figure S1. The specific codes used to build the study cohort are listed in Table S2.
Patients were followed until progression to the next stage, death, or dropout for the duration of data availability (follow-up). Patients were stratified into episodes, starting from the index date of a given CKD stage (3 or 4) until the next stage or end of data. Thereafter, a new index date and baseline were created for subsequent episodes. Patient episodes were further stratified by the blood eosinophil level into the following groups: <300 cells/µL (considered to be normal blood eosinophils for this study’s purpose), 300–499 cells/µL, 500–1499 cells/µL, and ≥1500 cells/µL.15 Patients having at least two blood eosinophil records during an episode were considered for the study. An approximation of the blood eosinophil level for each patient episode was made by taking the mean of the recorded blood eosinophil level across the length of respective patient episodes to offset the effect of fluctuations across the patient journey. An illustration of the study design is provided in Figure 1. Ethics approval from Institutional Review Boards was not required because this study utilized commercially available, anonymized administrative EHR data.
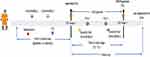 |
Figure 1 Study design. An illustrative patient journey with details of baseline, enrollment, follow-up, and assessment endpoints. Abbreviations: EHR, electronic health record; T1, time 1; T2, time 2. |
Data Collection
Patient characteristics, demographics, and comorbidities (including the Charlson Comorbidity Index [CCI])16 were assessed at baseline. Medications (Table S3) were assessed at baseline and first diagnosis of CKD (stages 3 and 4 or eGFR <60 mL/min). Estimated GFR levels were recorded during the follow-up for assessment of the CKD stage. Body mass index, smoking status, blood eosinophils, and other blood tests (eg, hemoglobin and serum bicarbonate) were noted in the follow-up until progression to the next stage.
Outcomes
The primary outcomes of the study were time to CKD progression and all-cause mortality. CKD progression was assessed using ICD-9-CM/ICD-10-CM diagnosis codes and eGFR over the follow-up period. The secondary outcome was healthcare resource utilization, defined as the number of inpatient days (including length of stay) and outpatient/office/emergency room visits.
Statistical Methods
Patients were stratified according to the starting CKD stage and grouped into one of the following stage-change categories: 3 to 3, 3 to 4, 3 to 5, 4 to 4, or 4 to 5. In cases of multiple eGFR tests conducted within 60 days, the 60-day mean eGFR value was utilized. In addition, any eGFR value found within 30 days of an ICD code for a CKD stage was superseded by the ICD diagnosis. Blood eosinophil values were averaged for each patient episode, with the average used to place the episode in the appropriate eosinophil groups as defined above. For each patient episode, CKD progression was measured by the time taken to transition between stages.
Categorical variables are presented as counts and percentages. Continuous variables were summarized using the means, medians, standard deviations (SD), quartiles (Q), and ranges. Descriptive statistics were provided for the overall characteristics of the study population at baseline. A multivariate Cox proportional hazards regression model was used to assess the impact of each variable on CKD progression expressed as a hazard ratio (HR) with 95% CIs while simultaneously adjusting for remaining variables. Kaplan–Meier curves were plotted to determine median survival estimates along with time-dependent outcome probabilities across various blood eosinophil groups. Statistics were performed using R studio (Version 1.1.456).
Results
A total of 383,708 cases were identified in which patients with CKD stages 3 and 4 either remained in the same stage or progressed to stages 4 or 5 throughout the study period (Figure S1). Of these cases, 68.4% (262,636) occurred in patients over age 65 and 66.6% (255,643) occurred in females. Approximately 77.5% (297,324) of episodes occurred in Caucasians, with 12.2% (46,722) and 2.4% (9236) noted in African Americans and Asians, respectively. Hypertension and diabetes were frequently observed comorbidities, reported in 56.0% (214,802) and 34.0% (130,282) of episodes, respectively. Common medications (Table S3) in CKD stage 3 and 4 episodes were antidiabetics (eg, sulfonylureas and biguanides) and statins, present in 34.0% (130,282) and 25.6% (98,219) of episodes, respectively. Medicare (167,292; 43.6%) and commercial (97,497; 25.4%) insurance provided coverage for the majority of the CKD stage 3 and 4 episodes. Patient characteristics expressed by CKD stages 3 and 4 and average eosinophil categories are provided in Table 1.
