Back to Journals » Patient Preference and Adherence » Volume 16
Assessment of Insomnia and Associated Factors Among Patients Who Have Recovered from COVID-19 in Vietnam
Authors Huynh G , Nguyen HV, Vo LY, Le NT, Nguyen HTN
Received 20 April 2022
Accepted for publication 1 July 2022
Published 8 July 2022 Volume 2022:16 Pages 1637—1647
DOI https://doi.org/10.2147/PPA.S371563
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Giao Huynh,1 Hau Viet Nguyen,2 Lan Y Vo,1 Ngoc Thi Le,3 Han Thi Ngoc Nguyen4
1Faculty of Public Health, University of Medicine and Pharmacy at Ho Chi Minh City, Ho Chi Minh City, Vietnam; 2Emergency Department, University Medical Center Ho Chi Minh City, Ho Chi Minh City, Vietnam; 3Faculty of Medicine, Nguyen Tat Thanh University, Ho Chi Minh City, Vietnam; 4Infection Control Department, University Medical Center Ho Chi Minh City, Ho Chi Minh City, Vietnam
Correspondence: Hau Viet Nguyen, Emergency Department, University Medical Center Ho Chi Minh City, 215 Hong Bang Street, District 5, Ho Chi Minh City, Vietnam, Tel +84918764092, Email [email protected] Han Thi Ngoc Nguyen, Infection Control Department, University Medical Center Ho Chi Minh City, 201 Nguyen Chi Thanh Street, District 5, Ho Chi Minh City, Vietnam, Tel +84397975519, Email [email protected]
Introduction: The COVID-19 pandemic has been affecting the lives of millions of people globally. Patients recovering from COVID-19 are facing, not only the symptom of long COVID, but also psychological problems, such as sleep disturbance. This study aims to assess the proportion of COVID-19 recovered adult patients that suffer from insomnia and associated factors in Vietnam.
Methods: A cross-sectional study was performed between January and March 2022 among patients who have recovered from a COVID-19 infection. Data were collected based on a self-administered questionnaire that included sociodemographic and standardized questionnaires from the Hospital Anxiety and Depression Scale (HADS), the Perceived stress scale (PSS) and the dependent variable using Insomnia Severity Index (ISI). Multivariable logistic regression was conducted to explore factors associated with the patients’ insomnia disorder.
Results: A total of 325 participants were included in this analysis, 34.5% of participants had insomnia. According to multivariable logistic regression, participants who were equal to and over 50 years of age, feeling alienated from others, and were not supported by families or relatives, reported significantly higher levels of insomnia disorders over those aged under 50 years, having closer ties with family and had received support from family or relatives. Besides, respondents who recorded mental health problems that included anxiety, depression and stress were more likely to get insomnia disorders than those without mental health symptoms (OR 2.7, 95% CI 1.1– 6.6) (OR 4.5, 95% CI 2.3– 8.9) (OR 2.3, 95% CI 1.1– 5.3), respectively, all p < 0.05.
Conclusion: There was a remarkable rate of COVID-19 recovered patients experiencing insomnia disorders. Older age, alienated relationships and not being supported by families or relatives, as well as had mental health problems, are factors that affected the patients’ insomnia, which showed that these sleep issues need to be screened and managed among adults who have recovered from COVID-19.
