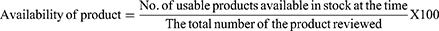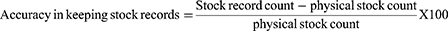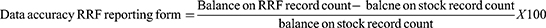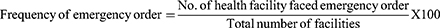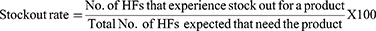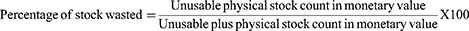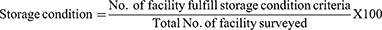Back to Journals » Integrated Pharmacy Research and Practice » Volume 11
Assessment of HIV Rapid Test Kits Inventory Management Practice and Challenges in Public Health Facilities of Addis Ababa, Ethiopia
Authors Bekele A , Gemechu F, Ayalew M
Received 12 January 2022
Accepted for publication 12 March 2022
Published 25 March 2022 Volume 2022:11 Pages 85—94
DOI https://doi.org/10.2147/IPRP.S356134
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Jonathan Ling
Azmeraw Bekele,1,2 Feneti Gemechu,3 Mihret Ayalew2,4
1Social and Administrative Pharmacy Unit, Institute of Health, Jimma University, Jimma, Ethiopia; 2School of Pharmacy, Jimma University, Jimma, Ethiopia; 3Department of Pharmacy, Adama Hospital Medical College, Adama, Ethiopia; 4Department of Pharmacology, Institute of Health, Jimma University, Jimma, Ethiopia
Correspondence: Azmeraw Bekele, Email [email protected]
Background: Many people with undiagnosed HIV live in sub-Saharan Africa and vulnerable laboratory systems undermine testing services.
Methods: A facility-based mixed-approach cross-sectional study was conducted from January 1 to February 1, 2020. A total of 23 health facilities were included in the study which stratified into hospitals and health centers. Six months of bin card records and request and resupply forms (RRFs) were reviewed. Data were collected through physical inventory, observation, and document review. After the data was imported into the MS Excel 2016 spreadsheet, it was analyzed using SPSS | Version 20 | Software. In addition, 12 semi-structured in-depth interviews were conducted and responses were analysed using a thematic approach.
Results: The entire health facility had RRF reports and bin card records while the availability of HIV rapid test kits was 75%. More than half, 38 (55.1%) of the bin card records were updated and the average data accuracy of bin cards was 84.1%. The data quality of the RRF reports was determined accurate 18 (78.3%), complete 15 (65.22%), and on-time 7 (30.3%). Sixteen (69.6%) health facilities experienced at least one stock out with an average daily stock out of 4%. The wastage rate was 0.0083%. Only 9 (39.1%) health facilities have fulfilled acceptable storage conditions.
Conclusion: Most of the health facilities did no longer fulfilled acceptable storage conditions. Similarly, the data quality of most bin card records and RRF reports was poor. This deprived inventory management practice was likely related to supply, staff, and documentation challenges.
Keywords: inventory management, rapid test kits, health facilities, Addis Ababa
Corrigendum for this paper has been published
Background
Global awareness of the plight of people living with HIV (human immunodeficiency virus) is estimated at 45%, well below the World Health Organization’s target. Many people with undiagnosed HIV live in sub-Saharan Africa,1 and vulnerable health and laboratory systems undermine testing services.2 In low-income countries with HIV infection, rapid diagnostic tests are widely used in diagnostic algorithms.1 The test accuracy can be affected by inadequate inventory control,3 leading to unnecessary false activation of false-positive diagnoses for antiretroviral therapy, or vice versa.4,5 Moreover, the burden of inventory management for HIV test products is increasing rapidly.6 Supply interruptions and out-of-stocks are important issues in managing the inventory of HIV test products.7 There are similar challenges in managing inventories of HIV test kits because of the multiple purposes of the test, the variety of test procedures, and the need for different types or brands of test kits.8,9 In Nigeria, HIV test kits had a lack of job aids and electronic records, lack of professional training, inadequate storage spaces, incomplete documentation, and some stock-outs.10 Likewise, in the Republic of Malawi, there was a problem in calculating the order quantity or not receiving the order quantity, and sixty-five percent of health facilities placed an emergency order within six months. Some of the logistics management information system (LMIS) tools did not include the HIV test kit in use, some data was missing, and incomplete.11 In Ethiopia’s inventory management processes, the HIV testing algorithm includes three rapid test kits (RTKs): STAT PACKTM (screening test), ABONTM (confirmatory test), and SDBIOLINETM (tie-breaker test). Indeed, there is underperforming inventory management of HIV RTKs in any health care system of Ethiopia results from patients going without knowing their HIV status and reduced confidence in the health care system.6,12 Thus, this study is aimed to assess the inventory management practice and related challenges of HIV rapid test kits in public health facilities of Addis Ababa.