 |
Table 1 Characteristics of Patients by Episodes and Average Blood Eosinophil Values |
Kidney function (eGFR) was analyzed across blood eosinophil levels in patients who progressed from CKD stages 3 and 4 (Table S4). Among patients whose CKD progressed from stages 3 and 4, those with normal blood eosinophils had a higher mean eGFR than patients with blood eosinophils ≥300 cells/µL. Among patients with stage 3 CKD who progressed to stages 4 or 5 (hereafter indicated as 4/5), the average eGFR was 33 mL/min in patients with normal blood eosinophils and 31 mL/min in those with ≥300 cells/µL. Similarly, among patients with stage 4 CKD who progressed to stage 5, the average eGFR was 16 mL/min higher with normal blood eosinophils and 15 mL/min in those with ≥300 cells/µL. The average time to CKD progression was 477 days in CKD stage 3 patients with normal blood eosinophils compared with patients with ≥300 cells/µL (ranging from 396 to 453 days). For stage 4 patients, the average time to CKD progression was 422 days in those with normal blood eosinophils than in those with ≥300 cells/µL (ranging from 320 to 382 days). Complete patient episode characteristics expressed by all blood eosinophil groups are provided in Table S4. Together, patients with blood eosinophils ≥300 cells/µL progressed more rapidly across the CKD stages analyzed.
Based on Kaplan–Meier analysis, patients with normal blood eosinophil levels progress slower from CKD stage 4 to 5 over 3 years compared to those with levels over 300 cells/µL (Figure 2). The results of Cox proportional hazards regression models are presented in Table 2. Patients with blood eosinophils of 300–499 cells/µL were more likely to progress from stage 3 to 4/5 (HR 1.30; p<0.0001) and from stage 4 to 5 (HR 1.28; p<0.0001) compared with patients with normal blood eosinophils. Patients with blood eosinophils between 500 and 1499 cells/µL were also associated with an increased probability of CKD progression from stage 3 to stages 4/5 (HR 1.37; p<0.0001) and from stage 4 to 5 (HR 1.38; p<0.0001).
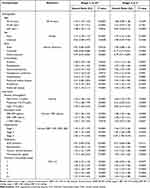 |
Table 2 Factors for CKD Progression |
The Cox proportional hazards regression model also identified factors associated with a greater risk of CKD progression (Table 2). The increased severity of anemia was associated with an increased likelihood of CKD progression: patients with mild, moderate, and severe anemia were more likely to progress from CKD stage 3 to 4/5 (HR 1.50, 1.73, and 1.99, respectively) and from stage 4 to 5 (HR 1.64, 2.16, and 2.90, respectively) compared with patients with normal hemoglobin levels (Table 2). Hypertension also presented a greater risk of CKD progression: patients with hypertension stages 1–3 were more likely to progress from stage 3 to 4/5 (HR 1.32, 1.87, and 2.96, respectively) and from stage 4 to 5 (HR 1.46, 1.87, and 4.30, respectively) compared with normotensive patients. Polycystic kidney disease, Goodpasture syndrome, glomerulonephritis, proteinuria, and sickle cell disease increased the likelihood of patients progressing from stage 3 to 4/5 and from stage 4 to 5. Moreover, CCI scores of 1, 2, 3, 4, and ≥5 were associated with HRs of 1.05, 1.28, 1.42, 1.53, and 1.57, respectively, for CKD progression from stage 3 to stages 4/5 using a CCI of 0 as reference. Patients with prescribed medications associated with hypertension and anemia, such as calcium channel blockers, beta-blockers, and hematopoietic agents, were more likely to progress from stage 3 to stages 4/5 as well as from stage 4 to 5. Patients aged ≥65 (HR 1.08) were less likely to progress from stage 3 to stages 4/5 as compared with those aged 45–54 (HR 1.37) and 55–64 (HR 1.34) when using young adult patients (18–44 years) as a reference. Males had a greater likelihood of progressing from stage 3 to stages 4/5 (HR 1.12) and from stage 4 to 5 (HR 1.37). Caucasians and other races exhibited a lower risk for CKD progression using African Americans as a reference for progression from stage 3 to 4/5 (HR 0.85 and 0.79, respectively) and from stage 4 to 5 (HR 0.77 and 0.79, respectively).
