Back to Journals » International Journal of General Medicine » Volume 14
Assessment of Subclinical Deterioration of Right Ventricular Function by Three-Dimensional Speckle Tracking Echocardiography in Breast Cancer Patients Undergoing Anthracycline-Based Chemotherapy
Authors Xu H, Mao L, Liu H, Zhang Y, Yang J
Received 3 January 2021
Accepted for publication 25 February 2021
Published 16 March 2021 Volume 2021:14 Pages 885—893
DOI https://doi.org/10.2147/IJGM.S300257
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Haiyan Xu,1 Ling Mao,2 Hailang Liu,1 Yuanyuan Zhang,3 Jing Yang1
1Department of Cardiology, The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University, Huai’an, Jiangsu, People’s Republic of China; 2Department of Thyroid and Breast Surgery, The Affiliated Huai’an Hospital of Xuzhou Medical University and the Second People’s Hospital of Huai’an, Huai’an, Jiangsu, People’s Republic of China; 3Department of Medical Laboratory, The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University, Huai’an, Jiangsu, People’s Republic of China
Correspondence: Jing Yang
Department of Cardiology, The Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University, Huai’an, 223300, Jiangsu, People’s Republic of China
Email [email protected]
Objective: This study was aimed at assessing the longitudinal strain changes of RV function using three-dimensional speckle tracking echocardiography (3D STE) in breast cancer patients receiving anthracycline chemotherapy.
Patients and Methods: A total of 95 women with breast cancer receiving epirubicin (360 mg/m2) underwent 3D STE at baseline, the end of chemotherapy and 12 months after chemotherapy. 3D STE assessment included RV ejection fraction (EF), LV global longitudinal strain (GLS), RV GLS, and RV free wall longitudinal strain (RV FWLS). Meanwhile, serum hs‐cTnI and NT-proBNP were measured. Chemotherapy-related cardiac dysfunction (CTRCD) was defined as an absolute decrease in 3D LVEF > 10% to a value < 50%, while a percent reduction of 3D LV GLS > 15% indicated subclinical CTRCD.
Results: Subclinical CTRCD occurred in 10 (10.5%) patients during follow-up. Compared to baseline, the 3D GLS of LV and GLS and FWLS of RV decreased significantly at 12months after chemotherapy (all p< 0.01). Variations of 3D RV GLS and RV FWLS had a good discrimination for predicting subclinical CTRCD. The variation of 3D RV FWLS was the only independent predictor of subclinical CTRCD (OR, 1.37; 95% CI, 1.12– 2.87; p = 0.028) in multivariate analysis. The cutoff value with − 17.5% of 3D RV FWLS variation had a high predictive accuracy for subclinical CTRCD, with an AUC of 0.74, 80.5% sensitivity and 65.8% specificity. The association between 3D RV FWLS and the cumulative dose of anthracyclines was calculated by Spearman’s test (r = − 0.71, p < 0.001).
Conclusion: Longitudinal strain analysis by 3D STE allows the identification of subclinical RV dysfunction when conventional indices of RV function are unaffected. 3D RV FWLS was superior to other parameters in early detection of the development of CTRCD in breast cancer patients receiving epirubicin therapy.
Keywords: right ventricle, global longitudinal strain, three-dimensional echocardiography, anthracycline, cardiotoxicity
Erratum for this paper has been published.
Introduction
Breast cancer is the most common malignancy amongst women and the 5‐year relative survival rate for this disease has significantly improved.1 Anthracyclines are the most widely used antineoplastic agents in breast cancer chemotherapy, which can significantly improve the survival rate of patients. On the other hand, anthracycline-induced cardiotoxicity may negatively impact prognosis.2 Chemotherapy-related cardiac dysfunction (CTRCD) is one of the anthracycline-induced cardiotoxicity, that may initially symptomatic or asymptomatic with continuous progressive decline in left ventricular ejection fraction (LVEF). Early detection of subclinical cardiotoxicity is important in breast cancer patients, in order to initiate timely cardioprotective treatment and avoid overt cardiac dysfunction and heart failure. Echocardiography is recommended as the method of choice for the detection of detection of CTRCD before, during and after cancer therapy.3 Many studies involving anthracycline-induced cardiotoxicity focused almost exclusively on left ventricular (LV) function.4–6 More recently, however, there has been a greater interest in the right ventricular (RV) function.