Methods
Study Design and Study Settings
A facility-based mixed-method cross-sectional study was conducted from January 1 to February 1, 2020. The quantitative and qualitative studies were conducted concurrently in which the qualitative study followed a concurrent explanatory approach13 for triangulation. This study was conducted in the public health facilities of Addis Ababa, the capital city of Ethiopia with a total population of 4,794,000 across 10 sub cities.14 The city provided major public healthcare services using 12 hospitals and 98 health centers.
Study Population
The study populations were HIV RTKs commonly managed by selected public health facilities. Relevant data were retrieved by reviewing logistic records and reports of HIV rapid test kits.
Eligibility Criteria
This study included public health facilities that were actively providing HIV testing services for more than or equal to six months. Three HIV RTKs and corresponding logistic records and reports were included in the study except for incomplete records and reports. Health posts are considered to be the dispensing unit of health centers and they are not analogous to hospitals or health centers and excluded to keep the consistency of information.
Sample Size and Sampling Procedures
The sample size of health facilities was determined based on the logistic indicators assessment tool that recommends a minimum of 15% of the target health facilities.15 A total of 93 health facilities (11 hospitals and 82 health centers) were actively providing HIV testing services. Yet, 15% of 93 health facilities were too small to represent the remaining health facilities. To increase the generalizability of the study, the sample size was increased by 25% (93×25%≈23) (11 hospitals and 12 randomized health centers). Thus, 23 health facilities were included in the study. In addition, 6 months of logistic records and reports were reviewed.15 According to Ethiopia’s integrated pharmaceutical logistics system, each hospital and health center is required to submit its RRF report to the Ethiopian pharmaceutical supply agency hub every two months.16 As a result, 69 RRF reports (23 health facilities are required to report 3 RRFs within six months) were reviewed. The numbers of bin cards were corresponding to the number of 3 RTKs so that 3 bin cards were reviewed from each health facility.
Data Collection Tools and Process
The data collection tool was adapted from a standardized tool developed by the United States Agency for International Development/Deliver Projects.15,18 The data collectors were pharmacists who had previous experience in managing program commodities and worked at the health centers. One of the authors has provided four hours of training and provided them with an interpretation of relevant logistic variables subjected to the study. Data were collected using physical inventory, observation, and document reviews. On the day of the facility visit, the data collectors first introduced themselves and remind the purpose of the study at each health facility.
Data Quality Assurance
A pilot study was conducted in five percent of health facilities that were excluded from the actual study to tailor the contents of the data collection tool. Data collectors were pharmacists and they have received four hours of training on what and how to collect the required data. One of the researchers was closely monitored the whole data collection process to keep the data complete and consistent.
Data Entry and Analysis
The complete and consistent data were entered into MS Excel 2016 spreadsheet and exported to social science statistics package (SPSS) | Version 20 | Software for analysis. Quantifiable variables were measured by using frequency, mean, and percentage values. Finally, results were presented using tables, graphs, and texts.