In patients with no CKD progression, mortality was similar between patients with normal blood eosinophils and those with 500–1499 cells/µL for both stage 3 (20.0% vs 19.3%) and stage 4 (25.0% vs 25.8%) (Table S4). However, there was a numerical increase in mortality for patients with blood eosinophils ≥300 cells/µL that progressed from CKD stage 3 to 4/5 (300–499 cells/µL: 24.1%; 500–1499 cells/µL: 24.1%; and ≥1500 cells/µL: 28.3%) and 4 to 5 (300–499 cells/µL: 24.3%; 500–1499 cells/µL: 26.2%; and ≥1500 cells/µL: 23.8%) compared with patients with normal blood eosinophils that progressed (stage 3 to 4/5, 22.6%; stage 4 to 5, 22.9%). A shorter, non-significant mean time to death was noted in patients with 500–1499 cells/µL that progressed from stage 4 (21 days) compared with patients with normal blood eosinophils.
Healthcare resource utilization parameters expressed by CKD stages 3 and 4 and eosinophil groups are provided in Table 3. Monthly outpatient visits ranged from 0.91 to 0.96 across eosinophil groups in stage 3 CKD patients who did not progress and from 1.03 to 1.33 in patients who progressed from stage 3 to 4/5. Inpatient admissions were notably higher for patients who progressed from stage 4 to 5 compared with those progressing from stage 3 to 4/5; these patients were more likely to have a length of stay of ≥3 days.
 |
Table 3 Healthcare Resource Utilization |
Discussion
We evaluated the rate of CKD progression in patients with different bEOS levels in a large US EHR database. Our data suggest an association of CKD progression from stages 3 and 4 in patients with blood eosinophils ≥300 cells/μL. Higher blood eosinophils also appeared to be associated with a shorter time to CKD progression. CKD progression was associated with a greater number of outpatient visits, however patients with high blood eosinophils had a greater number of inpatient days. Larger studies are needed to confirm the association between elevated blood eosinophil levels and mortality.
Elevated blood eosinophils have been associated with an increased risk of kidney pathology in specific patient populations.12–14 One study reported 15.9-fold greater adjusted odds for progression to end-stage renal disease (ESRD) in patients with peripheral eosinophilia (>4%) compared with those without peripheral eosinophilia, with the fastest decline in kidney function observed in patients with ≥10% eosinophilia.12 Similarly, CKD patients with spikes (>3%) in blood eosinophils exhibited a 58% higher risk for the composite endpoint of ESRD or death than non-CKD patients.14 The present study complements these findings with a larger sample in a real-world setting and suggests that blood eosinophils ≥300 cells/µL increase the risk for CKD progression. The effects reported in the above studies were larger compared with this study and may be attributed to a combination of small sample size, risk prediction for severe endpoints typically associated with blood eosinophils >1500 cell/μL17 (eg, ESRD or death), distribution of co-morbidities,18 and homogeneity in groups. However, the present study was performed using a US database; thus, the effects observed in our study are likely more representative of average CKD patients in the US.