Keywords: COVID-19, insomnia, recovery, sleep disturbance, Vietnam
Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), a series of acute, atypical respiratory illnesses that are capable of infecting humans like SARS-CoV and MERS-CoV outbreak previously, caused the coronavirus disease 2019 (COVID-19) and was detected in China in late December 2019.1 Since then, SARS-CoV-2 has rapidly evolved into a pandemic that posed a significant threat to public health and a high morbidity and mortality rate. As of 3 June 2022, over 529 million confirmed cases have been reported, while deaths are reported to be over 11.9 million globally.2 Public health interventions, as well as immunization, were performed to help limit transmission and prevent serious illness and death.3
Nevertheless, the consequences of COVID-19 present an unprecedented challenge by patients recovering from COVID-19 who are faced with, not only the symptoms of long COVID (describe signs and symptoms which continue after acute COVID-19 from 4 to 12 weeks), but also the psychological effects including anxiety disorders, depressive manifestations, sleep problems and others. These long-term psychiatric problems may, therefore, pose remarkable economic, healthcare and social burdens to the post-pandemic world.4,5 Previously, patients who had recovered from the SARS and MERS infections reported that the long-term impact on mental health, especially psychiatric complications in SARS confirmed patients, could last over 2 years after recovery.6,7 In the COVID-19 pandemic, Xu et al found that COVID-19 recovered patients had a remarkable rate of depression and insomnia, 9.92% and 26.45%, respectively.8 This revealed the negative impacts of the COVID-19 pandemic on psychological problems as well as insomnia. Patients with mental health disorders who had high-risk factors were more likely to develop insomnia. By contrast, the high rate of insomnia is also related to psychological reactions.9 Therefore, the early detection and proper intervention for insomnia and mental illness in the post-pandemic world holds great significance.
Insomnia is considered a widespread neuropsychological sleep-related disorder known to result in various issues including cognitive impairments, negative thoughts, emotional distress, and perceived sleep insufficiency, besides affecting the rate of other medical disorders.10 Clinically, insomnia is considered one of the major psychiatric disorders in adolescents, including the main symptom being perceived sleep dissatisfaction, and difficulty in initiating or maintaining sleep.11,12 Additionally, insomnia was commonly reported among various populations, not only in the acute phase of COVID-19 infection but also after recovery. Recently, Jahrami H’s study found that the global rate of sleep problems among populations during COVID-19 was 35.7%, with the rates of health-care workers and the general population being 36.0% and 32.3%, respectively.13 Patients with COVID-19 have a higher rate of sleep problems (74.8%).13 Moreover, research has suggested that there is a reportable change in the proportion of sleep disturbances among COVID-19 patients during this pandemic, such as Jahrami et al (74.8%),13 Deng et al (34%),14 Goldstein et al (11%),15 Huang et al (26%).16 Also, COVID-19 patients who had a higher incidence of hospital-acquired infection are more susceptible to developing mental disorders and an increased need for admission in ICU care.17
The adverse impact of the coronavirus disease 2019 (COVID-19) pandemic on sleep problems in the global population shows the need for urgent attention to this problem, especially the long-term implications on survivors from COVID-19 need to be determined.18,19 Several studies have revealed that proper sleep not only reduces the hazardous effects of non-communicable diseases (NCDs) but also leads to enhanced immunity to reduce the possible contraction of the COVID-19 infection.20,21 Moreover, sleep becomes more essential in the pandemic because the lack of sleep could impair psychological functioning and decision-making leading to mood changes and individuals becoming more susceptible to disease.22 Despite the importance of managing sleep disturbances in COVID recovered patients, detailed data in this area is currently lacking and unclear. Therefore, this study aims to assess the proportion of insomnia and associated factors among adults who have recovered from COVID-19 in Vietnam to evaluate the impact of COVID-19 on patients’ mental health, as well as implement interventions by prioritizing screening and treatment in these subjects.
Methods
Study Design and Participants
A cross-sectional study targeted a sample of patients who have recovered from COVID-19 who had a checkup in two hospitals at Ho Chi Minh City (HCMC), Vietnam between January 24th and March 7th 2022. All patients were conveniently selected for the survey. They agreed to be involved and signed informed consent before their enrollment. They were able to withdraw from the survey at any time and all personal information was kept confidential. After the study, patients who showed any mental health problems were offered further evaluation by a psychiatrist if so desired.
Inclusion and Exclusion Criteria
The inclusion criteria consisted of patients who (1) were aged ≥18 years (2) infected with the virus that causes COVID-19 at least 4 weeks after infection (confirmed by COVID-19 rapid antigen tests or real-time reverse transcription-polymerase chain reaction tests) and (3) agreed to partake in the study. Participants with any severe conditions that prevented them from participating in this study were excluded, including such conditions as impaired consciousness, dementia, had prior history of any psychotic disorders or insomnia problems or did not complete the questionnaire were excluded.
