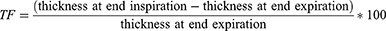Back to Journals » Journal of Multidisciplinary Healthcare » Volume 16
Assessment Tools for Pulmonary Involvement in Patients with Ankylosing Spondylitis: Is Diaphragmatic Ultrasonography Correlated to Spirometry?
Authors Dhahri R, Mejri I , Ghram A, Dghaies A, Slouma M, Boussaid S, Metoui L, Gharsallah I, Ayed K , Moatemri Z, Farahat RA , AlHamdani A , Dergaa I
Received 11 October 2022
Accepted for publication 23 December 2022
Published 11 January 2023 Volume 2023:16 Pages 51—61
DOI https://doi.org/10.2147/JMDH.S393061
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Rim Dhahri,1,2,* Islam Mejri,2,3,* Amine Ghram,3– 5 Abir Dghaies,1,2 Marwa Slouma,1,2 Soumaya Boussaid,2,4,6 Leila Metoui,1,2 Imen Gharsallah,1,2 Khadija Ayed,2,7 Zied Moatemri,2,3 Ramadan Abdelmoez Farahat,8,* Ahmad AlHamdani,9,* Ismail Dergaa9– 11,*
1Department of Rheumatology, Military Hospital of Instruction, Tunis, Tunisia; 2University of Tunis el Manar, Faculty of Medicine of Tunis, Tunis, Tunisia; 3Department of Pneumology, Military Hospital of instruction, Tunis, Tunisia; 4Healthy Living for Pandemic Event Protection (HL-PIVOT) Network, Chicago, IL, USA; 5Research Laboratory “Heart Failure, LR12SP09”, Hospital Farhat HACHED of Sousse, Sousse, Tunisia; 6Department of Rheumatology, Rabta Hospital, Tunis, Tunisia; 7Aviation Medicine Center of Expertise, Tunis, Tunisia; 8Faculty of Medicine, Kafrelsheikh University, Kafrelsheikh, Egypt; 9Primary Health Care Corporation (PHCC), Doha, Qatar; 10Research Unit Physical Activity, Sport, and Health, UR18JS01, National Observatory of Sport, Tunis, Tunisia; 11High Institute of Sport and Physical Education, University of Sfax, Sfax, Tunisia
*These authors contributed equally to this work
Correspondence: Ismail Dergaa, Primary Health Care Corporation (PHCC), Doha, Qatar, Email [email protected]; [email protected]
Objective: Spondyloarthritis (SpA) is a chronic inflammatory rheumatic disease that can lead to spinal ankylosis and consequently, restrictive pulmonary dysfunction. Thus, the present study aimed to assess the accuracy of diaphragm ultrasound compared to spirometry in the screening of restrictive pulmonary disorders in radiographic SpA patients.
Methods: We conducted a cross-sectional study of 50 patients with radiographic SpA, over six months. Sociodemographic data, clinical characteristics of the disease, as well as biological, radiological, and therapeutic data, were collected. Spirometry and diaphragm ultrasound were performed.
Results: The mean age of the study participants (N= 50) was 42.7± 11 years [range: 25– 66] with male predominance (N= 41). Spirometry showed a restrictive disorder in 32% of cases. The mean chest expansion (CE) value was 3.9± 1.81cm [range: 1– 9] with a median of 4 cm. A pathological value (< 5cm) was observed in 72% of cases. A significant positive correlation was found between the right inspiratory diaphragmatic thickness and forced vital capacity (FVC) (r= 0.36; p = 0.02) and the supine FVC (r=0.29; p = 0.04). The left inspiratory diaphragmatic thickness was correlated with the percentage of the FVC decrease (r= 0.35; p = 0.01) defined as the percentage of difference between FVC and supine FVC. The right expiratory diaphragmatic thickness was associated with the FVC (r=0.32; p = 0.02). A significant positive correlation was found between the CE and the presence of B lines (r=0.32; p = 0.02), but not between the CE and the FVC.
Conclusion: The present study showed that diaphragm ultrasonography is correlated with spirometric findings in radiographic SpA patients. Further studies are required to assess its reliability, specificity, and sensitivity in this pathology.
