Back to Journals » Clinical Ophthalmology » Volume 17
Assessment of Direct Costs of Admission Due to Presumed Microbial Keratitis in a Tertiary Referral Hospital in Thailand: A 7-Year Retrospective Study
Authors Chantra S , Chotcomwongse P, Jittreprasert S, Senarak W, Amornpetchsathaporn A, Kemchoknatee P , Ruamviboonsuk P
Received 8 June 2023
Accepted for publication 22 September 2023
Published 29 September 2023 Volume 2023:17 Pages 2845—2860
DOI https://doi.org/10.2147/OPTH.S425058
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Somporn Chantra,1,2 Peranut Chotcomwongse,1 Supachase Jittreprasert,1 Wirapha Senarak,1,* Anyarak Amornpetchsathaporn,1,* Parinee Kemchoknatee,1,2 Paisan Ruamviboonsuk1,2
1Department of Ophthalmology, Rajavithi Hospital, Bangkok, Thailand; 2College of Medicine, Rangsit University, Bangkok, Thailand
*These authors contributed equally to this work
Correspondence: Somporn Chantra, Department of Ophthalmology, Rajavithi Hospital, 2 Phayatai Road, Bangkok, 10400, Thailand, Tel +66 86-541-3765, Email [email protected]
Objective: To evaluate the direct healthcare cost of admission and examine the effects of cost drivers of treating presumed microbial keratitis (MK) at a tertiary referral hospital.
Design: Retrospective study.
Methods: A total of 741 patients who presented with MK were included. All information regarding costs was collected, and demographic data were employed for risk factor analysis.
Results: The total cost of treating MK over a 7-year period at Rajavithi Hospital was US$14,514,625.04, while the median cost was US$10,840.17 per patient (Q1– 3, US$5866.56– 24,172.28). The medical professional services were the highest cost category in terms of both total cost of treatment over 7 years and median cost per patient, accounting for US$6,474,718.43 and US$5235.20 (Q1– 3, US$2582.79– 10,474.24) respectively. In 2020, the total cost of treatment declined, corresponding with fewer hospitalized patients; however, the median cost per patient was the highest of all years, amounting to US$15,089.90 (Q1– 3, US$8064.17– 29102.50), while the median cost per patient from 2014 to 2019 was US$9969.96 (Q1– 3, US$5177.98– 21,942.68). Statistical significance was found in the median cost per patient in 2020 compared to the median cost per patient in 2014– 2019 (p-value 0.019). Risk factors associated with the more expensive cost of treatment were longer length of stay (LOS); age more than 60 years old; readmission; diabetes mellitus (DM); hypertension; and heart disease.
Conclusion: There were several key factors impacting the direct healthcare costs of severe MK treatment. Medical professional services emerged as the most substantial cost category, while longer hospital stays, older age groups, readmission cases, and comorbidities such as diabetes mellitus, hypertension, and heart disease were all linked to elevated treatment expenses. There were no statistically significant differences in the direct medical expenses during hospitalization associated with treating severe MK, whether the culture results were positive or negative, or regardless of the type of cultured organism utilized.
Keywords: corneal ulcer, infectious keratitis, healthcare cost, economic burden
Introduction
Microbial keratitis (MK) is an infection of the cornea which can lead to visual impairment and corneal blindness. According to a systematic review and meta-analysis on global causes of blindness and distance vision impairment from 1990–2020, it was estimated that approximately 1.3 million people had visual blindness, and 2.9 million suffered from visual impairment due to corneal opacity.1 It is difficult to uncover epidemiological information specific to MK since most of the data are recorded under the umbrella termed “corneal blindness or corneal opacity” which encompasses a variety of corneal disorders.1 Corneal infections, trauma, inflammation, degeneration, nutritional deficiencies, dystrophies, and other distinct factors, including onchocerciasis, trachoma, and iatrogenic sources, all contribute to corneal opacification.2 MK undeniably stands as a notable contributor to corneal opacification and blindness, exhibiting varying incidence rates among different populations.2,3 The Asia Cornea Society Infectious Keratitis Study collected data from thirteen study centers and 30 sub-centers in Asia and found that trauma was the commonest risk factor (accounting for 34.7%), and that clinical diagnosis of bacterial infection was slightly more common than that of fungal keratitis (38% vs 32.7%). Among the isolated microorganisms, fusarium was the most prevalent, followed by Pseudomonas aeruginosa and Aspergillus flavus.4 While the gold standard for diagnosis of MK is culture from corneal sampling, the median culture positivity rate from clinically diagnosed MK is only 50.3%;5 therefore, the initial treatment plan is based on presumptive clinical diagnosis, and subsequent treatment is adjusted in accordance with microbiology results. As MK may progress rapidly and be resistant to treatment, therapy should be commenced as a matter of urgency. Topical medication should be started promptly and surgical intervention may be needed to control the infection and prevent visual impairment.6 Some patients need to be admitted for in-patient treatment in certain scenarios, such as where there is any immediate visual threat; when patients have not responded well to previous treatment at an out-patient department (OPD); when it is advisable to pursue hourly treatment; when follow-up ensuring is requires; or when the patient is a child.7 Generally, the in-patient admission cost varies substantially depending on several factors, such as cause of admission, individual health situation, medications, surgical interventions, investigations, hospital fees and doctors’ charges.