The Qualitative Study
Twelve semi-structured in-depth key informant interviews were conducted upon information saturation. Key informants (seven pharmacy heads and five Ethiopian pharmaceutical supply agency (EPSA) coordinators) were selected based on their position for the underlying information. The interview was interactive and conducted by one of the researchers using a semi-structured interview guide adopted from the logistics indicator assessment tool (Appendix).19 Probing techniques were applied to get more information and each interview took an average of 20 minutes. The thematic analysis of response was manually performed using an excel spreadsheet. After the researchers became familiarized; preliminary codes were assigned to describe the content, contents were classified, the topics were subjected to preliminary codes and then, the themes were identified and named. The verbatim phrases were pulled out using participants’ words. Finally, results were presented using texts.
Quantifiable Variables and Their Measurements
The following standard indicators were used to measure quantifiable variables, while the overall inventory management status is not feasible to measure over the individual indicators.
- The timelines of RRF reports: as per the standard operating procedure of the integrated pharmaceutical logistics system of Ethiopia;
- Hospitals and health centers should submit their RRF report to the Ethiopian pharmaceutical agency or zone health department until the 10th day of the month.
- Health centers that send their RRF report via the district health office should submit their RRF to the district health office until the 5th day of the month of the reporting period.
Operational Definitions
Acceptable storage condition: Health facility storage condition is said to be acceptable if that facility has fulfilled at least 80% of criteria for acceptable storage conditions.20
Bin card update: It had to be updated within the previous 30 days. Also if the bin card was last updated with a balance of zero and the facility has not received any of the products.21 If drugs had no transaction ahead of 30 days, it is considered that the bin card was not updated.
Data quality of logistics management information (LMIS) tools: measured using the three dimensions; timeliness, completeness, and accuracy.22
Completeness of reports: a report was considered to be complete if all the columns for each product listed in the report are filled unless the facility does not manage the product.21
Timelines of reports: perusing the standard operating procedure of the integrated pharmaceutical logistics system of Ethiopia.21
Data accuracy of records and reports: happened whether there is no discrepancy between stock balances on bin card (manual, electronic) and the physical count or there is no discrepancy between the balances on the bin card and balances on the RRF report over the range of selected items. A high accuracy rate (80% and above) indicates good inventory practice.20
Value of unusable stock: the monetary value of unusable stock due to expiry, damage, or spoilage.
Results
Availability and Updating Practice of Bin Card Records
The current study indicated that bin card records were available at all health facilities; on average 38 (55.1%) were updated. STAT-PAK had the most updated bin cards available (Table 1).
 |
Table 1 Availability and Updating Practices of Bin Card Records of HIV RTKs in Selected Public Health Facilities of Addis Ababa, 2020 |
Availability of Rapid HIV Test Kits
About 75% of HIV RTKs were available with the average values between 29 (80.5%) to 23 (69.7%). STATPAKTM was the most available HIV RTKs with a mean of 91.3% and ABON was the least available RTKs with a mean of 65.2% (Figure 1).
 |
Figure 1 Availability of HIV rapid test kits during the facility visit in selected public health facilities of Addis Ababa, 2020. |
Stock Out of Rapid HIV Test Kits
Sixteen (69.6%) health facilities experienced stock out at least for one of the two HIV RTKs within six months with the mean daily stock out of 4%. SD BIOLINTM was the most stock out HIV RTK fluctuated among months of stock out (Figure 2).
 |
Figure 2 Stock out durations of HIV rapid test kits within six months in selected public health facilities of Addis Ababa, 2020. |
Data Quality of LMIS Records and Reports
Data Accuracy of Bin Card Records
This study showed that the bin card updating practice was nearly similar among hospitals 9 (39.1%) and health centers 8 (34.8%). The change in data accuracy of bin cards was low among HIV RTKs (Figure 3). The average bin card accuracy was close to 84%.
 |
Figure 3 Data accuracy of bin card records of HIV rapid test kits in selected public health facilities of Addis Ababa, 2020. |
Data Quality of RRF Reports
The majority, 18 (78.3%) of the health facilities generated accurate RRF reports. Most of the hospitals 7 (30.4%) and some of the health centers 8 (34.8%) were submitted their RRF reports on time with a reporting rate of 15 (65.22%) but merely 7 (30.3%) health facilities conveyed complete RRF reports (Figure 4).
 |
Figure 4 Data quality of RRF reports of HIV rapid test kits in selected public health facilities of Addis Ababa, 2020. |
Emergency Orders by Health Facilities
Nine (39.1%) out of 23 health facilities placed more than 3 emergency orders for two HIV RTKs within the past 6 months and 7 (30.4%) health facilities placed three or fewer emergency orders so that there were frequent under stock reports.