Keywords: spondyloarthritis, ultrasonography, diaphragm, restrictive pulmonary disorder, spirometry
Introduction
Spondyloarthritis (SpA) is a heterogeneous group of chronic rheumatic diseases characterized by inflammation of the spine and sacroiliac joints.1 Radiographic SpA is one common form of SpA, affecting mainly the axial skeleton, causing inflammatory back pain and resulting in varying degrees of structural and functional impairment. Spinal ankylosis can lead to respiratory complications including restrictive pulmonary disease induced by chest wall excursion limitations and/or interstitial lung disease, both of which can cause serious impairment.2,3 Inflammation in the thoracic vertebrae and costovertebral joints leads to gradual fusion and ossification of the joints, resulting in increased dorsal kyphosis and rigidity of the thorax.1,4 Furthermore, the expansion of the rib cage gradually decreases, leading to an atrophy of the intercostal muscles.5 Thus, breathing becomes mainly diaphragmatic, resulting in mechanical limitations and reduced lung volumes.
In fact, the most prevalent pattern of pulmonary dysfunction is restrictive ventilatory impairment.6 The gold-standard definition of restrictive pulmonary disease requires measurement of total lung capacity (TLC). Since measurement of TLC may not be available for all clinicians, spirometry as an effective test to exclude a restrictive pattern, can be performed.7
Indeed, the pulmonary involvement in radiographic SpA patients can be evaluated by spirometry to measure lung volumes,5 with a sensitivity of 60%. Despite its importance, spirometry detects restrictive involvement but cannot determine whether the restriction is due to chest wall limitations or interstitial lung disease.7,8 Because chest expansion is limited in patients with SpA, ventilation becomes more dependent on the diaphragm, and respiration becomes compensated by the diaphragm, especially during hyperventilation.9 Hence, better be to assess the diaphragmatic involvement separately. In this context, Ultrasonography (US) is consistent. In fact, US has been increasingly used in respiratory medicine. US offers many advantages: it is easy to be performed, usable at the bedside of the patient, and without irradiation. It also allows studying the morphological and dynamic aspects of each hemidiaphragm and the thoracic and sub-diaphragmatic environment. Its field of exploration is extremely wide and the results of the examination are immediately available.10 Recent studies have evaluated the reliability of US in assessing sagittal spine mobility, chest expansion,11 and diaphragmatic motion9 in radiographic SpA patients compared with controls. To the best of our knowledge, no study has evaluated the reliability of US in assessing the diaphragm involvement in ventilatory mechanics impairment in radiographic SpA patients and its correlation with the disease’s activity, yet. Therefore, we aimed to determine the accuracy of diaphragm US compared to spirometry in screening pulmonary disorders in patients with radiographic SpA and to assess the correlation between activity of radiographic SpA, spirometric and US findings.
Methods
Study Design and Patient Selection
We conducted a simple blind cross-sectional study from August 2020 to January 2021 in the department of rheumatology of the military hospital of Tunis. A sample size of 48 patients was required to achieve statistical significance (power: 0.8; alpha: 0.05), calculated using a predictive formula. We included 50 patients aged over 18, with a diagnosis of radiographic SpA according to Assessment of Spondyloarthritis International Society (ASAS) criteria12 with no history of a known chronic pulmonary disease. All participants were informed about the purpose of the study. The non-inclusion criteria were an anterior respiratory disease such as interstitial lung disease or chronic obstructive pulmonary disease. We excluded non-consenting patients and those with an incomplete assessment (spirometry and diaphragmatic ultrasound). All patients gave their written consent before recruitment. The study procedure was in accordance with the Declaration of Helsinki and was approved by the Military Hospital Research Ethics Committee with the number: (ID 11/21/CLPP).
Clinical Assessment
Patients’ demographic, clinical, and therapeutic features (age, medical history, smoking status, disease duration) were collected. Anthropometric measures were evaluated by the body mass index (BMI) in addition to chest expansion (CE) metering and Bath Ankylosing Spondylitis Metrology Index (BASMI).13
The CE was measured with a tape measure placed around the chest at the xiphoid process level. The difference (cm) in the chest circumference between maximum inspiration and expiration was noted (best of two attempts).14 The lower normal limit of CE was set to 5 cm.15
Disease activity was assessed by inflammatory markers, including the C-reactive protein (CRP) (mg/l) and erythrocyte sedimentation rate (ESR) (mm/h)), the Ankylosing spondylitis Disease Activity Score (ASDAS)-(CRP),16 and Bath Ankylosing Spondylitis Disease Activity Index (BASDAI).17 Patient-reported physical function was measured with the Bath Ankylosing Spondylitis Functional Index (BASFI).18 Radiological scoring was assessed by the Bath Ankylosing Spondylitis Radiology Index (BASRI).19
Data Related to Pulmonary Involvement
Respiratory symptoms (chronic cough, dyspnea) were noted. Chest X-ray was performed on all included patients.