The cost of health care is growing constantly. A report from the World Health Organization (WHO) showed that global spending on health rose continually between 2000 and 2018 and reached US$ 8.3 trillion or 10% of global GDP.8 With regard to ophthalmology, it has been forecast that the global visual loss burden will increase, with the number of people suffering from blindness or visual impairment expected to rise as the global population grows and ages.9 To estimate the probable cost of MK treatment in the future, an evaluation of the economic impact of MK treatment is necessary. It has been established that the factors associated significantly with high overall admission costs for MK treatment include length of stay (LOS),10 old age,11 and fungal etiology.12–14
The purposes of this study were: firstly, to identify the direct healthcare cost of admission and future trends of presumed MK treatment at a tertiary referral hospital in Thailand; and secondly, to examine the effects of cost drivers on direct admission costs.
Methods
Study Site and Data Collection
The protocol of this research was reviewed and approved by the ethics committee of Rajavithi Hospital (No. 64100/ 2564). Rajavithi Hospital is located in the center of Bangkok, Thailand, and it is the largest public hospital under the Ministry of Public Health management and ownership. We have 3 cornea specialists, who are responsible for the assessment of more than 500 referral cornea and external eye disease cases each year. This was a retrospective cross-sectional study using data from medical record audits at Rajavithi Hospital. We identified all patients who were hospitalized with presumed MK between January 1, 2014 and December 31, 2020. We included cases of diagnosis of corneal ulcer (H160), injury of conjunctiva and corneal abrasion without mention of foreign body (S050), and ocular laceration without prolapse or loss of intraocular tissue (S053). All cases were coded according to the International Classification of Diseases, 10th Revision, Clinical Modification Codes (ICD-10-CM). All cases were reviewed. We excluded cases which lacked data required for evaluation, as well as diagnoses without signs of MK. All included cases were confirmed by at least 1 of 3 cornea specialists.
Demographic data from patients’ records included gender, age at admission, health insurance coverage, region of residence, nationality, underlying diseases, LOS, number of admissions, initial best corrected visual acuity (BCVA), and treatment according to presumed organism. VA data on first admission date (initial VA) were collected. We classified the distance vision impairment of our study cases into 4 groups according to the international classification of diseases in 2020 as follows.15 Distance vision impairment: mild - VA ≥ 20/60. Moderate - VA < 20/60 and ≥ 20/200. Severe - VA < 20/200 and ≥ 20/400. Blindness - VA < 20/400. We collected clinical findings at presentation in all cases in this study, including size, depth, and location of the corneal infiltration, and then classified them using a modified version of Jones’ grading criteria.16
Direct costs of admission are those expenses directly attributed to patient care. To better differentiate the root of treatment expense, we classified costs into 5 categories: hospital services costs; medical professional services costs; investigation costs; operative treatment costs; and non-operative treatment costs. The details of each cost category are described in Table 1.
 |
Table 1 Categories of Direct Cost of Admission for Presumed MK |
The Thailand Consumer Price Index (CPI) for medical care was used to convert the cost data to year 2020 values, and the adjusted figure was used for analysis. Using the 2020 exchange rate of 1 US$ = 35.0 THB, all costs were first calculated in Thai Baht (THB) and then converted to US dollars (US$).
All methods were conducted in adherence to pertinent guidelines and regulations. The requirement for informed consent from participants was waived by the Institutional Review Board of the Rajavithi Hospital ethics committee due to the retrospective nature of our study. We affirm that the data was anonymized and treated with strict confidentiality. This study was in compliance with the principles outlined in the Declaration of Helsinki.