Data Collection
A self-administered questionnaire was used to collect data, which included 4 parts: (1) sociodemographic information included questions about age, gender, educational level, occupation, marital status, socioeconomic status, history of chronic medical illness, living condition, COVID-19 vaccine status, relationship in families, family/relative support; (2) the Hospital Anxiety and Depression Scale (HADS) included 14 items, divided into two subscales containing 7 questions each to evaluate anxiety and depression. Each item is used a 4-point scale (0–3 scores) and the cut-off points for anxiety and depression have a total score of 8 points.23,24 (3) the 10-item Perceived Stress Scale (PSS) is validated and widely used to assess stress perception in patients. Responses to each question are used a 5-point Likert scale from 0 (never) to 4 (very often). However, items 4, 5, 7, and 8 were reverse scored. The total scores range from 0 to 40. The HADS and PSS indicated a high reliability with a Cronbach’s α being 0.85 and 0.72, respectively. The threshold used to consider the stress of a patient is when the score is ≥13.25 (4) the dependent variable was measured by the Insomnia Severity Index (ISI).26,27 The scale consisted of 7 items to measure the perceived severity of nocturnal and daytime symptoms of insomnia during the previous 2 weeks. The reliability of the scale in our study was high, with a Cronbach’s α = 0.90. Each item is used a 5-point Likert scale, yielding a total score ranging from 0 to 28. The cut-off score for diagnosing insomnia disorder is ≥8 while the absence of insomnia scored in the range from 0 to 7.26 The total score of insomnia disorder is categorized as follows; mild insomnia (8–14), moderate insomnia (15–21), and severe insomnia (22–28).27
Statistical Analysis
All data were analyzed with Stata statistics version 14. Descriptive statistics used frequency and percentages or mean and standard deviations to analyze response to questions. Chi-square tests or Fisher’s exact tests were analyzed for categorical variables to explore the potential factors associated with insomnia and all items with a p-value of 0.2 or less were entered into the multivariable logistic regressions. All p < 0.05 was considered statistically significant. The model fitness was assessed by the Hosmer-Lemeshow test (χ2= 8.7, 8 degrees of freedom, p = 0.371) and indicated that its fit was satisfactory. Multicollinearity of the independent variables was assessed by using Variance Inflation Factor (VIF) and the mean VIF was 1.41.
Ethic
All participants signed an informed consent before participating in the survey. Our survey was complied with the Declaration of Helsinki as well as the approval of the Ethics Committee at the University of Medicine and Pharmacy at Ho Chi Minh City, Vietnam (number: 108/UMP-BOARD).
Results
A total of 325 patients who met all participation criteria and completed the questionnaire were included in the survey. Table 1 presents the sociodemographic characteristics of the sample. The sample mainly comprised participants who were female (61.8%), the mean of age was 37.5 (±11.9), aged under 50 years (70.5%) and were married (62.5%). More than one-third of the participants were officers/sellers (40.3%), had chronic conditions (32.6%) and had high school and higher education (45.6%). The majority of patients had socioeconomic status in the middle level (81.5%), living with family or others (94.1%), were fully COVID-19 vaccinated (89.2%), had close relationships with family (92.6%) and received family or relative support (94.2%). There were under one-fourth of participants who reported anxiety (20.3%) and stress (23.4%) while a total of 30.8% of patients had depression.
 |
Table 1 Participants’ Characteristics (N = 325) |
Figure 1 shows the proportion of participants who had an insomnia disorder (ISI ≥ 8) and categorized the level of insomnia disorder, including the absence of insomnia (0–7), mild insomnia (8–14), moderate insomnia (15–21), and severe insomnia (22–28). There was approximately one-third of respondents who had insomnia symptoms (34.5%) categorized as mild, moderate or severe symptoms of insomnia, which were recorded as 18.2%, 12.6% and 3.7%, respectively.