The complex anatomical shape and contraction pattern of the RV are difficult to perform on traditional echocardiography. New echocardiographic techniques, such as 3-dimensional (3D) and speckle tracking echocardiography (STE) offer novel insights into RV function assessment. 3D echocardiography could provide most accurate data for the RV volume and RV ejection fraction (RVEF).7,8 Several studies have reported that 3D RV volumes and EF correlated significantly with cardiac magnetic resonance (CMR).9–11 CMR is the gold standard technique for measuring RV size and function but less for tissue characterization due to its thin walls. Some studies have shown that strain deterioration precedes overt changes in RV systolic function and is consistently predictive of the development of heart failure.12,13
This study was aimed at assessing the longitudinal strain changes of RV function using 3D STE in breast cancer patients receiving anthracycline chemotherapy.
Methods
Subjects
A total of 100 female patients with newly diagnosed breast cancer undergoing chemotherapy with anthracyclines between January 2016 and July 2018 at the Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University were enrolled prospectively into this single-center study. Patients with coronary artery disease (previous myocardial infarction, established coronary heart disease at least one coronary artery stenosis ≥50% by coronary catheterization or coronary computed tomography), arterial hypertension (BP ≥ 140/90 mmHg; BP ≥ 140/90 mmHg and/or using anti-hypertensive medication), cardiomyopathy (hypertrophic cardiomyopathy, dilated cardiomyopathy, restrictive cardiomyopathy), arrhythmias (sick sinus syndrome, atrial fibrillation or atrial flutter, frequent premature ventricular beats, complete atrioventricular block), more than mild valvular stenosis or regurgitation, prosthetic valves or pacemakers, diabetes mellitus (diagnostic criteria of the American Diabetes Association), chronic kidney disease (diagnostic criteria of Kidney Disease Outcomes Quality Initiative), congenital heart disease, and LVEF<50% before chemotherapy were excluded. Three patients were excluded due to uncontrolled hypertension and two patients were excluded due to poor 3D image quality. Thus, the final study group consisted of 95 breast cancer patients. The study received ethics approval from the ethics committee of the Affiliated Huaian No.1 People’s Hospital of Nanjing Medical University (No.2015182), and written informed consent was obtained from all participants. This study was conducted in accordance with the Declaration of Helsinki.
All patients received a 4‐cycle EC (epirubicin 90 mg/m2 + cyclophosphamide 600 mg/m2) chemotherapy regimen, with an inter-cycle interval of 21 days. None of the patients received other cardiotoxic agents, radiation therapy, or cardiac protective protocols during this study. Demographic data, cardiac biomarkers and echocardiographic parameters were documented. Two‐dimensional (2D) echocardiography, 3D‐STE, serum high-sensitive cardiac troponins troponin I (hs-cTnI) and N-terminal portion pro-natriuretic peptide type B (NT-proBNP) were routinely performed during the following 3 stages: at baseline, at the end of the chemotherapy and 12 months after chemotherapy simultaneously.
Echocardiographic Data Acquisition
Standard transthoracic echocardiography was performed on all patients using a GE Vivid E9 ultrasound machine (GE Healthcare, Horten, Norway) equipped with M5S (1.7–3.3 MHz). Real-time 3D image was performed using the same ultrasound machine by the 4V‐D probe (1–5 MHz). Standard 2D and 3D echocardiography were performed according to the American Society of Echocardiography guidelines.14
End-diastolic volume (EDV), end-systolic volume (ESV), and LVEF were manually calculated according to the biplane Simpson’s method in the 2D mode. Volume parameters were standardized by body surface area (BSA). The right ventricular diameters were measured in the focused RV apical four-chamber view (basal, mid-cavity and longitudinal RV diameter) at the ventricular end-diastole. Right ventricular end-diastolic area and end-systolic area were measured from the focused RV apical four-chamber view to calculate RV fractional area change (RV FAC). In focused RV apical four-chamber view, tricuspid annular plane systolic excursion (TAPSE) was acquired by M-mode. Tricuspid inflow (E) was measured by pulsed-wave Doppler imaging, while tricuspid annular (TV) peak systolic velocity (S’) and early diastolic velocity (e’) were measured by pulsed-wave tissue Doppler with a sample volume placed at the lateral segment of the tricuspid annulus. Systolic pulmonary artery pressure (SPAP) was measured using continuous wave Doppler in inflow tract of right ventricle.