Pulmonary Function Tests (PFT)
All participants underwent a PFT, evaluated by a spirometer (Spida 5) as follows:
- FVC (Forced Vital Capacity): is the maximal volume of air delivered during an expiration made as forcefully and completely as possible starting from full inspiration; expressed in liters.
- FEV1 (Forced Expiratory Volume in one second): is the volume, expressed in liters, delivered in the first second of the FVC maneuver.
- FEV1/FVC%: is the absolute ratio; derived from observed values (not percent predicted) (liters/liters×100). Primarily used in the diagnostics of obstructive ventilator disease.
- The FVC in the supine position was also performed. Indeed, the supine position has an impact on diaphragmatic function because of the increase in intra-abdominal pressure limiting the movement of the diaphragm, which leads to a decrease in lung volumes and FVC. This decrease is also due to the compression of the ribs by the bodyweight preventing full thoracic expansion.20
Patients were categorized as having a restrictive ventilatory pattern if FVC<80% with FEV1/FVC≥70%.21,22 The restriction is mild (FEV1>70%), moderate (FEV1 between 50 and 69%), or severe (FEV1 ≤ 49%).23 The percentage of decrease in FVC in supine (Δ FVC) was calculated using the formula: [(FVC- FVC supine)/FVC] *100. Δ FVC was considered significant under physiological conditions if it was greater than 7%.24
US Measurements
An experienced investigator carried out US examinations using a Doppler echocardiography (SONON) equipped with two probes connected to a tablet via Wi-Fi.
Search for B Lines
A low-frequency convex probe (3.5 MHz): for the search for B-lines and measurement of diaphragmatic excursion (DE) with the patient in a sitting position. These lines correspond to a hydroaeric comet-tail artifact, hyperechoic, arising from the pleural line. Their presence is a sign of interstitial disorder.25,26 However, B-lines <3/field of view were considered normal.25,26
Diaphragmatic Measurements
They were performed on the patients in a sitting position with the probe facing the thoraco-abdominal transition zone.27 This zone corresponds to a linear image created by the sudden rupture of echogenicity between the lung and the underlying organs.10
A High-Frequency Linear Probe (7.5 MHz)
For the measurement of the diaphragmatic thickening (DT) between inspiration and expiration with the patient in the supine position.
Measurement of the Diaphragmatic Excursion
The diaphragmatic excursion was measured for both hemidiaphragm (right diaphragmatic excursion (RDE) and left diaphragmatic excursion (LDE)) in B mode using a low-frequency probe (3.5 MHz). It corresponds to the maximum distance traveled by the diaphragm between forced inspiration and forced expiration, expressed in millimeters (mm). It was considered abnormal if the DE was <47mm in men and <36mm in women.28
Measurement of the Diaphragmatic Thickness (Tdi)
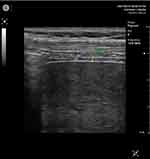
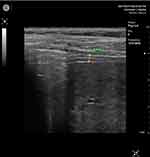
The patient was in a supine position with flexed legs and hands under the head. In intercostal incidence, the probe was placed at the level of the 7th, 8th, or 9th intercostal space, on the anterior axillary line. Inspiratory and expiratory diaphragmatic thickness were measured for both hemidiaphragm: diaphragmatic thickness at end inspiration (tdi-insp) and end-expiration (tdi-ex) (Figures 1 and 2).
To assess atrophy and contraction of the diaphragm, it is necessary to evaluate the thickness and thickening fraction (TF)27 at the end of forced inspiration (tdi-insp) and the end of forced expiration (tdi-exp). That was made for both hemi-diaphragms: Right diaphragmatic thickness at the end of forced inspiration (Right tdi-insp), Left diaphragmatic thickness at the end of forced inspiration (Left tdi-insp), Right diaphragmatic thickness at the end of forced expiration (Right tdi-exp), and Left diaphragmatic thickness at the end of forced expiration (Left tdi-exp). We used a high-frequency probe (7.5 MHZ) in B mode.
The tdi-exp provides information on diaphragm trophicity. Its value could be:29 Greater than 2.5mm (Normal), between 2.5 and 1.5mm (Hypotrophy of the diaphragm), and less than 1.5mm (Atrophy of the diaphragm).
The thickening Fraction (TF) was calculated by the formula:
The pathological value of TF was defined as below: if the TF < 36%: diaphragm hypotrophy and if the TF < 20%: diaphragm atrophy.27
Statistical Analysis
Statistical analyses were performed using IBM SPSS statistics 25 (Armonk, NY: IBM Corp). Descriptive statistics were indicated as ‘means and standard deviations for variables with normal distribution, ‘median, minimum-maximum for non-normal distributed variables, and “frequency and percentage [n (%)]” for categorical variables.