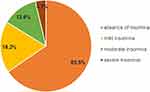 |
Figure 1 Prevalence of insomnia. |
Table 2 indicates the associated factors between demographics and insomnia. There shows a relationship between insomnia disorders and age, occupation, relationships in families and the support of families or relatives (all p < 0.05). The association between mental health and insomnia is presented in Table 3 which shows that anxiety, depression and stress were related to insomnia disorders (all p < 0.001). The factors associated with insomnia in the multivariate logistic regression analysis are reported in Table 4. Participants who were equal to or over 50 years of age, reported feeling alienated from others and were not supported by family or relatives reported significantly higher levels of insomnia disorders than those aged under 50 years, who had closer connections with family and received support from family and relatives (OR 2.8, 95% CI 1.4–5.7), (OR 4.1, 95% CI 1.1–15.7), (OR 3.9, 95% CI 1.2–13.2), respectively, all p < 0.05. Besides, respondents who indicated they had anxiety (20.3%), depression (30.8%) and stress (23.4%) were more likely to report an insomnia disorder than those without any mental health symptoms (OR 2.7, 95% CI 1.1–6.6) (OR 4.5, 95% CI 2.3–8.9) (OR 2.3, 95% CI 1.1–5.3), respectively, all p < 0.05.
 |
Table 2 Associated Factors Between Demographic and Insomnia (N = 325) |
 |
Table 3 Associated Factors Between Mental Health and Insomnia (N = 325) |
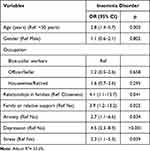 |
Table 4 Multivariable Logistic Regression Analysis Between Demographic and Insomnia (N = 325) |
Discussion
Since the emergence of the COVID-19 pandemic, there have been serious and significant impacts on all aspects of life around the world. The effects of vaccines, therapeutics or public health strategies have contributed to controlling the spread of the disease. However, the burden of COVID-19 can be persistent on both physical and mental health and other medical complication in the short-term and long-term post recovery. Amongst these post recovery issues, sleep disorders were commonly reported during the pandemic, not only in the acute phase of COVID-19 infection but also after recovery, which lead to having continuing issues on the survivors’ life. Therefore, this study aims to explore the prevalence of insomnia among COVID-19 patients post recovery and associated factors in the context of the global health crisis as this challenge continues worldwide.
These results revealed that approximately one-third of participants had insomnia symptoms (34.5%) with the majority of them having symptoms from mild to moderate (18.2% and 12.6%, respectively). The levels observed in this investigation are far below those previously observed rates of insomnia among COVID-19 inpatients, or those discharged and examined one-month after hospital treatment. These groups reported an insomnia range from 42.8% to 74.8%.13,28–30 Moreover, our findings are lower than the results in the umbrella review that found approximately 36.36% of health-care workers had insomnia during the COVID-19.31 A possible explanation for this might be that poor sleep is linked to susceptibility to infection and a worsening clinical course among patients. In the acute phase, the patients seem to be fearful and anxious about social isolation procedures, as well as mandatory lockdowns, which combined, seem to cause an incidence of sleep disturbance in the population.13,32,33 Owing to the fact of long-term social isolation during this time, COVID-19 patients’ suicidal ideation and suicide attempts also increased accordingly.34 Besides, COVID-19 patients who require intensive care at a specialist hospital (ICU) could potentially become a high-risk group for developing psychiatric disorders.35 Since proper sleep plays a crucial role in accelerating patient recovery to enhance immunity to protect against different viral infections, it is crucial that health professionals screen for and treat insomnia among survivors from COVID-19 to improve both normal sleep patterns and the mental health of these people.