Full-volume 3D images were taken for all patients and stored in digital format for off-line analysis (4.6.0.411, TomTec Imaging Systems GMBH, Germany). Four consecutive beats data during a single breath hold were digitally stored to ensure optimal data quality. After manual delineation of the mitral valve edges and apex, the software automatically and simultaneously depicted the endocardial and epicardial borders through the entire cardiac cycle in three different vectors. The LV global longitudinal strain (GLS) was automatically calculated as weighted averages of the regional values from the 17 myocardial segments. RV data were analyzed by 4D RV-Function Tom Tec software. Endocardial borders were traced in four planes: the apical four chamber view, and the three short-axis planes at near the apex, the mid-level, and the base of the right ventricle. The RV endocardium was traced semi-automatically with refinement by manual adjustment at end-diastolic and end-systolic phase respectively. Ultimately, the software produces a complete data set comprising RV EDV, RV ESV, RVEF and RV GLS. RV free wall longitudinal strain (RV FWLS) was computed by averaging the peak systolic strain values of the three segments of the free wall (from base to apex). All images were acquired and analyzed offline by two experienced echocardiographer who were blinded to the clinical characteristics of the patients.
A randomly selected cohort of 10 patients was analyzed to evaluate intra-observer and inter-observer variabilities for 3D strain measurements by an interval of >1 month. Intra-observer and interobserver variability of 3D STE data were calculated as intraclass correlation coefficients (ICCs). The inter-observer ICCs were 0.88 for LV GLS, 0.86 for RV GLS and 0.85 for RV FWLS, respectively. Similarly, intra-observer measurement showed ICCs of 0.92 for LV GLS, 0.89 for RV GLS, 0.87 for RV FWLS indicating satisfactory reproducibility of strain measurements by 3D STE.
An absolute decrease in 3D LVEF > 10% to a value <50% was considered as CTRCD, while a percent reduction of 3D LV GLS > 15% indicated subclinical CTRCD.3 Percentage variation of strain values refers to the absolute value of the strain parameters between the baseline and 12 months after the chemotherapy.
Cardiac Biomarkers Assays
Concentrations of hs-cTnI and NT-proBNP were measured on an Elecsys 2010 analyzer (Roche Diagnostics Corporation, Indianapolis, IN, USA) using a commercially available electrochemiluminescence immunoassay.
The 99th percentile reference limit of hs-cTnI is 10 pg/mL. NT-proBNP >125 pg/mL was considered elevated. Blood samples corresponding to the baseline and follow-up timepoints were included for analysis. Biomarker assessment were routinely performed within 24 hours after epirubicin cumulative dosages of 180 mg/m2 and 360 mg/m2 during follow-up time.
Statistical Analysis
Continuous variables were summarized as mean and standard deviation or median and interquartile range (IQR) as appropriate, whereas categorical measures were summarized as percentage and frequencies. Differences between groups were compared using one-way ANOVA for continuous variables (Mann–Whitney U-test for variables with non-normal distribution). The correlation between 3D STE parameters and the cumulative doses of epirubicin was calculated by Spearman’s test. The correlation between 3D STE parameters and cardiac biomarkers and the correlation between 3D LV GLS and RV strains were calculated by Pearson’s test. Receiver operating curve (ROC) analysis was performed to assess the discrimination of 3D strain values for predicting subclinical CTRCD using area under the curve (AUC). The optimum cutoff value that maximized both sensitivity and specificity for discriminating subclinical CTRCD was determined. All statistical tests were 2-sided and a p value <0.05 was considered statistically significant. Statistical analysis was performed using the SPSS Version 20.0 software package (SPSS 20.0, Chicago, IL, USA).