The independent-sample t-test was used to compare two groups with continuous normally distributed variables. Nonparametric tests were used for variables that did not have a normal distribution. The significance level was set as p < 0.05. Pearson’s correlation coefficient (r) was used to determine associations and correlations between US findings, PFT findings, and the continuous variables. The correlation strength scale was set as follow: r: +0.6 to 1.0: strong positive; r: +0.3 to 0.6: moderate positive; r: 0.0 to +0.3: weak positive; r: 0 to −0.3: weak negative; r: −0.3 to −0.6: moderate negative; and r:-0.6 to −1.0: strong negative.
Results
Patients’ Clinical Characteristics (Table 1)
Fifty patients were enrolled (41 men, and 9 women). Their mean age was 42.7 ± 11 years [range: 25–66] with a median of 42 years. Fifty-four percent of the patients were smokers (19 packs/year on average). The mean disease duration was 10 ± 8.4 years [range: 1–33] with a median of 8 years. The mean BASDAI and ASDAS scores were 2.8 ± 1.61 [range: 0–6.5] and 2.4 ± 1.01 [range: 0.1–4.9], respectively. Twelve percent of patients had very active disease, 50% had active disease, 24% a moderate activity, and 12% had inactive disease. Eighty percent of the patients were on non-steroidal anti-inflammatory drugs. Thirty percent of them were on biological drugs. Table 1 shows the main characteristics of our patients. Respiratory symptoms such as shortness of breath after exercise were reported by 20% of patients. Three patients reported a chronic dry cough. Pulmonary auscultation was normal in all patients. The chest X-ray showed chest enlargement in 12% of cases and an elevated diaphragmatic hemi-cupola in 4% of cases.
 |
Table 1 Baseline Clinical and Demographic Characteristics of Spondyloarthritis Patients |
The mean CE value was 3.9±1.81 cm [range: 1–9] with a median of 4 cm. A pathological value (<5 cm) was observed in 72% of cases, of which 24% had a significant decrease of CE (<3 cm).
The spirometry showed a restrictive disorder in 32% of cases. The restriction was mild in 75%, moderate in 12.5%, and severe in 12.5% of cases. In the supine position, the decrease in FVC was significant in 32% of cases. No obstructive or mixed disorders were noticed.
Pulmonary US showed B lines (more than three) in five patients aged between 26 and 56 years. Only two of them had low FVC (Table 1).
The diaphragmatic US showed an elevated hemi-cupola in four patients (Table 1). Only one patient had a low RDE. Tdi-exp was reduced in 54% of cases on the right side and 48% of cases on the left side. The TF was reduced in 56% of cases on the right side and 60% of cases on the left side (Table 1).
Correlation Between US and PFT Findings (Table 2)
A significant positive moderate correlation was found between the right tdi-insp and the FVC (r = 0.36; p = 0.02) and the FVC in the supine position (r = 0.29; p = 0.04). A significant positive moderate correlation was found between the left tdi-insp and the percentage of decrease in FVC in supine (Δ FVC) (r = 0.35; p = 0.01). A significant positive moderate correlation was found between the right tdi-exp and the FVC (r = 0.32; p = 0.02). There was no correlation between the DE nor the TF measures and the spirometry findings.
 |
Table 2 Pearson Correlation Analysis Between US and PFT Findings |
Correlation Between the CE and the US and the PFT Findings (Table 3)
A significant positive moderate correlation was found between the CE and the presence of B lines (r = 0.32; p = 0.02). There was no significant correlation between CE, FVC and Δ FVC (Table 3).
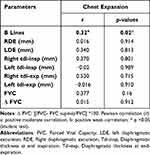 |
Table 3 Pearson Correlation Analysis Between Chest Expansion, Ultrasound and Spirometry Data |
Discussion
The present study showed a significant positive moderate correlation between the right tdi-insp, tdi-exp, and FVC. This correlation was confirmed in the supine position for the right tdi-insp. Furthermore, a significant positive moderate correlation was found between the left tdi-insp and the percentage of decrease in FVC (Δ FVC). There was a tendency for the correlation significance between the presence of B lines and FVC. A significant positive moderate correlation was found between the CE and the presence of B lines. No significant correlation was found between diaphragm TF and spirometry findings.
To the best of the author’s knowledge, this is the first study assessing the contribution of diaphragmatic US in the screening of restrictive respiratory disorder in radiographic SpA patients. Only one previous study used the US to evaluate diaphragmatic excursion in radiographic SpA patients [9]. However, this is the first study to examine diaphragmatic thickening in radiographic SpA patients and compare US findings to spirometric ones.