This study also identified patient characteristics associated with higher CKD progression from stages 3 and 4. In our analysis, older patients had a lower likelihood of CKD progression. Aging is associated with nephron atrophy, impaired glomerular filtration, and an overall reduction in nephron function, which can occur in the absence of kidney pathology.19,20 Thus, lower eGFR values may be less indicative of pathology in aged patients. While females are at greater risk for developing CKD,18 males were at greater risk of CKD progression in this study. This finding was similar to a meta-analysis of four studies of 7724 patients that investigated the effects of male sex on CKD progression, which found that males had a 37% greater risk of progression from late-stage CKD to ESRD.21 African Americans were at greater risk of CKD progression compared with other races in this analysis. This finding strengthens a similar conclusion drawn by a scoping review of racial disparities in CKD that was based on smaller studies.22 The review attributed the risk to poorer kidney function than Caucasians, as well as to cardiovascular, diabetic, and genetic (apolipoprotein E) differences.22
In this study, patients who had prescriptions for cardiovascular (calcium channel blockers and beta-blockers) and hematopoietic drugs were more likely to progress from CKD stages 3 and 4. Patients with hypertension, anemia, and diabetes had a greater risk for CKD progression according to disease severity, which was consistent with previous studies.21,23 Patients who had never smoked or had quit smoking were 5–10% less likely to progress from stages 3 and 4. Finally, greater CCI scores were associated with a greater risk of CKD progression. Taken together, comorbid conditions, especially those that make up metabolic syndrome, increase the risk of CKD progression. Importantly, the agreement of connected measures within this study (eg, increased risk of CKD progression in patients with hypertension and antihypertensive prescriptions), as well as with the existing literature, provides a greater level of confidence in our findings of an association between elevated blood eosinophils and risk of CKD progression.
There was a general trend for greater healthcare resource utilization in CKD patients with blood eosinophils ≥300 cells/µL compared with patients with normal blood eosinophils. Specifically, blood eosinophils ≥300 cells/µL appeared to be associated with more inpatient days in the progression period; however, there was substantial variability across blood eosinophil groups. More research is needed to fully investigate the impact of blood eosinophils and CKD progression on healthcare resource utilization.
This analysis describes the relationship between blood eosinophils and the progression of CKD as well as associated risk factors. We can speculate upon the role of eosinophils in increasing the risk of progression, but a clear mechanism for eosinophil-mediated CKD progression has not been elucidated despite numerous publications describing increased morbidity with elevated blood eosinophils in CKD.24–26 It is intriguing to consider the role of eosinophil-depleting biologics, such as benralizumab, in hopes of curtailing the progression of CKD in the setting of elevated blood eosinophils, but this requires further research. Mechanistic studies using animal models are necessary to characterize pathophysiological roles for eosinophils as related to CKD progression.
Strengths and Limitations
The Optum PANTHER EHR database contains medical diagnoses and procedures, medications, patient and provider characteristics, laboratory values, vital signs, provider notes, and health-care costs via an integrated insurance claims database, which increases the reliability of data for projection to the general US population. Moreover, this study utilized a long period of follow-up, which enabled the monitoring of disease progression over time. Limitations included retrospective examination of EHR to assess the risk of CKD progression according to patient and/or disease characteristics. The veracity of results depends on the accuracy of administrative coding, from which errors can arise. We were unable to differentiate between primary and secondary eosinophilia because of available data, and our sample size for the analysis of healthcare resource utilization was limited. Because of the lack of blood eosinophil records in the baseline period (12 months) of most patient episodes, the approximation of blood eosinophil levels was made using the records in the follow-up period. There was also the potential for incomplete capture of mortality data through electronic health records.
Conclusions
This large real-world study of a US EHR database suggests that higher blood eosinophil levels (≥300 cells/µL) may contribute to the increased risk of CKD progression from stages 3 and 4, independent of known risk factors. This study also identified factors contributing to the risk of CKD progression, which included sex, race, hypertension, anemia, and prescriptions for cardiovascular and hematopoietic drugs. Larger studies are needed to assess whether elevated eosinophils are associated with poor CKD outcomes and if eosinophil-depleting therapies provide a benefit in this patient population.