Wastage to HIV Rapid Test Kits
The current study indicated that wasted stock of HIV RTKs due to expiry, damage, and loss was accounted for 51,493.23 Ethiopian Birr (ETB) with a wastage rate of 0.0083%. A large value of wasted HIV RTKs found at the health centers 41,940.49 ETB than at the hospitals 9,552.74 ETB. SDBIOLINTM was profoundly wasted in hospitals with a value of 13.1% whereas ABONTM was much wasted at the health centers 10.4% more than SDBIOLINTM wasted values at the hospitals.
Storage Conditions of Health Facilities
Out of the total health facilities, only 9 (39.1%) have fulfilled at least 80% of the criteria for acceptable storage conditions. Of which, 7 (30.4%) were hospitals and 2 (8.7%) were health centers (Figure 5). Thirteen (56.5%) health facilities used stored and organize HIV RTKs according to the first-in-first-out stock rotation principle.
 |
Figure 5 Storage conditions of HIV rapid test kits in selected public health facilities of Addis Ababa, 2020. |
Qualitative Results
Twelve semi-structured in-depth face-to-face key informant interviews were conducted to identify challenges related to the inventory management practice of HIV RTKs. Thus, semi-structured interviews were analysed using a thematic approach and deductively categorized into supply, staff, and documentation-related challenges.
Supply Related Challenges
The common challenges identified at the Ethiopian pharmaceutical supply agency were stock disruption, customs delay, and uncertainty of suppliers following undetermined factors like that of coronavirus disease-19. The addition of new HIV test kits and changes to the testing algorithms slowed the selection process and increased the wastage of HIV RTKs. Moreover, the stock status monitoring was identified as problematic. One of the EPSA coordinators explained that;
I worked for last four years and I noted that our clients are constantly complaining. This is likely due to the lack of an integrated information management system used to track the stock status of critical inventories throughout corresponding health facilities. Moreover, supply planning of HIV test kits was going to be unsettled.
The issues listed by the coordinators were pointed out as challenging opportunities by some other respondents at some health facilities where they had good coordination among the health stakeholders ahead of inconsistent monitoring activities.
Staff Related Challenges
The study identified a lack of sufficient manpower and low staff commitment as the major issues in securing inventory information. Facility professionals had slight skills regarding the features of software currently in use with a lack of user-friendly platforms. Many errors have encountered during the recording and reporting of test kit items had important negative effects though some efforts were made to modest the effect. Some staffs were not fully understood the complexity of existing inventory management practices and were poor to track the regular activities. One of the key informants reported that;.
Most of our staffs are increasingly disappointed with low service compensations and they are not fully acting at their responsibility. They are dissatisfied with their current position though the facility is tried to expand their satisfaction through the commencement of Kaizen which is a continuous improvement approach involving all employees and makes the working environment more productive.
Contrariwise, the solution mentioned by the key informant regarding the Kaizen approach was identified as a missed opportunity in three-quarters of the health facilities.
Documentation Related Challenges
This study identified that the inventory tracking system in public health facilities was primarily manual and encountered human errors. Some health facilities made whip reports and followed by an inadequate supply of requested test kit items up to half of the quantity requested. The visibility of data between the supplier agency and respective health facilities was limited. The right amount of inventory, at the right time, and in the right place was rarely reported. One of the key informants said that;
… I took my current position eight years before, and our agency is accounted for the majority of imported items, and a lot of backorders are not filled from lower-level health facilities. This is promoting negative responses such as over requesting practice though our agency is trying to implement mobile solutions to exchange demanding information.
The key informant response from the supplier agency regarding mobile solution was contrasting with other health facility respondents who criticized the supplier for its low commitment.