In this study, a restrictive pulmonary disorder was found in 32% of cases, with mild restrictions in 75% of cases. No obstructive or mixed disorders were noted. Our results were similar to those of previous studies.1,30,31 Restrictive respiratory involvement is often asymptomatic in radiographic SpA patients.32 It is mainly related to the rigidity of the thoracic wall.3 During inspiration, the diaphragmatic cupola lowers, resulting in a shortening and thickening of the diaphragm.33 However, in radiographic SpA patients, chest wall compliance decreases, and the normal configuration of the chest wall becomes distorted.34 The chest wall rigidity is the consequence of the costovertebral, manubriosternal, and sternoclavicular joint ankylosis. That could be exacerbated later by an exaggeration of the dorsal kyphosis.1,4 Therefore, the expansion of the rib cage gradually decreases and the chest remains in an inspiratory position, leading to an atrophy of the intercostal muscles and, specifically, the diaphragm.5,34 The implication of these pathways lead to a myogenic remodeling of the diaphragm, which consists in a hypotrophy of fibers. This hypotrophy can evolve to an atrophy without any neurogenic involvement.34 Unlu et al evaluated the diaphragmatic motion in 33 radiographic SpA patients compared to 14 healthy controls.9 The diaphragmatic motion was lower in radiographic SpA patients (68.9 ±17mm versus 77.8 ±22.4mm), without a significant difference (p=0.14). In the present study, the DE was lower (65.28±11.56 for the RDE and 65.68±4.7 for the LDE), without a significant correlation to the spirometry findings. This difference between the two studies could be explained by the fact that our radiographic SpA patients had more active disease (50% versus 27%). It is even paramount to be vigilant in interpreting these results, given the small sample size of the two studies.
A pathological value of CE was observed in 72% of our study patients, 24% of whom had a significant decrease in CE (<3 cm). In a study by Yurdakul et al, assessing the sagittal spine mobility and chest expansion in 50 radiographic SpA patients and 50 controls, the CE was decreased in radiographic SpA patients, but the difference was not significant compared to controls.11
The US was proven as a good tool for the diagnosis and monitoring of pulmonary disorders.35,36 However, no studies have reported the usefulness of the US in the diagnosis and follow-up of interstitial lung disease in radiographic SpA patients. Indeed, it is a rare involvement in this pathology. In our study, B lines were found in 5 patients and were not correlated to the presence of pulmonary restrictive disorder. Nevertheless, there was a tendency to the correlation significance between the presence of B lines and the FVC (p = 0.06). In our study, a significant correlation between the CE and the US findings (B line) was observed (p = 0.02), but not with FVC. These results should be taken with caution due to the small sample size of the study. Given the lack of data in the literature, broader-spectrum studies are needed to confirm or refute our results.
US is currently an indispensable tool in assessing diaphragmatic dysfunction.37,38 US measurement of diaphragmatic thickness was validated by comparing cadaveric US diaphragmatic thickness with the same segment of resected diaphragm measured with a ruler.33 In the same study, diaphragmatic thickening was calculated in healthy volunteers and found to be correlated with tidal volume.
Half of our patients had a decreased inspiratory or expiratory diaphragmatic thickness indicating diaphragmatic dysfunction. A significant positive correlation was found between right diaphragmatic thickness and FVC (Table 2). Our results suggest that right hemidiaphragm dysfunction could be a predictive factor for decreased FVC, and thus possible restrictive ventilatory disorder. This could be explained by the hypothesis that the right hemidiaphragm is limited by the liver;10,39 thus, if a right diaphragmatic dysfunction is detected on the US, then the restrictive ventilatory disorder is significant enough to be detected by spirometry.
No previous studies have examined diaphragmatic thickness in radiographic SpA subjects and its association with restrictive disorder evaluated by PFT. However, diaphragmatic thickness and TF were studied in other pathologies.40,41 Abd El Aziz AA et al evaluated diaphragmatic thickness in 40 patients with chronic obstructive pulmonary disease, it was decreased with a significant positive correlation with FVC and FEV1.40 Likewise, Hiwatani et al41 evaluated diaphragmatic thickening in 36 patients with amyotrophic lateral sclerosis, and there was a correlation between end-inspiratory and end-expiratory forced diaphragmatic thickening and spirometry data if FVC < 80%. These studies, like ours, confirm that the US can be helpful in the screening of restrictive pulmonary disorders and can be used in AS patients.