Statistical Methods
We performed statistical calculations using SPSS V.26.0 (IBM SPSS Statistics for Windows). Descriptive statistics were used to summarize the demographic characteristics and clinical findings of the study participants. Continuous variables, such as age at admission, LOS, number of admissions, are presented as median and interquartile range (Q1–Q3). Categorical variables, including gender, underlying diseases, treatment based on presumed organism, group of initial visual acuity, age group, and readmission group are presented as frequencies and percentages. For continuous data, distribution was firstly evaluated using skewness and histogram plots. Normal distribution was assumed if skewness was between −1.96 and +1.96. To compare these variables between two groups, Student’s t-test and Mann–Whitney test were used for parametric and non-parametric tests respectively. Kruskal-Wallis test was also performed with nonparametric test to determine differences among 3 groups or more. Spearman correlation coefficient was used to determine correlations between age at first visit, LOS and cost of treatment. The interpretation was as follows: weak (r=0.00–0.40), moderate (r=0.41–0.69), and strong (r=0.70–1.00). R2 was also assessed to determine the strength of the relationship of this correlation, and statistical significance was set at p-value<0.05.
Results
The electronic inpatient records of the 1102 included patients were reviewed. We excluded 345 patients where the main cause of diagnosis was due to other causes, including non-infectious peripheral ulcerative keratitis (PUK), endophthalmitis, traumatic rupture globe and intraocular foreign body (IOFB), as shown Figure 1. The cost data for 16 patients were not completely available, and these cases were therefore excluded, leaving 741 patients with presumed MK with a total of 888 admission visits in the study. The numbers of patients who presented in each year, from 2014 to 2020, were 85, 121, 134, 103, 119, 120, and 59 patients respectively, as shown in Figure 2. All of the cases were classified as severe MK. Severe IK is defined as a diameter greater than 5 mm, a depth greater than 50%, dense infiltrates reaching the deep layers of the corneal stroma, hypopyon, and possibly scleral involvement.16
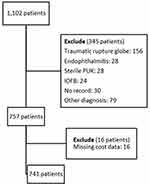 |
Figure 1 Flow of the inclusion and exclusion criteria. |
 |
Figure 2 The number of MK patients presenting per year. |
Costs and Cost Trends
The total cost of treating MK over these 7 years at Rajavithi Hospital was US$14,514,625.04. The median cost over the 7 years was US$10,840.17 per patient (Q1–3, US$5866.56–24,172.28), ranging from US$864.37 to US$156,048.35. Details of each sub-category are listed in Table 2 and Table 3. We found that the highest median cost per patient and total cost over 7 years was medical professional services costs, accounting for US$5235.20 (Q1–3, US$2582.79–10,474.24) and US$6,474,718.43 respectively. Figures 3 and 4 displays trends of total costs and median costs per patient in each admission year. The total cost showed a slightly decreasing trend. In 2020, we found that total cost of treatment declined, corresponding with fewer hospitalized patients; however, the median cost per patient in 2020 was the highest of all years, amounting to US$15,089.90 (Q1–3, US$8064.17–29102.50), while the median cost per patient from 2014 to 2019 was US$9969.96 (Q1–3, US$5177.98–21,942.68). Statistically significant differences were observed in the median total cost per patient in 2020 when compared to the median total cost per patient in 2014–2019. Additionally, statistically significant differences were found in non-operative treatment and hospital services costs per patient in 2020 compared to the median total cost per patient in 2014–2019, while no significant differences were observed in other cost categories.
 |
Table 2 Annual Total Cost in Each Cost Category by Admission Year (2014–2020) |
 |
Table 3 Average Cost per Patient in Each Cost Category by Admission Year (2014–2020) |
 |
Figure 3 Trends of total cost of treatment in each admission year. |
 |
Figure 4 Trends of median cost per patient in each admission year. |
Gender
Of the 741 patients, 462 (62.3%) were male and 279 (37.7%) were female. The median treatment costs per patient showed no statistically significant difference between males and females (US$11,713.92 (Q1–3, US$6107.50–24,687.30) vs US$9030.89 (Q1–3, US$5473.66–25,502.42) respectively, p-value=0.225) (Table 4).
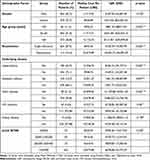 |
Table 4 Patient Demographics, Readmission, Underlying Diseases, and Treatment Costs |
Age
The mean age of the patients in this study was 52.4 (range, 7–95) years old. We explored the correlation between age and cost and found a weak but significant correlation between patient age and cost in the study (R2=0.0034, p<0.001). Pearson’s correlation coefficient revealed a small, but significant, positive relationship. (r= 0.34, p<0.001).