Discussion
Assessing real practices, identifying obstacles and opportunities can expand or shift the focus of the health service and link clients to treatment, support, and prevention programs.23 Ethiopian public health Institute is recommended to use accurate testing materials that are well stored and are not expired with good quality of records.24 To this regard, our finding indicated that each health facility had a bin card and nearly half of them were updated. While the mean availability of HIV RTKs was 75% in which STAT-PAKTM was the most stable item. This is in line with findings from Bayelsa State, Nigeria10 but lower than a finding from Zambezia that the availability of the same items was above 89%, yet there were threats and stock out of one or both tests kits.25 Indeed, the current study indicated that there needs to be particular attention to ABONTM and SDBIOLINTM stocked out for 162 days that hurt achieving targets (90-90-90) of the United Nation on HIV/AIDS program. When the RTK is stocked out, the client could be returned to their home without getting service for another day, goes to another health facility, or live without service. The respondents from the present study identified that stock out for HIV RTKS have been related to customs delay and unexpected contract terminations extending to unpredictable demand, damage, or loss of product like that of Nigeria.10 Otherwise, in Zambezia and Mozambique, inadequate quantification has been a major factor for stock-outs.26 It is the fact that accurate data is the source of accurate information to make an accurate decision. To this end, our finding indicated that the mean data accuracy of bin cards was 84.1%. Even though the mean bin card accuracy has encountered a maximum of 6% intolerable error (±10 error is tolerable), it is in line with the national target of 80%20 which is 16.1% higher than the study conducted in Addis Ababa27 but less than the international target value by 15.9%.17 This is possibly due to the larger the sample size in the previous study conducted in Addis Ababa likely incorporated more low-performing health facilities. Indeed, the data quality of logistic records and reports is crucial to keep the six rights in the logistics system. This to be in mind, the current study showed that the data accuracy 18 (78.3%), timeliness 15 (65.22%), and completeness 7 (30.3%) of RRF reports were below the national20 and international17 target values. It also disagrees with another cross-sectional study conducted in a similar setting that RRF reports found to be complete 24 (92.6%) and accurate 5 (60%).27 The qualitative findings of the current study identified that most health facilities mainly used a manual reporting system with encountered significant errors. Though our study facilities encountered deprived data quality, health facilities had good wastage management practices with an acceptable wastage rate of 0.0083% (< 2%).28 On the other hand, in southern Ethiopia, the wastage rate was unacceptable at 2.5%.29 Such difference between the current and the study conducted in southern Ethiopia could be due to the difference in setting and sample size where the previous study could incorporate poorly performing facilities with less logistical infrastructure compared to health facilities located in Addis Ababa. The present study showed that only 9 (39.1%) health facilities have fulfilled the criteria for acceptable storage conditions supported by the qualitative finding that there was a deterioration of stocks. This is lower than a study that 25 (83.3%) health facilities have been fulfilled the criteria for acceptable storage conditions29 though it might not be in line with the theoretical explanations that the current study was conducted in settings having more infrastructures than the previous study. It could be related to the lack of manpower, weak cooperation, and low commitment of staff identified by the current qualitative study. This study was limited to public health facilities and the sample size was not sufficient to illuminate reliable inferential statistical analysis.
Conclusion
The current study revealed that health facilities suffered from a minimum of 4% stock out per day. Most of the data accuracy of bin cards and data quality of RRF reports was poor and intolerable. Such poor practices were likely related to supply, staff, and documentation challenges. On the other hand, the wastage rate was acceptable STATPAKTM was fully available throughout six months though it could not be the only means to assure rapid HIV testing services. Another study is recommended to determine factors associated with poor inventory management practice.
Abbreviations
HIV, human immunodeficiency virus; EPSA, Ethiopia Pharmaceuticals Supply Agency; LIAT, logistic indicator assessment tool; LMIS, logistic management information system; RTK, rapid test kit; UNAIDS, United Nations Programme on HIV/AIDS.
Data Sharing Statement
All necessary data included in the manuscript.