To the best of the authors’ knowledge, the strong point of the present study was the first Tunisian monocenter study conducted to assess the diaphragm involvement in ventilatory mechanics impairment in radiographic SpA patients and its correlation with the disease’s activity. However, our study has some limitations which should be considered. First, the sample size was small because COVID-19 pandemic prevented us from completing the respiratory function tests to deduce the sensitivity, specificity, and positive and negative predictive values of US compared to spirometry, allowing generalization of our results. Second, the identification of restrictive impairment was limited to spirometry, suggesting a restrictive respiratory disorder on the harmonious decrease of the FVC and FEV1 without being highly sensitive or specific. Third, the present monocenter study was a limit to generalize our findings, because few physicians master the diaphragmatic US in Tunisia. Finally, future study should exclude smoking patients because it smoking affects negatively respiratory function, independently of the etiology.30 Our study included 54% smokers. Thus, it is considered as a confounding factor.
Conclusion
Our study demonstrates that diaphragm ultrasonography is an inexpensive and easily accessible tool to screen for restrictive lung disease in radiographic SpA patients. The present study showed that diaphragmatic dysfunction is correlated with FVC abnormalities. To date, the use of US in radiographic SpA patients is not codified. Further studies with larger sample sizes are required to assess the reliability, specificity, and sensitivity of diaphragm US to screen respiratory restriction.
Abbreviations
ASDAS-(CRP), Ankylosing spondylitis Disease Activity Score; BASDAI, Bath Ankylosing Spondylitis Disease Activity Index; BASFI, Bath Ankylosing Spondylitis Functional Index; BASMI, Bath Ankylosing Spondylitis Metrology Index; BASRI, Bath Ankylosing Spondylitis Radiology Index; BMI, Body mass index; CE, Chest expansion; CRP, C-reactive protein; DE, Diaphragmatic excursion; ESR, Erythrocyte sedimentation rate; FVC, Forced vital capacity; FEV1, Forced Expiratory Volume in one second; MRI, Magnetic resonance imaging; US, Ultrasonography; PFT, Pulmonary function test; LDE, Left diaphragmatic excursion; RDE, Right diaphragmatic excursion; SpA, Spondyloarthritis; Tdi, Diaphragmatic thickness; Tdi-insp, Diaphragmatic thickness at end-inspiration; Tdi-exp, Diaphragmatic thickness at end-expiration.
Ethical Approval
The study procedure was in accordance with the Declaration of Helsinki and was approved by the military hospital Research Ethics Committee with the number: (ID 11/21/CLPP).
Acknowledgments
Open access was provided by Qatar National Library.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; they took part in drafting, revising, or critically reviewing the article; they gave final approval of the version to be published; they agreed on the journal to which the article had been submitted; and they agreed to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not for- profit sectors.
Disclosure
All authors declared no potential conflicts of interest.
References
1. Berdal G, Halvorsen S, van der Heijde D, Mowe M, Dagfinrud H. Restrictive pulmonary function is more prevalent in patients with ankylosing spondylitis than in matched population controls and is associated with impaired spinal mobility: a comparative study. Arthritis Res Ther. 2012;14(1):R19. doi:10.1186/ar3699
2. Alaee S, Jones Q. Case of drug-induced interstitial lung disease secondary to Adalimumab. BMJ Case Rep. 2018;2018. doi:10.1136/bcr-2018-224375