Age Group
To better highlight the cost of MK treatment, we stratified patients into 3 groups according to their age at MK diagnosis as group1 (<30 years old), group 2 (30 to 60 years old), group 3 (>60 years old or more). There were 84 (11.4%), 384 (51.8%) and 273 (36.8%) in each group, respectively, and their median costs per patient were US$4294.22 (Q1–3, US$3881.52–8013.55), US$11,713.71 (Q1–3, US$5567.38–24,434.15) and US$13,123.24 (Q1–3, US$8121.85–29,699.60) respectively. All differences among these groups were statistically significant p-value <0.001 via Kruskal Wallis test (Table 4).
Readmission
There were 888 admission visits by the 741 patients in this study, ranging from 1 to 6 admissions per patient. Patients were grouped into 2 categories: single admission (626 patients, 87.6%), and readmission (115 patients, 12.4%). In the readmission category, 92, 17, 4, 1, and 1 patients had 2, 3, 4, 5, and 6 hospital readmission visits, respectively.
The total cost per patient was US$8692.56 (Q1–3, US$5229.29–19,305.94) in single admission group and US$33,019.89 (Q1–3, US$18,834.73–46,095.59) in readmission group, statistically significant difference with p<0.001. Patients in the readmission group tended to stay longer than those in the single admission group, at 34 days (Q1–3, 21–48 days) and 15 days (Q1–3,7–23 days, p<0.001) respectively (Table 4).
Underlying Disease
A total of 297 (40.1%) patients had prior underlying diseases. Among these 297 patients, hypertension was the most common underlying disease, accounting for 185 patients followed by diabetes mellitus (DM) 103 patients, heart disease 38 patients, HIV 30 patients and kidney disease 15 patients. The cost of treating MK patients with hypertension, DM, and heart disease were higher than therapy for those without these diseases with statistically significance difference p< 0.001, p=0.007, and p<0.001, respectively, while the other two underlying conditions showed no statistically significant differences, as shown in Table 4.
Initial Best Corrected Visual Acuity
A total of 620 patients (83.7%) initially presented with blindness. Of the rest, 33 (4.5%), 53 (7.2%) and 32 (4.3%) had severe visual impairment, moderate visual impairment, and normal to mild visual impairment, respectively. Patients with blindness incurred the highest cost of all groups. Statistical significance was found when comparing moderate visual impairment vs severe visual loss (p=0.04) and moderate visual impairment vs blindness (p<0.001), as shown in Table 3.
Length of Stay
The median LOS was 12 days, ranging from 1 to 86 days (Q1–3, 7–27 days). As shown in Figure 5, LOS showed a good linear relationship with median cost per patient (R2=0.738), and Spearman correlation coefficient revealed a strong and significant positive relationship (r= 0.902, p<0.001).
 |
Figure 5 Plot graph illustrating the relationship between the length of stay and cost of treatment. |
Treatment Groups
When cultured, out of 227 patients (30.6%), there were no signs of growth for any organisms. In the case of 341 patients (46.0%), positive bacterial cultures were observed, while 156 patients (21.0%) exhibited fungal infections. A smaller portion, 10 patients (1.3%), showed positive cultures for viruses, and 7 patients (0.9%) had parasitic infections, all of which were caused by Acanthamoeba species. Table 5 compares clinical characteristics among patients with different culture results. For patients with negative and positive cultures, no statistically significant differences were observed in age at presentation, number of surgical treatments, LOS, and BCVA at presentation, with p-values of 0.086, 0.133, 0.955, and 0.698, respectively. Similarly, among patients with positive cultures, there were no statistically significant differences in these clinical characteristics, with p-values of 0.070, 0.630, 0.580, and 0.641, respectively. Table 6 presents a comparison of the direct MK treatment costs per patient in each cost category for inpatients, categorized by culture-proven causative organisms. There was no statistically significant difference (p-value 0.312) between the total direct treatment costs for patients with negative cultures (median US$9998.88, Q1–Q3 US$5580.95–21,660.23) and those with positive cultures (median US$10,836.74, Q1–Q3 US$5966.79–25,056.15). Regardless of whether the expenses were incurred as part of hospital services costs, medical professional services costs, investigation costs, operative treatment costs, or non-operative treatment costs (p-values 0.680, 0.984, 0.105, 0.604, and 0.263). Among culture-positive cases, there was no statistically significant difference in direct healthcare costs in each category. However, it’s worth noting that cases of parasite infections among culture-positive patients resulted in the highest expenditure for inpatient care, primarily in terms of medical professional services and non-operative care, with each of these being statistically non-significant at p-values 0.918 and 0.344, respectively.