Other findings showed that patients who were equal to or over 50 years of age were more likely to have insomnia than those under 50 years of age. This finding is consistent with that of previous studies29,36 but contrary to Wang et al, which suggested that younger age sufferers had a risk factor for having an insomnia disorder.28 This finding may be explained by the fact that the majority of COVID-19-related deaths were older adults who had a high risk of insomnia.37,38 After the outbreak of COVID-19, older patients were greatly affected, not only physically but also mentally. They were faced with multiple pressures such as reinfection, property loss and potential death so were more prone to suffer from insomnia. Interestingly, our findings found that participants feeling alienated from others and who were not supported by family or relatives reported a higher rate of developing insomnia symptoms. These results are in line with those of previous studies that indicated that people who have a supportive social and psychological network from family or relatives had a lower risk of insomnia.36,39 This result may be explained by the lack of contact with family and loved ones during quarantine or hospitalization, which is consistent with the previous assertion that social isolation and loneliness are linked to poor mental health outcomes.19 As a result, social support is an important resource related to one’s mental well-being, which contributes to mitigating the negative life experiences, especially in the scenario of the current pandemic.
A positive association between mental health and insomnia disorder was observed in this study. Consistent with the previous study, a higher level of anxiety, depression and stress in patients was more likely to also report insomnia problems.28–30,40,41 From this, and similar to the SARS epidemic, psychiatric complications of infected patients can last for more than 2 years, and the medical costs of hospitalized patients with psychiatric comorbidities have increased greatly, which is causing a heavy economic burden to the individuals, families and society as a whole.7,42 Thus, psychological health plays an important role in the rehabilitation process of COVID-19 patients. It appears to be necessary to simultaneously address sleep problems and psychological distress during the recovery phase of patients infected with the COVID-19.
Several limitations should be considered in this study. First, the research convenience involved patients attending a checkup at two hospitals in HCMC, which may result in a limitation or a generalization of the current results. Second, the cross-sectional design may leave open the possibility of not determining the cause-effect relationships between insomnia and sociodemographics, and mental health issues, as well as evaluating the rate of insomnia problems in the past. However, our results have contributed to implementing interventions for surviving patients after they have recovered from COVID-19.
Implications
The study emphasizes the importance of strategies to assist patients who recovered from COVID-19 in overcoming not only sleep problems but also the mental health of these people. Future research should focus on information relating to sleep issues to understand the changes in sleep caused by COVID-19.
Conclusion
This study found a remarkable rate of COVID-19 recovered patients experiencing insomnia problems, and these reported sleep disturbances were affected by a range of different domains including older age, alienated relationships and not being supported by family or relatives, as well as mental health problems. Government and health-care providers need to design and implement appropriate psychological strategies and treatments to assist patients recovering from COVID-19.
Data Sharing Statement
Data relating to this study are available from the first author upon request.
Acknowledgments
We thank all the nurses as well as the patients in two hospitals in Ho Chi Minh City for the time and effort they devoted to this study. We also thank Raymond A Kuschert for assisting in editing this manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that was in the conception, design of the study, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Lotfi M, Hamblin MR, Rezaei N. COVID-19: transmission, prevention, and potential therapeutic opportunities. Clin Chim Acta. 2020;508:254–266. doi:10.1016/j.cca.2020.05.044
2. WHO. Weekly epidemiological update on COVID-19-8 February 2022. Available from: https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19—8-february-2022.
3. CDC. Benefits of getting a COVID-19 vaccine. Available from: https://www.cdc.gov/coronavirus/2019-ncov/vaccines/vaccine-benefits.html.
4. NICE. COVID-19 rapid guideline: managing the long-term effects of COVID-19. Available from: https://www.nice.org.uk/guidance/ng188.