3. Dincer U, Cakar E, Kiralp MZ, Bozkanat E, Kilac H, Dursun H. The pulmonary involvement in rheumatic diseases: pulmonary effects of ankylosing spondylitis and its impact on functionality and quality of life. Tohoku J Exp Med. 2007;212(4):423–430. doi:10.1620/tjem.212.423
4. Fisher LR, Cawley MI, Holgate ST. Relation between chest expansion, pulmonary function, and exercise tolerance in patients with ankylosing spondylitis. Ann Rheum Dis. 1990;49(11):921–925. doi:10.1136/ard.49.11.921
5. Romagnoli I, Gigliotti F, Galarducci A, et al. Chest wall kinematics and respiratory muscle action in ankylosing spondylitis patients. Eur Respir J. 2004;24(3):453–460. doi:10.1183/09031936.04.00123903
6. Sampaio-Barros PD, Cerqueira EM, Rezende SM, et al. Pulmonary involvement in ankylosing spondylitis. Clin Rheumatol. 2007;26(2):225–230. doi:10.1007/s10067-006-0286-2
7. Cerrahoglu L, Unlu Z, Can M, Goktan C, Celik P. Lumbar stiffness but not thoracic radiographic changes relate to alteration of lung function tests in ankylosing spondylitis. Clin Rheumatol. 2002;21(4):275–279. doi:10.1007/s100670200073
8. Feltelius N, Hedenström H, Hillerdal G, Hällgren R. Pulmonary involvement in ankylosing spondylitis. Ann Rheum Dis. 1986;45(9):736–740. doi:10.1136/ard.45.9.736
9. Ünlü E, Pamuk ÖN, Erer B, Dönmez S, Çakir N. Diaphragmatic movements in ankylosing spondylitis patients and their association with clinical factors: an ultrasonographic study. Rheumatol Int. 2012;32(2):435–437. doi:10.1007/s00296-010-1657-1
10. Richard P. Explorer le diaphragme: l'échographie est essentielle [Exploring the diaphragm: ultrasound is essential]. Rev Mal Respir. 2017;34(6):645–660. French. doi:10.1016/j.rmr.2017.02.005
11. Yurdakul OV, Rezvani A. Can ultrasound be an assessment tool for sagittal spine mobility and chest expansion in patients with ankylosing spondylitis? Medicine. 2018;97(39):e12609. doi:10.1097/MD.0000000000012609
12. Poddubnyy D, Rudwaleit M. Early spondyloarthritis. Rheum Dis Clin North Am. 2012;38(2):387–403. doi:10.1016/j.rdc.2012.04.007
13. Jenkinson TR, Mallorie PA, Whitelock HC, Kennedy LG, Garrett SL, Calin A. Defining spinal mobility in ankylosing spondylitis (AS). The Bath AS Metrology Index. J Rheumatol. 1994;21(9):1694–1698.
14. van der Heijde D, Lie E, Kvien TK, et al. ASDAS, a highly discriminatory ASAS-endorsed disease activity score in patients with ankylosing spondylitis. Ann Rheum Dis. 2009;68(12):1811–1818. doi:10.1136/ard.2008.100826
15. Calin A, Nakache JP, Gueguen A, Zeidler H, Mielants H, Dougados M. Defining disease activity in ankylosing spondylitis: is a combination of variables (Bath Ankylosing Spondylitis Disease Activity Index) an appropriate instrument? Rheumatology. 1999;38(9):878–882. doi:10.1093/rheumatology/38.9.878
16. Gouilly P, Reggiori B, Gnos PL, Schuh O, Muller K, Dominguez A. À propos de la mesure de l’ampliation thoracique: on measuring thoracic expansion. Kinésithérapie la Revue. 2009;9(88):49–55. doi:10.1016/S1779-0123(09)70808-3
17. Moll JM, Wright V. An objective clinical study of chest expansion. Ann Rheum Dis. 1972;31(1):1–8. doi:10.1136/ard.31.1.1
18. Calin A, Garrett S, Whitelock H, et al. A new approach to defining functional ability in ankylosing spondylitis: the development of the Bath Ankylosing Spondylitis Functional Index. J Rheumatol. 1994;21(12):2281–2285.
19. MacKay K, Mack C, Brophy S, Calin A. The Bath Ankylosing Spondylitis Radiology Index (BASRI): a new, validated approach to disease assessment. Arthritis Rheum. 1998;41(12):2263–2270. doi:10.1002/1529-0131(199812)41:12<2263::AID-ART23>3.0.CO;2-I
20. Vilke GM, Chan TC, Neuman T, Clausen JL. Spirometry in normal subjects in sitting, prone, and supine positions. Respir Care. 2000;45(4):407–410.