Ethics Approval and Consent to Participation
Ethical approval of the study was obtained from the Ethical Review Board of Jimma University, Institute of Health (Ref. No. IRB 000241/2012). Both written and verbal informed consent was obtained from all participants, before enrolling them in the study, including publication of anonymized responses while personal identifiers were not used during reporting and publishing the result.
Acknowledgment
The authors would like to acknowledge Jimma University for facilitating the study.
Funding
This research received no specific fund.
Disclosure
The authors declared they have no conflicts of interest for this work.
References
1. World Health Organization. Consolidated Guidelines on HIV Testing Services. 5Cs: Consent, Confidentiality, Counselling, Correct Results and Connection. Geneva, Switzerland: WHO; 2015.
2. Staveteig S, Wang S, Head SK, Bradley SEK, Nybro E. Demographic patterns of HIV testing uptake in Sub-Saharan Africa. DHS Comparative Reports No. 30. Calverton, Maryland, USA: ICF International; 2013.
3. Crucitti T, Taylor D, Beelaert G, Fransen K, Van Damme L. Performance of a rapid and simple HIV testing algorithm in a multicenter Phase III microbicide clinical trial. Clin Vaccine Immunol. 2011;18(9):1480–1485. PMID: 21752945; PubMed Central PMCID: PMCPMC3165239. doi:10.1128/CVI.05069-11
4. Pavie J, Rachline A, Loze B, et al. Sensitivity of five rapid HIV tests on oral fluid or finger-stick whole blood: a real-time comparison in a healthcare setting. PLoS One. 2010;5(7):e11581. PMID: 20657834; PubMed Central PMCID: PMCPMC2906506. doi:10.1371/journal.pone.0011581
5. Shanks L, Siddiqui MR, Kliescikova J, et al. Evaluation of HIV testing algorithms in Ethiopia: the role of the tie-breaker algorithm and weakly reacting test lines in contributing to a high rate of false positive HIV diagnoses. BMC Infect Dis. 2015;15(1):39. PMID: 25645240; PubMed Central PMCID: PMCPMC4331460. doi:10.1186/s12879-015-0769-3
6. Allers C, O’Hearn T, Kagone M. Sierra Leone: Supply Chain Assessment for ARV Drugs and HIV Test Kits. Arlington, Va: USAID | DELIVER PROJECT Task Order 1; April 2007.
7. Pasquet A, Messou E, Gabillard D, Minga A, Depoulosky A. Impact of drug stock-outs on death and retention to care among HIV-infected patients on combination antiretroviral therapy in Abidjan. PLoS One. 2010;5(10):2010. doi:10.1371/journal.pone.0013414
8. Doris B, Chafulumira F, Felling B, Msipa P, Mwenda R. LaboratoryServices and Supply Chain Assessment. Arlington, Va: USAID | DELIVER PROJECT, Task Order 1; 2009.
9. Augusto ÂD, Iriemenam NC, Kohatsu L, et al. High level of HIV false positives using EIA-based algorithm in survey: importance of confirmatory testing. PLoS One. 2020;15(10):e 0239782. doi:10.1371/journal.pone.0239782
10. Omo-Emmanuel U, Chinedum O, Emmanuel IO, Michael O, Negedu-Momoh O. Evaluation of laboratory logistics management information system in HIV/AIDS comprehensive health facilities in Bayelsa State, Nigeria. Int J Curr Res Med Sci. 2017;3(1):21–38.
11. Allers C, Mwirotsi A. Republic of Malawi: HIV Test Kit Supply Chain Assessment and Quantification. Arlington, Va: USAID | DELIVER PROJECT Task Order 1; April 2008.
12. Desale A, Taye B, Belay G, Nigatu A. Assessment of laboratory logistics management information system practice for HIV/AIDS and tuberculosis laboratory commodities in selected public health facilities in Addis Ababa, Ethiopia. Pan Afr Medl J. 2013;15. doi:10.11604/pamj.2013.15.46.1969
13. Schoonenboom J, Johnson R. How to construct a mixed methods research design. Kolner Z Soz Sozpsychol. 2017;69(S2):107–131. doi:10.1007/s11577-017-0454-1
14. Addis Ababa, Ethiopia population- population stat [Internet]. Populationstat.com; 2020 [cited 13 August 2021]. Available from: https://populationstat.com/ethiopia/addis-ababa.