 |
Table 5 Comparison of Clinical Characteristics Among Patients with Different Culture Results |
 |
Table 6 Comparison of Direct MK Treatment Costs for Inpatients Based on Culture-Proven Causative Organisms |
Discussion
This was a 7-year retrospective study investigating the economic burden of treating hospitalized MK and its associated factors. Over the past 7 years, Rajavithi Hospital spent approximately 14 million US$ on treating 741 patients who initially presented with MK. The median cost per patient was US$10,840.17 (US$5866.56–24,172.28) which is higher than that of a previous study published in 2013 from Thailand (median cost per patient 20,699 THB = 673.55 USD, average exchange rate at the time of publication in 2013 of 30.731 THB/ 1 USD).14 In the United States, MK costs the health care system an estimated 175 million dollars in direct health expenditure.5 Compared with other countries, our median cost was higher than the average in Taiwan study in 2000–2013 (New Taiwan Dollar (NTD) 34,261.20 ± 40,906.62 (US$ 1027 ± 1227.20), but lower than that found in a UK study in January to December 2013 (2855 British Pound (GBP) (US$4465.22)).10,11 Keay et al reported that the cost of treating contact lens -related MK was for severe cases with vision loss, 1596 AUD (US$1085.28) for severe cases without vision loss, and 795 AUD (US$540.60) for mild MK17 (ANOVA, p= 0.001). Another study in Belgium reported a mean total cost of 3093,19 EUR. US$3835,56) for treating contact lens -related MK.18 In reality, expenditure in different countries cannot be directly compared because of the varying costs of living, medication prices and guaranteed minimum incomes.
The highest cost incurred in treating one individual patient in our study was US$156,048.35. This patient was initially admitted with MK at the eye department, but after 2 days, he developed hospital-acquired pneumonia and then sepsis. Due to his multiple underlying diseases, he was intubated and referred to an intensive care unit (ICU) where he received many systemic medications, blood transfusion and hemodialysis as required. Finally, he was safely discharged after spending 58 days in the hospital.
Hospitalization is one of the medical strategies that can help improve adherence to drug regimens. It is usually needed in difficult settings, such as with older age groups, with people who do not have access to means of transport, or individuals who lack adequate home healthcare. However, hospitalization may result in higher treatment cost, and it may also increase the risk of hospital-associated infection being transmitted to the patients, who could also face loss of income during their stay and possibly experience psychological problems. Many studies have revealed a reduction in the number of hospitalized COVID-19 cases due to lack of hospital beds, medical supplies and personnel.19,20 Bhatt et al reported that the number of hospitalized cardiovascular patients markedly decreased during the COVID-19 situation, and patient LOS has also been shorter during this time.21 However, ours is the first study to determine the reduction of hospitalized MK patients during the COVID-19 pandemic. Lowering LOS and accelerating patient discharge can yield multiple benefits in a variety of ways.22
As shown in Figure 3, total costs in 2020 displayed a significantly downward trend. The reason may be that, in Thailand as in other countries, we faced the pandemic crisis of COVID-19 throughout the entire year. The total cost burden declined as the number of patients markedly decreased; however, Figure 4 shows that the median cost per patient in 2020 rose above that of other years. This implies that, firstly, the hospitalized patients in 2020 were more severe cases and needed more treatment expense. Secondly, it reflects fact that the advent of the COVID-19 pandemic impeded the referral system by limiting our ability to transfer patients from peripheral to central areas, and some patients decided not to seek medical care in Bangkok hospitals due to the overcrowding resulting from the number of COVID-19 cases there. The implementation of self-treatment strategies or home health care could yield benefits, not only for patients but also for the hospitals.
In this retrospective study, the factors associated with higher costs included longer LOS, elderly, readmitted patients, DM, hypertension, and heart disease. LOS has been found in several studies to be the most significant factor affecting cost of medical care.10,14 A study by Moussa et al concurred that longer LOS is significantly associated with higher cost deficit.10 At Rajavithi Hospital, hospital and medical professional costs are charged at a fixed rate of 400 THB and 650 THB per day respectively. Therefore, the longer the patient stay, the higher the cost in both categories. Figure 5 also explicitly shows a strong and significant linear correlation between LOS and medical cost per patient. The longest LOS was 86 days, and this was the case of a female who was hospitalized on 5 separate visits. She was treated with penetrating keratoplasty (PKP) at her first visit; unfortunately, the disease was not brought under control and became recurrent on the graft. Later, another PKP was performed, and the total cost of all treatment courses amounted to US$109,087.03.