21. Miller A. Lung function testing: selection of reference values and interpretative strategies. Am Rev Respir Dis. 1992;146(5 Pt 1):1368–1369. doi:10.1164/ajrccm/146.5_Pt_1.1368b
22. Godfrey MS, Jankowich MD. The Vital Capacity Is Vital: epidemiology and Clinical Significance of the Restrictive Spirometry Pattern. Chest. 2016;149(1):238–251. doi:10.1378/chest.15-1045
23. Bemba ELP, Moyikoua R, Ouedraogo AR, et al. Profil spirométrique et radiographique des patients anciens tuberculeux pulmonaires traités et guéris au service de pneumologie du CHU de Brazzaville [Spirometric and radiographic profile of former pulmonary tuberculosis patients treated and cured in the pneumology department of the Brazzaville University Hospital]. Rev Pneumol Clin. 2017;73(5):217–224. French. doi:10.1016/j.pneumo.2017.08.009
24. Wanger J, Clausen JL, Coates A, et al. Standardisation de la mesure des volumes pulmonaires. Rev Mal Respir. 2007;24(3):51–64. doi:10.1016/S0761-8425(07)91118-3
25. Lichtenstein DA. Ultrasound in the management of thoracic disease. Crit Care Med. 2007;35(5 Suppl):S250–261. doi:10.1097/01.CCM.0000260674.60761.85
26. Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The comet-tail artifact. An ultrasound sign of alveolar-interstitial syndrome. Am J Respir Crit Care Med. 1997;156(5):1640–1646. doi:10.1164/ajrccm.156.5.96-07096
27. Santana PV, Cardenas LZ, Albuquerque ALP, Carvalho CRR, Caruso P. Diaphragmatic ultrasound: a review of its methodological aspects and clinical uses. J brasileiro de pneumologia. 2020;46(6):e20200064. doi:10.36416/1806-3756/e20200064
28. Boussuges A, Gole Y, Blanc P. Diaphragmatic motion studied by m-mode ultrasonography: methods, reproducibility, and normal values. Chest. 2009;135(2):391–400. doi:10.1378/chest.08-1541
29. Carrillo-Esper R, Pérez-Calatayud ÁA, Arch-Tirado E, et al. Standardization of Sonographic Diaphragm Thickness Evaluations in Healthy Volunteers. Respir Care. 2016;61(7):920–924. doi:10.4187/respcare.03999
30. El Maghraoui A, Chaouir S, Abid A, et al. Lung findings on thoracic high-resolution computed tomography in patients with ankylosing spondylitis. Correlations with disease duration, clinical findings and pulmonary function testing. Clin Rheumatol. 2004;23(2):123–128. doi:10.1007/s10067-003-0845-8
31. El Ismaili ATA, Amara B, Serraj M, El Biaze M, Benjelloun MC. L’atteinte pulmonaire dans la spondylarthrite ankylosante (à propos de 52 cas) [Pulmonary involvement in ankylosing spondylitis (about 52 cases)]. Rev Mal Respir. 2018;35:A121–122. French. doi:10.1016/j.rmr.2017.10.266
32. Kim DY, Lee SJ, Ryu YJ, Lee JH, Chang JH, Kim Y. Progressive Pulmonary Fibrocystic Changes of Both Upper Lungs in a Patient with Ankylosing Spondylitis. Tuberc Respir Dis (Seoul). 2015;78(4):459–462. doi:10.4046/trd.2015.78.4.459
33. Wait JL, Nahormek PA, Yost WT, Rochester DP. Diaphragmatic thickness-lung volume relationship in vivo. J Applied Physiol. 1989;67(4):1560–1568. doi:10.1152/jappl.1989.67.4.1560
34. Vanderschueren D, Decramer M, Van den Daele P, Dequeker J. Pulmonary function and maximal transrespiratory pressures in ankylosing spondylitis. Ann Rheum Dis. 1989;48(8):632–635. doi:10.1136/ard.48.8.632
35. Sansone F, Attanasi M, Di Filippo P, Sferrazza Papa GF, Di Pillo S, Chiarelli F. Usefulness of Lung Ultrasound in Paediatric Respiratory Diseases. Diagnostics. 2021;11:10.
36. Sofia S, Boccatonda A, Montanari M, et al. Thoracic ultrasound and SARS-COVID-19: a pictorial essay. J Ultrasound. 2020;23(2):217–221. doi:10.1007/s40477-020-00458-7
37. Bongioanni P. Diaphragm ultrasonography as a tool in assessing respiratory muscle involvement in amyotrophic lateral sclerosis/motor neuron disease. J Clin Ultrasound. 2022;50(1):136–137. doi:10.1002/jcu.23093
38. Ziaeifar M, Sarrafzadeh J, Noorizadeh Dehkordi S, Arab AM, Haghighatkhah H, Zendehdel Jadehkenari A. Diaphragm thickness, thickness change, and excursion in subjects with and without nonspecific low back pain using B-mode and M-mode ultrasonography. Physiother Theory Pract. 2021;1–11.
39. Houston JG, Angus RM, Cowan MD, McMillan NC, Thomson NC. Ultrasound assessment of normal hemidiaphragmatic movement: relation to inspiratory volume. Thorax. 1994;49(5):500–503. doi:10.1136/thx.49.5.500
40. Abd El Aziz AA, Elwahsh RA, Abdelaal GA, Abdullah MS, Saad RA. Diaphragmatic assessment in COPD patients by different modalities. Egyptian J Chest Dis Tuberculosis. 2017;66(2):247–250. doi:10.1016/j.ejcdt.2017.03.006
41. Hiwatani Y, Sakata M, Miwa H. Ultrasonography of the diaphragm in amyotrophic lateral sclerosis: clinical significance in assessment of respiratory functions. Amyotroph Lateral Scler Frontotemporal Degener. 2013;14(2):127–131. doi:10.3109/17482968.2012.729595
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.