15. USAID | DELIVER PROJECT, Task Order 1. Logistics Indicators Assessment Tool (LIAT): HIV Test Kits. Arlington, Va: USAID | DELIVER PROJECT, Task Order 1; 2009.
16. PFSA. Standard operating procedures (SOP) manual for the integrated pharmaceuticals logistics system in health facilities of Ethiopia. Addis Ababa, Ethiopia; Nov, 2015. Available from: www.pfsa.gov.et/webadmin/IPLS.
17. World Health Organization. Harmonized monitoring and evaluation indicators for procurement and supply management systems: early-warning indicators to prevent stock-outs and overstocking of antiretroviral, antituberculosis and antimalaria medicines. Available from: apps.who.int/iris/handle/10665/44546. Accessed March 20, 2022.
18. Assessment Tool for Laboratory Services. USAID Global Health Supply Chain Program [Internet]. Ghsupplychain.org; 2017 [cited 19 August 2021]. Available from: https://www.ghsupplychain.org/assessment-tool-laboratory-services-2017.
19. USAID deliver project, task order 1. Logistics system assessment tool (LSAT) [Internet]. Arlington, Va: USAID DELIVER PROJECT, Task Order 1; 2009. Available from: https://publications.jsi.com/JSIInternet/Inc/Common/_download_pub.cfm?id=14130&lid=3.
20. Federal Ministry of Health. National pharmacy service, pharmaceuticals supply chain and medical equipment monitoring and evaluation frame work. Addis Ababa; 2019.
21. Shewarega A, Dowling P, Necho W, et al. Ethiopia: National Survey of the Integrated Pharmaceutical Logistics System. Arlington, Va.: USAID | DELIVER PROJECT, Task Order 4, and Pharmaceuticals Fund and Supply Agency (PFSA); 2015.
22. John Snow, Inc. The Supply Chain Manager’s Handbook, a Practical Guide to the Management of Health Commodities. Arlington, VA 22209 USA: John Snow, Inc; 2019.
23. Consolidated guidelines on HIV testing services [Internet]. www.who.int; 2015 [cited September 13, 2021]. Available from: http://www.who.int/hiv/pub/vct/hiv-self-testing-guidelines/en/.
24. Federal ministry of health. National guidelines for comprehensive HIV prevention, care and treatment. Adis ababa; 2017: 20.
25. Wahlfeld CC, Muicha A, Harrison P, et al. HIV rapid diagnostic test inventories in Zambézia province, Mozambique: a tale of 2 test kits. Int J Health Policy Manag. 2019;8(5):292–299. doi:10.15171/ijhpm.2019.07
26. USAID GHSC-PSM Single Award IDIQ. Assessment of the HIV Rapid Test Kits: Supply Chain in Zambezia and Maputo Provinces. Maputo, Mozambique: Task Order No. AID-OAA-TO-15-00007 Subcontract number; 2017:6–36.
27. Tilahun A, Geleta DA, Abeshu MA, Geleta B, Taye B. Assessment of integrated pharmaceutical logistic system for the management HIV/AIDS and tuberculosis laboratory diagnostic commodities in public health facilities in Addis Ababa, Ethiopia. J Pharma Care Health Sys. 2016;3:158. doi:10.4172/2376-0419.1000158
28. USAID | DELIVER PROJECT, Task Order 1. Quantification of Health Commodities: HIV Test Kit Companion Guide, Forecasting Consumption of HIV Test Kits. Arlington, Va.: USAID | DELIVER PROJECT, Task Order 1; 2009.
29. Damtie TA, Ibrahim AJ, Yikna BB. Supply chain management performance of HIV/AIDS commodities and factors affecting it at health facilities of SNNPRS of Ethiopia; from the perspective of achieving 90- 90-90 Strategies. Integr Pharm Res Pract. 2020;9:11–21. doi:10.2147/IPRP.S228162
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.