Abbreviations
CCI, Charlson Comorbidity Index; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; EHR, electronic health records; EID, eosinophilic immune dysfunction; ESRD, end-stage renal disease; HR, hazard ratio; ICD-9-CM, International Classification of Diseases, 9th Revision; ICD-10-CM, International Classification of Diseases, 10th Revision.
Data Sharing Statement
The data that support the findings of this study are available from Optum®, but restrictions apply to the availability of these data, which were used under license for the current study. They may be obtained in accordance with AstraZeneca’s data sharing policy described at https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure and with permission from Optum®.
Ethics Approval and Consent to Participate
This study was conducted in accordance with the Declaration of Helsinki, ICH GCPs, and the Guidelines for Good Pharmacoepidemiology Practice (GPP) published by the International Society of Pharmacoepidemiology (ISPE) and the applicable legislation on non-interventional studies and observational studies. All data within the US administrative database, Optum™ PANTHER, is certified as de-identified by an independent statistical expert following HIPAA statistical de-identification rules and managed according to Optum® customer data use agreements. Institutional Review Board approval was not required because the data is exempt under the United States Code of Federal Regulations (45 CFR 46.101(b) (4)).
Acknowledgments
The authors would like to acknowledge Superior Medical Experts for editorial assistance.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded by AstraZeneca. In collaboration with ZS Associates, AstraZeneca was involved in the study design, interpretation of data, review of the manuscript, and decision to submit for publication. All authors had full access to the data and shared responsibility for the decision to submit the article for publication.
Disclosure
Danuta Kielar, Andrew M. Jones, Xia Wang, Heide Stirnadel-Farrant, and Rohit K. Katial are or were employed by AstraZeneca at the time of study conduct and may own stock/stock options. Manu Garg, Chandrakant Sharma, Abhinav Bansal, Shubhankar Thakar, and Qin Ye are consultants from ZS, which received funding from AstraZeneca to conduct this study.
References
1. Ramirez GA, Yacoub M-R, Ripa M, et al. Eosinophils from physiology to disease: a comprehensive review. Biomed Res Int 2018;2018:1–28. doi:10.1155/2018/9095275
2. Niccoli G, Ferrante G, Cosentino N, et al. Eosinophil cationic protein: a new biomarker of coronary atherosclerosis. Atherosclerosis. 2010;211(2):606–611. doi:10.1016/j.atherosclerosis.2010.02.038
3. Lacy P, Moqbel R. Immune effector functions of eosinophils in allergic airway inflammation. Curr Opin Allergy Clin Immunol. 2001;1(1):79–84. doi:10.1097/00130832-200102000-00014
4. Furuta GT, Atkins FD, Lee NA, et al. Changing roles of eosinophils in health and disease. Ann Allergy Asthma Immunol. 2014;113(1):3–8. doi:10.1016/j.anai.2014.04.002
5. Di Stefano F, Amoroso A. Clinical approach to the patient with blood eosinophilia. Eur Ann Allergy Clin Immunol. 2005;37(10):380–386.