5. The Lancet Global Health. Mental health matters. Lancet Glob Health. 2020;8(11):e1352. doi:10.1016/S2214-109X(20)30432-0
6. Park HY, Park WB, Lee SH, et al. Posttraumatic stress disorder and depression of survivors 12 months after the outbreak of Middle East respiratory syndrome in South Korea. BMC Public Health. 2020;20(1):605. doi:10.1186/s12889-020-08726-1
7. Mak IW, Chu CM, Pan PC, Yiu MG, Chan VL. Long-term psychiatric morbidities among SARS survivors. Gen Hosp Psychiatry. 2009;31(4):318–326. doi:10.1016/j.genhosppsych.2009.03.001
8. Xu F, Wang X, Yang Y, et al. Depression and insomnia in COVID-19 survivors: a cross-sectional survey from Chinese rehabilitation centers in Anhui province. Sleep Med. 2022;91:161–165. doi:10.1016/j.sleep.2021.02.002
9. Li Y, Qin Q, Sun Q, Sanford LD, Vgontzas AN, Tang X. Insomnia and psychological reactions during the COVID-19 outbreak in China. J Clin Sleep Med. 2020;16(8):1417–1418. doi:10.5664/jcsm.8524
10. Vaziri Z, Nami M, Leite JP, Delbem ACB, Hyppolito MA, Ghodratitoostani I. Conceptual framework for insomnia: a cognitive model in practice. Front Neurosci. 2021;15:628836. doi:10.3389/fnins.2021.628836
11. Winkelman JW, Solomon CG. Clinical practice. Insomnia disorder. N Engl J Med. 2015;373(15):1437–1444. doi:10.1056/NEJMcp1412740
12. Qaseem A, Kansagara D, Forciea MA, Cooke M, Denberg TD; Clinical Guidelines Committee of the American College of Physicians. Management of chronic insomnia disorder in adults: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2016;165(2):125–133. doi:10.7326/M15-2175
13. Jahrami H, BaHammam AS, Bragazzi NL, Saif Z, Faris M, Vitiello MV. Sleep problems during the COVID-19 pandemic by population: a systematic review and meta-analysis. J Clin Sleep Med. 2021;17(2):299–313. doi:10.5664/jcsm.8930
14. Deng J, Zhou F, Hou W, et al. The prevalence of depression, anxiety, and sleep disturbances in COVID-19 patients: a meta-analysis. Ann N Y Acad Sci. 2021;1486(1):90–111. doi:10.1111/nyas.14506
15. Goldstein CA, Rizvydeen M, Conroy DA, et al. The prevalence and impact of pre-existing sleep disorder diagnoses and objective sleep parameters in patients hospitalized for COVID-19. J Clin Sleep Med. 2021;17(5):1039–1050. doi:10.5664/jcsm.9132
16. Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397(10270):220–232. doi:10.1016/S0140-6736(20)32656-8
17. Zhang J, Xu D, Xie B, et al. Poor-sleep is associated with slow recovery from lymphopenia and an increased need for ICU care in hospitalized patients with COVID-19: a retrospective cohort study. Brain Behav Immun. 2020;88:50–58. doi:10.1016/j.bbi.2020.05.075
18. Vindegaard N, Benros ME. COVID-19 pandemic and mental health consequences: systematic review of the current evidence. Brain Behav Immun. 2020;89:531–542. doi:10.1016/j.bbi.2020.05.048
19. Brooks SK, Webster RK, Smith LE, et al. The psychological impact of quarantine and how to reduce it: rapid review of the evidence. Lancet. 2020;395(10227):912–920. doi:10.1016/S0140-6736(20)30460-8
20. Budreviciute A, Damiati S, Sabir DK, et al. Management and prevention strategies for non-communicable diseases (NCDs) and their risk factors. Front Public Health. 2020;8:574111. doi:10.3389/fpubh.2020.574111
21. Paules CI, Marston HD, Fauci AS. Coronavirus infections-more than just the common cold. JAMA. 2020;323(8):707–708. doi:10.1001/jama.2020.0757
22. Medic G, Wille M, Hemels ME. Short- and long-term health consequences of sleep disruption. Nat Sci Sleep. 2017;9:151–161. doi:10.2147/NSS.S134864
23. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
24. Bjelland I, Dahl AA, Haug TT, Neckelmann D. The validity of the hospital anxiety and depression scale. An updated literature review. J Psychosom Res. 2002;52(2):69–77. doi:10.1016/s0022-3999(01)00296-3
25. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav. 1983;24(4):385–396. doi:10.2307/2136404
26. Bastien CH, Vallieres A, Morin CM. Validation of the insomnia severity index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. doi:10.1016/s1389-9457(00)00065-4
27. Morin CM, Belleville G, Belanger L, Ivers H. The insomnia severity index: psychometric indicators to detect insomnia cases and evaluate treatment response. Sleep. 2011;34(5):601–608. doi:10.1093/sleep/34.5.601
28. Wang Y, Zhu LY, Ma YF, et al. Association of insomnia disorder with sociodemographic factors and poor mental health in COVID-19 inpatients in China. Sleep Med. 2020;75:282–286. doi:10.1016/j.sleep.2020.06.011
29. Alimoradi Z, Brostrom A, Tsang HWH, et al. Sleep problems during COVID-19 pandemic and its’ association to psychological distress: a systematic review and meta-analysis. EClinicalMedicine. 2021;36:100916. doi:10.1016/j.eclinm.2021.100916
30. Mazza MG, De Lorenzo R, Conte C, et al. Anxiety and depression in COVID-19 survivors: role of inflammatory and clinical predictors. Brain Behav Immun. 2020;89:594–600. doi:10.1016/j.bbi.2020.07.037
31. Sahebi A, Abdi K, Moayedi S, Torres M, Golitaleb M. The prevalence of insomnia among health care workers amid the COVID-19 pandemic: an umbrella review of meta-analyses. J Psychosom Res. 2021;149:110597. doi:10.1016/j.jpsychores.2021.110597
32. Xiang YT, Yang Y, Li W, et al. Timely mental health care for the 2019 novel coronavirus outbreak is urgently needed. Lancet Psychiatry. 2020;7(3):228–229. doi:10.1016/S2215-0366(20)30046-8
33. Lin CY, Brostrom A, Griffiths MD, Pakpour AH. Investigating mediated effects of fear of COVID-19 and COVID-19 misunderstanding in the association between problematic social media use, psychological distress, and insomnia. Internet Interv. 2020;21:100345. doi:10.1016/j.invent.2020.100345
34. Calati R, Ferrari C, Brittner M, et al. Suicidal thoughts and behaviors and social isolation: a narrative review of the literature. J Affect Disord. 2019;245:653–667. doi:10.1016/j.jad.2018.11.022
35. Sommer IE, Bakker PR. What can psychiatrists learn from SARS and MERS outbreaks? Lancet Psychiatry. 2020;7(7):565–566. doi:10.1016/S2215-0366(20)30219-4
36. Guo J, Yang L, Xu Y, et al. Prevalence and risk factors associated with insomnia symptoms among the Chinese general public after the coronavirus disease 2019 epidemic was initially controlled. Nat Sci Sleep. 2021;13:703–712. doi:10.2147/NSS.S307996
37. Demartini B, Nistico V, D’Agostino A, Priori A, Gambini O. Early psychiatric impact of COVID-19 pandemic on the general population and healthcare workers in Italy: a preliminary study. Front Psychiatry. 2020;11:561345. doi:10.3389/fpsyt.2020.561345
38. Li Q, Guan X, Wu P, et al. Early transmission dynamics in Wuhan, China, of novel coronavirus-infected pneumonia. N Engl J Med. 2020;382(13):1199–1207. doi:10.1056/NEJMoa2001316
39. Roberts RE, Roberts CR, Chen IG. Impact of insomnia on future functioning of adolescents. J Psychosom Res. 2002;53(1):561–569. doi:10.1016/s0022-3999(02)00446-4
40. Weibel S, Jermann F, Weiner L, et al. Insomnia in adult attention-deficit/hyperactivity disorder: a comparison with borderline personality disorder population in a clinical setting and control participants. Compr Psychiatry. 2017;76:119–128. doi:10.1016/j.comppsych.2017.04.009
41. Zandifar A, Badrfam R, Yazdani S, et al. Prevalence and severity of depression, anxiety, stress and perceived stress in hospitalized patients with COVID-19. J Diabetes Metab Disord. 2020;19(2):1431–1438. doi:10.1007/s40200-020-00667-1
42. Dubey S, Biswas P, Ghosh R, et al. Psychosocial impact of COVID-19. Diabetes Metab Syndr. 2020;14(5):779–788. doi:10.1016/j.dsx.2020.05.035
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