Results
Baseline Characteristics
The characteristics of the patients are shown in Table 1. The mean age of these patients was 53.2±8.7 years, with a range of 34–68 years old. Among the patients, 36 patients had done coronary computed tomography and 70 patients had done holter electrocardiogram. All patients had normal LVEF values at baseline. Subclinical CTRCD occurred in 10 (10.5%) patients during follow-up, of whom 4 patients (4.2%) were diagnosed as CTRCD according to the 3D LVEF criterion. At the end of the chemotherapy, only four patients were diagnosed as subclinical CTRCD according to the 3D LV GLS criterion. Among all patients, no one developed clinical cardiac toxicity during the period of follow-up.
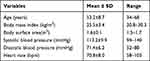 |
Table 1 Clinical Characteristics at Baseline |
Standard Echocardiography
Mean values of the standard echocardiographic parameters at baseline and during follow-up are shown in Table 2. There were no significant differences in LVEF, RV basal diameter, RV middle diameter, RV longitudinal diameter at the end of chemotherapy and 12months after chemotherapy (all p> 0.05). 3D LV GLS showed a significant reduction during anthracycline chemotherapy (p<0.05). TAPSE and RV FAC decreased significantly at 12months after chemotherapy (both p<0.05). The ratios of tricuspid annular E/A and TV E/e’ showed no significant variation during the follow-up period (both p > 0.05). The tricuspid annular S′ showed no significant differences between pre- and post-therapies as well (p >0.05). No significant change in SPAP was observed (p > 0.05).
 |
Table 2 Standard Echocardiographic Parameters |
Three-Dimensional Speckle Tracking Echocardiography
The results of 3D STE analysis are shown in Table 3. At the end of chemotherapy, 3D RV GLS, RV FWLS and LV GLS were deteriorated significantly, while no significant differences were observed in other 3D STE parameters (all p > 0.05). Compared with the baseline, there were no significant differences in 3D RV EDVI, RV ESVI and RVEF at 12months after chemotherapy (all p > 0.05). However, 3D RV GLS and RV FWLS deteriorated markedly at 12months after chemotherapy (both p < 0.05). There was no significant difference in 3D LVEF between baseline and 12months after chemotherapy, while 3D GLS of LV decreased significantly at the end of follow-up (p< 0.01).
 |
Table 3 Three-Dimensional Speckle Tracking Echocardiography Parameters |
Serum Biochemical Markers
At the end of chemotherapy, the hs-cTnI concentrations rose from 1.3 (0.7, 2.7) to 6.8 (4.5, 9.0) pg/mL (p = 0.039). However, the median value of NT-proBNP concentrations showed no significant difference during the chemotherapy (p > 0.05). Spearman correlation analysis showed that hs-cTnI concentrations were significantly correlated with 3D RV GLS (r = −0.35, p = 0.031) and RV FWLS (r = −0.39, p = 0.025) at the end of chemotherapy. There was no correlation between NT-proBNP concentrations and 3D RV GLS, either NT-proBNP concentrations and 3D RV FWLS (both p > 0.05).
Relationship of Three-Dimensional Strain and Subclinical Cancer Therapeutics-Related Cardiac Dysfunction
There was no difference in 3D RVEF (−10.8% vs −5.4%, p=0.383) and RV GLS (−18.2% vs −11.3%, p=0.159) variation values in patients with and without subclinical CTRCD. On the other hand, 3D RV FWLS variation (- 20.6% vs - 13.1%, p = 0.020) were significantly greater in patients who developed subclinical CTRCD at 12months after chemotherapy (Figure 1).