In our study, there was no statistically significant difference in the costs of treating males and females. Ballouz et al findings were in agreement with this, with gender not being associated with medical burden and treatment length.23 Another study from Taiwan, in contrast, reported that male gender predominated in an IPD group.11 The mean age of patients in this research was 52.4 years, but people of all ages have been shown to be affected by MK, and in our study, the age at the presentation ranged from 7 to 95 years old. The age group causing highest treatment expense was patients aged more than 60 years, and this is similar to the results of a previous study from Koh et al, who found that highest health care expenditure was incurred in treating those older than 65 years. They also reported that patients older than 40 years of age accounted for approximately 80% of the cost of treating MK.11 However, Boonpasart et al, who conducted a study in King Chulalongkorn Memorial Hospital, Thailand, found that the majority of patients were in the age groups 21–30 and 51–60 years of age.24
Regarding VA at initial presentation, we firstly hypothesized that worse VA would result in higher median costs per patient; however, statistical differences were identified only between moderated visual impairment VS severe visual impairment group and moderate visual impairment VS blindness group. This could be because the number of patients in the group with blindness was markedly larger than those of other groups.
The culture positivity rate in the literature varies, ranging between 24% and 77%.2,5,6,25–27 Despite our clinic’s status as a tertiary referral center and the fact that the majority of our patients were referred after receiving broad-spectrum antimicrobial therapy prior to presentation, we observed a positive culture rate of 69.4%. This rate aligns with the existing literature and represents a relatively high percentage. Our study revealed that among culture-positive patients, bacteria were the most common causative agents, accounting for 66.3% of cases, followed by fungal infections at 30.4%. This finding is consistent with previous research findings in Thailand.7,28 The predominant age group in both culture-positive and culture-negative patients was 30–60 years old. This observation aligns with the higher prevalence of MK within this age range, likely due to increased exposure to relevant risk factors. This finding is consistent with a comprehensive review of 14 epidemiologic studies conducted across diverse regions, including Asia, the United Kingdom, Europe, North America, South America, Africa, the Middle East, and Australia. The review highlighted a consistent trend, with the majority of MK cases occurring in individuals aged 30 to 55 years.2
Regarding treatment modalities, 63.8% of patients in our study received medical therapy alone, while 36.2% required surgical interventions such as therapeutic keratoplasty or evisceration/enucleation. Interestingly, no significant difference was found in the frequency of surgical interventions between the culture-positive group (34.6% of 514 patients) and the culture-negative group (39.6% of 227 patients). These results are in line with some previous studies that also compared culture results.29,30 However, it’s worth noting that certain other studies have reported a higher need for surgical intervention in the culture-positive group compared to the culture-negative group.31–33 These discrepancies may be attributed to variations in demographic data and risk factors among the study populations.29,31
In our study, we found that the median direct cost for treating a single episode of severe keratitis was US$10,840.17, with an interquartile range spanning from US$5866.56 to US$24,172.28. Notably, there was no significant difference in direct costs observed between cases with negative cultures and those with positive cultures. Among the latter group, individuals with parasitic infections incurred the highest direct costs, primarily due to expenses related to medical professional services. However, our statistical analysis did not reveal a significant difference in costs among the culture-positive cases. All the parasite cultures in our study were determined to be Acanthamoeba species. The treatment for Acanthamoeba keratitis typically involves the administration of multiple medications. These medications encompass a range of antimicrobial drugs such as chlorhexidine, propamidine, as well as other therapeutic agents including analgesics, cycloplegics, and sometimes antifungal agents.34,35 Importantly, the cost of these medications can accumulate significantly, especially if they must be used over an extended period.
Radhakrishnan et al reported that fungal keratitis carries the highest treatment costs compared to other types of infections, primarily due to its prolonged treatment duration and the elevated expenses associated with antifungal medications.36 We did not identify any statistically significant differences in the total cost of treating MK between patients with positive cultures and those with negative cultures. Additionally, among the subset of patients with positive cultures, we found no statistically significant distinctions in terms of medical professional costs, which constitute the primary expense in the treatment of MK. This observation may help explain the similar LOS between culture-negative and culture-positive patients in our study. The median LOS was 12 days for culture-negative patients, 12 days for bacterial infections, 12.5 days for fungal infections, 10 days for viral infections, and 7 days for parasitic infections. These comparable LOS figures suggest that the duration of hospitalization, a significant contributor to medical professional costs, was similar for both culture-positive and culture-negative patient groups in our research. Koh YY found that DM was associated with severity of condition requiring hospitalization or surgical interventions.11 Our study showed that higher costs were incurred not only in treatment of DM, but also of hypertension and heart disease.