6. Liao W, Long H, Chang CC, et al. The eosinophil in health and disease: from bench to bedside and back. Clin Rev Allergy Immunol. 2016;50(2):125–139. doi:10.1007/s12016-015-8507-6
7. Jacobsen EA, Jackson DJ, Heffler E, et al. Eosinophil knockout humans: uncovering the role of eosinophils through eosinophil-directed biological therapies. Annu Rev Immunol. 2021;39(1):719–757. doi:10.1146/annurev-immunol-093019-125918
8. Akuthota P, Weller PF. Spectrum of eosinophilic end-organ manifestations. Immunol Allergy Clin North Am. 2015;35(3):403–411. doi:10.1016/j.iac.2015.04.002
9. Hou L, Lloyd-Jones DM, Ning H, et al. White blood cell count in young adulthood and coronary artery calcification in early middle age: coronary artery risk development in young adults (cardia) study. Eur J Epidemiol. 2013;28(9):735–742. doi:10.1007/s10654-013-9842-7
10. Zhao X, Jiang L, Xu L, et al. Predictive value of in-hospital white blood cell count in Chinese patients with triple-vessel coronary disease. Eur J Prev Cardiol. 2019;26(8):872–882. doi:10.1177/2047487319826398
11. Dembic M, Hedley PL, Torp-Pedersen C, et al. Pregnancy-associated plasma protein-A (papp-A) and the proform of the eosinophil major basic protein (prombp) are associated with increased risk of death in heart failure patients. Scand J Clin Lab Invest. 2017;77(5):352–357. doi:10.1080/00365513.2017.1325926
12. Tariq A, Okamato K, Tariq A, et al. Eosinophilia and risk of incident end stage kidney disease. BMC Nephrol. 2020;21(1):14. doi:10.1186/s12882-020-1685-3
13. Ishii R, Fujita S, Kizawa S, et al. Association between absolute blood eosinophil count and CKD stages among cardiac patients. Heart Vessels. 2016;31(2):198–205. doi:10.1007/s00380-014-0590-8
14. Agarwal R, Light RP. Patterns and prognostic value of total and differential leukocyte count in chronic kidney disease. Clin J Am Soc Nephrol. 2011;6(6):1393–1399. doi:10.2215/CJN.10521110
15. Yilmaz I, Turk M. What should be the cutoff value of blood eosinophilia as a predictor of inhaled corticosteroid responsiveness in patients with chronic obstructive pulmonary disease? Am J Respir Crit Care Med. 2017;196(9):1229–1230. doi:10.1164/rccm.201705-0892LE
16. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
17. Kuang FL. Approach to patients with eosinophilia. Med Clin North Am. 2020;104(1):1–14. doi:10.1016/j.mcna.2019.08.005
18. Hill NR, Fatoba ST, Oke JL, et al. Global prevalence of chronic kidney disease - A systematic review and meta-analysis. PLoS One. 2016;11(7):e0158765. doi:10.1371/journal.pone.0158765
19. Romagnani P, Remuzzi G, Glassock R, et al. Chronic kidney disease. Nat Rev Dis Primers. 2017;3:17088. doi:10.1038/nrdp.2017.88
20. Mallappallil M, Friedman EA, Delano BG, et al. Chronic kidney disease in the elderly: evaluation and management. Clin Pract. 2014;11(5):525–535. doi:10.2217/cpr.14.46
21. Tsai WC, Wu HY, Peng YS, et al. Risk factors for development and progression of chronic kidney disease: a systematic review and exploratory meta-analysis. Medicine. 2016;95(11):e3013. doi:10.1097/MD.0000000000003013
22. Hounkpatin HO, Fraser SDS, Honney R, et al. Ethnic minority disparities in progression and mortality of pre-dialysis chronic kidney disease: a systematic scoping review. BMC Nephrol. 2020;21(1):217. doi:10.1186/s12882-020-01852-3
23. McClellan WM, Flanders WD. Risk factors for progressive chronic kidney disease. J Am Soc Nephrol. 2003;14(7 Suppl 2):S65–S70. doi:10.1097/01.ASN.0000070147.10399.9E
24. Gauckler P, Shin JI, Mayer G, et al. Eosinophilia and kidney disease: more than just an incidental finding? J Clin Med. 2018;7(12):529. doi:10.3390/jcm7120529
25. Nagata M, Nakagome K, Soma T. Mechanisms of eosinophilic inflammation. Asia Pac Allergy. 2020;10(2):e14. doi:10.5415/apallergy.2020.10.e14
26. Mihai S, Codrici E, Popescu ID, et al. Inflammation-related mechanisms in chronic kidney disease prediction, progression, and outcome. J Immunol Res. 2018;2018:2180373. doi:10.1155/2018/2180373
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