 |
Figure 1 Bar graphs of three-dimensional speckle tracking echocardiography (3D STE) variation according to the presence or absence of subclinical chemotherapy-related cardiac dysfunction (CTRCD). |
Table 4 shows the results of univariate and multivariate logistic regression analyses of predictors of subclinical CTRCD. In the univariate model, variations of 3D RVEF, RV GLS and RV FWLS were associated with a higher incidence of subclinical CTRCD. In the multivariate model, only the variation of 3D RV FWLS remained the independent predictor of subclinical CTRCD (OR, 1.37; 95% CI, 1.12–2.87; p = 0.028).
 |
Table 4 Logistic Regression Analysis for Predictors of Chemotherapy-Related Cardiac Dysfunction |
The area under the curve of 3D RV FWLS, RV GLS and RVEF are 0.74, 0.68, and 0.64, respectively. ROC curve analysis showed that the optimal-cut off of 3D RV FWLS variation for the discrimination of patients with subclinical CTRCD was −17.5% with a sensitivity of 80.5% and a specificity of 65.8% (Figure 2).
Correlation Between 3D LV GLS and 3D RV Strains
The association between 3D LV GLS and 3D RV FWLS was calculated by Pearson’s test (r = 0.47, p < 0.001) (Figure 3). Correlation coefficients for 3D RVEF and RV GLS: RVEF (r = - 0.32, p = 0.019), RV GLS (r = 0.36, p = 0.086). RV FWLS changes was moderately related to decrease in 3D LVEF > 10% to a value <50% (r =0.42, p < 0.001).
 |
Figure 2 The association between three-dimensional left ventricular global longitudinal strain (3D LV GLS) and three-dimensional right ventricular free wall longitudinal strain (3D RV FWLS). |
Correlation Between 3D RV FWLS and Anthracycline Doses
The association between 3D RV FWLS and the cumulative dose of anthracyclines was calculated by Spearman’s test (r = −0.71, p < 0.001). Cumulative anthracycline dose was correlated with 3D RV GLS (r = −0.53, p = 0.034) and RVEF (r = - 0.60, p = 0.026).
Discussion
In this study, we assessed the longitudinal strain changes of RV function in patients with breast cancer underwent anthracycline chemotherapy using 3D STE. RV GLS and RV FWLS reduced significantly at 12months after anthracycline therapy. 3D STE is superior to classic echocardiographic indices to identify the subclinical CTRCD at the end of anthracycline chemotherapy. In addition, 3D RV FWLS had high sensitivity and specificity in differentiating patients with subclinical CTRCD after chemotherapy from baselines. The accumulating dose of epirubicin positively correlated with the decline of 3D RV FWLS.
The anthracycline-related clinical and subclinical LV dysfunction has been well studied in breast cancer patients.6,15 However, RV dysfunction is not considered in the diagnosis of CTRCD. Given the established prognostic significance of RV dysfunction on outcome of patients with heart failure,16,17 anthracycline-related toxic effects on the right ventricle have become the focus of greater interest. Although the assessment of the right ventricle by conventional 2D echocardiography has prognostic value, its accuracy and reproducibility are limited. The CMR is the gold standard imaging tool for assessing RV volume and RVEF, while echocardiography is the most widely used clinically. As echocardiographic techniques have evolved, recent studies have validated the accuracy of 3DTTE-determined RV volumes and RVEF against CMR.
Speckle-tracking echocardiography is a novel method for assessment of LV myocardial deformation. GLS has been proven to identify subclinical LV cardiotoxicity as it is more sensitive than traditional echocardiographic parameters.3,18 Similar to LV strain, STE-derived RV longitudinal strain has been shown to be feasible, reproducible and prognostic in oncological patients.13,19,20 Due to less load dependent and less angle dependent than conventional parameters, RV FWLS has recently been proposed to evaluate RV dysfunction.
In our study, 3D RV GLS and RV FWLS decreased during and after anthracycline chemotherapy. The 3D RVEF had stable and normal during the whole procedure. At 12 months after chemotherapy, 3D RV FWLS was significantly reduced in group who developed subclinical CTRCD compared with the group without subclinical CTRCD (- 20.6% vs - 13.1%, p = 0.020). Furthermore, this study showed the variation of 3D RV FWLS as an excellent independent predictor of subclinical CTRCD in multivariate logistic regression analysis (OR = 1.37; 95% CI: 1.12–2.87, p = 0.028).