Our goal in treating MK is to control infection and to limit visual impairment. Nevertheless, the economic burden both direct (ie diagnostic disorder, vision aids or devices, undiagnosed vision loss, educational, assistance programs) and indirect (ie productivity loss, informal care, nursing home, entitlement programs, tax deductions, transfer deadweight loss, and the constraints of salary during hospitalization) should also be considered.37 The health care coverage system was derived mainly from the government’s policy statement on expenditure, and as the government is responsible for paying for all standard health expenses of the Thai population, the ability to achieve a balance between costs and treatment outcomes is a primary concern affecting patients, physicians, and policymakers alike.
Strength and Limitations
This study provides the up-to-date treatment cost of MK for the past 7 years, including the COVID-19 situation. In considering 741 patients, this is one of the largest studies performed to evaluate the cost of MK treatment. We also indicate the primary sources of cost by classifying the subgroups of cost. With regard to limitations, firstly, this study may have underestimated the true cost of treating MK by omitting the non-hospitalized patients who were treated as OPD cases. COVID-19 markedly reduced the number of hospitalized patients, so this study’s findings may not truly reflect the real-world situation of MK, but it does raise some question regarding the current treatment strategy. Secondly, we included both MK-related and non-MK-related treatment cost; for example, the costs of treating unfavorable complications were also included for all patients which, overall, may have been an overestimation. Thirdly, since all cases in the study were categorized as severe MK, necessitating hospitalization. We did not endeavor to calculate the treatment costs across varying severity levels. It’s evident that managing mild and moderate MK cases would incur lower expenses due to the absence of hospitalization, potentially resulting in reduced treatment costs for MK at different severity levels. Fourthly, we gathered data on all eligible patients from their admission to the hospital until their discharge. The study did not encompass the direct cost of ongoing treatment in the outpatient department until their microbial keratitis was fully healed or until evisceration/enucleation took place. It’s evident that the total direct treatment cost until complete healing of their MK might surpass what has been reported in this study. Lastly, the study’s retrospective nature was another limiting feature.
Conclusion
Our findings highlight several key factors impacting the direct healthcare costs of severe microbial keratitis treatment. Notably, medical professional services emerged as the most substantial cost category, while longer hospital stays, older age groups, readmission cases, and comorbidities such as diabetes mellitus, hypertension, and heart disease were all linked to elevated treatment expenses. Remarkably, there were no statistically significant differences in the direct medical expenses associated with treating severe MK, whether the culture results were positive or negative, or regardless of the type of cultured organism utilized.
Acknowledgment
We wish to express our gratitude to Mr. John Flanagan for his proofreading of this research project, which contributed to its clarity and accuracy. The support received from the Rajavithi research grant (Grant number 64100/2564) has been instrumental in advancing our study. We also extend our thanks to Assoc. Prof. Dusit Sujirarat, Krissana Arsayot, and Panida Yoopetcch for their assistance in conducting statistical analysis and providing constructive feedback, which significantly enhanced the quality and rigor of this research manuscript.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Flaxman SR, Bourne RRA, Resnikoff S, et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Heal. 2017;5(12):e1221–34. doi:10.1016/S2214-109X(17)30393-5
2. Ting DSJ, Ho CS, Deshmukh R, Said DG, Dua HS. Infectious keratitis: an update on epidemiology, causative microorganisms, risk factors, and antimicrobial resistance. Eye. 2021;35(4):1084–1101. doi:10.1038/s41433-020-01339-3
3. Song X, Xie L, Tan X, et al. A multi-center, cross-sectional study on the burden of infectious keratitis in China. PLoS One. 2014;9(12):e113843. doi:10.1371/journal.pone.0113843
4. Khor WB, Prajna VN, Garg P, et al. The Asia cornea society infectious keratitis Study: a prospective multicenter study of infectious keratitis in Asia. Am J Ophthalmol. 2018;195:161–170. doi:10.1016/j.ajo.2018.07.040
5. Ung L, Bispo PJM, Shanbhag SS, et al. The persistent dilemma of microbial keratitis: global burden, diagnosis, and antimicrobial resistance. Surv Ophthalmol. 2019;64(3):255–271. doi:10.1016/j.survophthal.2018.12.003
6. Lin A, Rhee MK, Akpek EK, et al. Bacterial keratitis preferred practice pattern. Ophthalmology. 2019;126(1):1–55. doi:10.1016/j.ophtha.2018.10.018
7. Tananuvat N, Suwanniponth M. Microbial keratitis in Thailand: a survey of common practice patterns. J Med Assoc Thai. 2008;91(3):316–322.