There is no consensus in the literature regarding the RV GLS value that can predict cardiotoxicity. Keramida et al13 found that the cutoff value of RV GLS percent change that identified cardiotoxicity was 14.8% with a sensitivity of 66.7%, a specificity of 70.8% and the AUC of 0.68 in breast cancer patients. Cherata et al21 demonstrated that 17% reduction of RV FWLS had a sensitivity of 55% and a specificity of 70% with AUC of 0.75 to identify patients with CTRCD. ROC analysis in this study showed that variation of RV FWLS has better sensitivity and specialty in detection of subclinical cardiotoxicity. The AUC for 3D RV GLS reduction was 0.68. An important debate in RV strain methodology is whether GLS or FWLS should be evaluated. Chang et al22 reported that RV FWLS represented an independent predictor of occult RV systolic dysfunction in breast cancer patients receiving epirubicin therapy. In our study, RV FWLS had a slightly higher predictive value for subclinical CTRCD than RV GLS. The optimal cut-off of 3D RV FWLS percent change for the discrimination of patients with subclinical CTRCD was 17.5% with a sensitivity of 80.5% and a specificity of 65.8% and the AUC was 0.74. RV FWLS evaluate RV function more accurately than RV GLS since intraventricular septum is a constituent part of the LV.
RV GLS deterioration seems to develop almost simultaneously with LV GLS deterioration in breast cancer patients receiving trastuzumab with or without anthracyclines.13,20 In our study, 3D GLS of LV and RV decreased significantly at the end of anthracycline chemotherapy and 12 months after chemotherapy. Several studies20,22,23 evaluated the prognosis of RVEF, RV FAC, TAPSE, and tricuspid annular S′ in patients receiving anthracycline treatment for breast cancer. Data on RV systolic function are discordant. In our study, RV FAC and TAPSE decreased significantly in patients at 12 months after chemotherapy. Both tricuspid annular S′ and TAPSE are angle dependent and only reflect the longitudinal function of the basal portion of the RV. RV FAC is the most commonly used two-dimensional method to assess RV function. RV FAC may better reflect RV function but dependents on image plane, requiring complete visualization of the entire RV structure. 3D RVEF has been reported to have incremental prognostic value over two-dimensional RV function parameters.7 However, RVEF deterioration may be a late manifestation of RV dysfunction. Furthermore, we found that 3D RV strain was more sensitive than above traditional 2D parameters and 3D RVEF. There was significant correlation between 3D RV FWLS and 3D LV GLS after anthracycline chemotherapy.
Diastolic function was also explored in our study. E/e’ ratio is a reliable marker in assessing RV diastolic function. There is a direct correlation between E/e’ and RV diastolic filling pressures in right heart catheterization studies.24 Increased E/e’ values was related to increased RV filling pressures. E/e’ ratio was increased after four cycles of anthracycline chemotherapy in our study, although the difference was not statistically significant. The assessment of cardiac diastolic function by echocardiography maybe have some breakthroughs in future.
The hs‐cTnI is likely the most sensitive and specific marker of myocardial injury induced by anthracycline chemotherapy.25,26 The present study showed the hs-cTnI concentrations significantly rose from 1.3 to 6.8 ng/mL at the end of chemotherapy (p = 0.039). Although the hs-cTnI concentrations had correlation with 3D RV GLS and RV FWLS, it did not predict cardiotoxicity. The challenge with the available published data is hs‐cTnI does not reflect the damage extent of heart function as well as the strategy to undertake in case of an abnormal result.27 In this study, NT-proBNP was also not the predicter of cardiotoxicity after chemotherapy. NT-proBNP may be useful, but its role in detecting cardiotoxicity is not established in chemotherapy.
Cardiotoxicity of anthracyclines is always dose-dependent,15,22 that is, the incidence of myocardial injury is positively correlated with the cumulative dose of the drug. The effect of anthracycline dose on myocardial deformation using 3D STE was evaluated in the present study. Consistent with previous studies, a high negative relation between 3D RV FWLS and cumulative anthracycline dose was observed.
The main limitations of this study were the limited number of patients and not frequent and short follow-up in single-center. A larger prospective multicenter study with a longer follow‐up will be needed to confirm our results. In addition, evaluation of RV function and deformation parameters was performed using 3D STE, accuracy of which is highly dependent on image quality. Insufficient temporal resolution may affect the accuracy of strain data. However, a multi-beat, full-volume 3-DE acquisition using 4 consecutive cardiac cycles was performed to achieve adequate temporal resolution in this study.
Conclusion
In this study, longitudinal strain analysis by 3D STE allows the identification of subclinical RV dysfunction when conventional indices of RV function are unaffected. 3D RV FWLS was superior to other parameters in early detection of the development of CTRCD in breast cancer patients receiving epirubicin therapy. Deformation imaging of the RV using 3D STE may be a promising modality for detection of subclinical cardiotoxicity during anthracycline chemotherapy.
Funding
The research was supported by the Huai’an Municipal Science and Technology Bureau (CN) (HAB201816, HAB201723).
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Siegel R, Naishadm D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2019;69:7–34. doi:10.3322/caac.21551
2. Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–1084. doi:10.1056/NEJM200004133421502
3. Zamorano JL, Lancellotti P, Rodriguez Muñoz D, et al. 2016 ESC position paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC committee for practice guidelines: the task force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 2016;37:2768–2801. doi:10.1093/eurheartj/ehw211
4. Lorenzini C, Lamberti C, Aquilina M, et al. Reliability of left ventricular ejection fraction from three-dimensional echocardiography for cardiotoxicity onset detection in patients with breast cancer. J Am Soc Echocardiogr. 2017;30:1103–1110. doi:10.1016/j.echo.2017.06.025
5. Narayan HK, Finkelman B, French B, et al. Detailed echocardiographic phenotyping in breast cancer patients: associations with ejection fraction decline, recovery, and heart failure symptoms over 3 years of follow-up. Circulation. 2017;135:1397–1412. doi:10.1161/CIRCULATIONAHA.116.023463
6. Zhang KW, Finkelman BS, Gulati G, et al. Abnormalities in 3-dimensional left ventricular mechanics with anthracycline chemotherapy are associated with systolic and diastolic dysfunction. JACC Cardiovasc Imaging. 2018;11:1059–1068. doi:10.1016/j.jcmg.2018.01.015
7. Nagata Y, Wu VC, Kado Y, et al. Prognostic value of right ventricular ejection fraction assessed by transthoracic 3D echocardiography. Circ Cardiovasc Imaging. 2017;10:e005384. doi:10.1161/CIRCIMAGING.116.005384
8. Addetia K, Maffessanti F, Muraru D, et al. Morphologic analysis of the normal right ventricle using three-dimensional echocardiography-derived curvature indices. J Am Soc Echocardiogr. 2018;31:614–623. doi:10.1016/j.echo.2017.12.009
9. Leibundgut G, Rohner A, Grize L, et al. Dynamic assessment of right ventricular volumes and function by real-time three-dimensional echocardiography: a comparison study with magnetic resonance imaging in 100 adult patients. J Am Soc Echocardiogr. 2010;23:116–126. doi:10.1016/j.echo.2009.11.016
10. Muraru D, Spadotto V, Cecchetto A, et al. New speckle-tracking algorithm for right ventricular volume analysis from three-dimensional echocardiographic data sets: validation with cardiac magnetic resonance and comparison with the previous analysis tool. Eur Heart J Cardiovasc Imaging. 2016;17:1279–1289. doi:10.1093/ehjci/jev309
11. Ishizu T, Seo Y, Atsumi A, et al. Global and regional right ventricular function assessed by novel three-dimensional speckle-tracking echocardiography. J Am Soc Echocardiogr. 2017;30:1203–1213. doi:10.1016/j.echo.2017.08.007
12. Song FY, Shi J, Guo Y, et al. Assessment of biventricular systolic strain derived from the two-dimensional and three-dimensional speckle tracking echocardiography in lymphoma patients after anthracycline therapy. Int J Cardiovasc Imaging. 2017;33:857–868. doi:10.1007/s10554-017-1082-6
13. Keramida K, Farmakis D, Bingcang J, et al. Longitudinal changes of right ventricular deformation mechanics during trastuzumab therapy in breast cancer patients. Eur J Heart. 2019;21:529–535. doi:10.1002/ejhf.1385
14. Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28:1–39 e14. doi:10.1016/j.echo.2014.10.003
15. Jacobse JN, Steggink LC, Sonke GS, et al. Myocardial dysfunction in long-term breast cancer survivors treated at ages 40–50 years. Eur J Heart Fail. 2020;22:338–346. doi:10.1002/ejhf.1610
16. Bosch L, Lam CSP, Gong L, et al. Right ventricular dysfunction in left-sided heart failure with preserved versus reduced ejection fraction. Eur J Heart Fail. 2017;19:1664–1671. doi:10.1002/ejhf.873
17. Muraru D, Badano LP, Nagata Y, et al. Development and prognostic validation of partition values to grade right ventricular dysfunction severity using 3D echocardiography. Eur Heart J Cardiovasc Imaging. 2020;21:10–21. doi:10.1093/ehjci/jez233
18. El-Sherbeny WS, Sabry NM, Sharbay RM. Prediction of trastuzumab-induced cardiotoxicity in breast cancer patients receiving anthracycline-based chemotherapy. J Echocardiogr. 2019;17:76–83. doi:10.1007/s12574-018-0394-4
19. Chen L, Huang J, Wu W, Ta S, Xie X. The impact of right ventricular function on prognosis in patients with stage III non small cell lung cancer after concurrent chemoradiotherapy. Int J Cardiovasc Imaging. 2019;35:1009–1017. doi:10.1007/s10554-019-01590-0
20. Calleja A, Poulin F, Khorolsky C. et al. Right ventricular dysfunction in patients experiencing cardiotoxicity during breast cancer therapy. J Oncol. 2015;609194. doi:10.1155/2015/609194
21. Cherata DA, Donoiu I, Diaconu R, et al. Longitudinal strain analysis allows the identification of subclinical deterioration of right ventricular function in patients with cancer therapy-related left ventricular dysfunction. Discoveries (Craiova). 2019;7:e94. doi:10.15190/d.2019.7
22. Chang WT, Shih JY, Feng YH, et al. The early predictive value of right ventricular strain in epirubicin-induced cardiotoxicity in patients with breast cancer. Acta Cardiol Sin. 2016;32:550–559. doi:10.6515/acs20151023a
23. Abdar Esfahani M, Mokarian F, Karimipanah M. Alterations in the echocardiographic variables of the right ventricle in asymptomatic patients with breast cancer during anthracycline chemotherapy. Postgrad Med J. 2017;93:271–274. doi:10.1136/postgradmedj-2016-134286
24. Husain N, Gokhale J, Nicholson L, Cheatham JP, Holzer RJ, Cua CL. Noninvasive estimation of ventricular filling pressures in patients with single right ventricles. J Am Soc Echocardiogr. 2013;26:1330–1336. doi:10.1016/j.echo.2013.08.002
25. Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J Am Coll Cardiol. 2014;63:809–816. doi:10.1016/j.jacc.2013.10.061
26. Jones M, O’Gorman P, Kelly C, Mahon N, Fitzgibbon MC. High-sensitive cardiac troponin-I facilitates timely detection of subclinical anthracycline-mediated cardiac injury. Ann Clin Biochem. 2017;54:149–157. doi:10.1177/0004563216650464
27. Simoes R, Silva LM, Cruz A, et al. Troponin as a cardiotoxicity marker in breast cancer patients receiving anthracycline-based chemotherapy: a narrative review. Biomed Pharmacother. 2018;107:989–996. doi:10.1016/j.biopha.2018.08.035
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