8. World Health Organization. Global spending on health: weathering the storm 2020. Working paper; 2020.
9. Bourne RRA, Steinmetz JD, Flaxman S, et al. Trends in prevalence of blindness and distance and near vision impairment over 30 years: an analysis for the global burden of disease study. Lancet Glob Heal. 2021;9(2):e130–43. doi:10.1016/S2214-109X(20)30425-3
10. Moussa G, Hodson J, Gooch N, et al. Calculating the economic burden of presumed microbial keratitis admissions at a tertiary referral centre in the UK. Eye. 2021;35(8):2146–2154. doi:10.1038/s41433-020-01333-9
11. Koh YY, Sun CC, Hsiao CH. Epidemiology and the estimated burden of microbial keratitis on the health care system in Taiwan: a 14-year population-based study. Am J Ophthalmol. 2020;220:152–159. doi:10.1016/j.ajo.2020.07.026
12. Prajna VN, Nirmalan PK, Saravanan S, Srinivasan M. Economic analysis of corneal ulcers in South India. Cornea. 2007;26(2):119–122. doi:10.1097/ICO.0b013e31802b36dc
13. Keay L, Edwards K, Naduvilath T, et al. Microbial keratitis: predisposing factors and morbidity. Ophthalmology. 2006;113(1):109–116. doi:10.1016/j.ophtha.2005.08.013
14. Kampitak K, Patrasuwan S, Kongsomboon K. Cost evaluation of corneal ulcer treatment. J Med Assoc Thail. 2013;96(4):456–459.
15. Bourne RRA, Steinmetz JD, Saylan M, et al. Causes of blindness and vision impairment in 2020 and trends over 30 years, and prevalence of avoidable blindness in relation to VISION 2020: the right to sight: an analysis for the global burden of disease study. Lancet Glob Heal. 2021;9(2):e144–60. doi:10.1016/S2214-109X(20)30489-7
16. Gurnani B, Kaur K. Bacterial keratitis. In: StatPearls. StatPearls Publishing LLC; 2022.
17. Keay L, Edwards K, Dart J, Stapleton F. Grading contact lens-related microbial keratitis: relevance to disease burden. Optom Vis Sci. 2008;85(7):531–537. doi:10.1097/OPX.0b013e31817dba2e
18. Verhelst D, Koppen C, Van Looveren J, Meheus A, Tassignon MJ. Clinical, epidemiological and cost aspects of contact lens related infectious keratitis in Belgium: results of a seven-year retrospective study. Bull Soc Belge Ophtalmol. 2005;297:7–15.
19. Roth AR, Lazris A. Benefits of avoiding unnecessary medical care during the COVID-19 pandemic. Am Fam Physician. 2021;103(10):584–585.
20. Portela MC, Cristina C, Pereira DA, Maria S, Lima L. Patterns of hospital utilization in the unified health system in six Brazilian capitals: comparison between the year before and the first six first months of the COVID-19 pandemic. BMC Health Serv Res. 2021;21:1–13. doi:10.1186/s12913-020-05996-8
21. Bhatt AS, Moscone A, Mcelrath EE, Varshney AS. Fewer hospitalizations for acute cardiovascular conditions during the COVID-19 pandemic. J Am Coll Cardiol. 2020;76(January):280–288. doi:10.1016/j.jacc.2020.05.038
22. Caminiti C, Meschi T, Braglia L, et al. Reducing unnecessary hospital days to improve quality of care through physician accountability: a cluster randomised trial. BMC Health Serv Res. 2013;13(1):1. doi:10.1186/1472-6963-13-14
23. Ballouz D, Maganti N, Tuohy M, Errickson J, Woodward MA. Medication burden for patients with bacterial keratitis. Cornea. 2019;38(8):933–937. doi:10.1097/ICO.0000000000001942
24. Boonpasart S, Puangsricharern V, Jittpoonkusol T, Kasetsuwan N, Pariyakanok L. Infectious keratitis at King Chulalongkorn Memorial Hospital: a-12-year retrospective study of 391 cases. J Med Assoc Thail. 2002;85(1):217–230.
25. Ting DSJ, Settle C, Morgan SJ, Baylis O, Ghosh S. A 10-year analysis of microbiological profiles of microbial keratitis: the North East England Study. Eye. 2018;32(8):1416–1417. doi:10.1038/s41433-018-0085-4
26. Peng MY, Cevallos V, McLeod SD, Lietman TM, Rose-Nussbaumer J. Bacterial keratitis: isolated organisms and antibiotic resistance patterns in San Francisco. Cornea. 2018;37(1):84–87. doi:10.1097/ICO.0000000000001417
27. Kaliamurthy J, Kalavathy CM, Parmar P, Nelson Jesudasan CA, Thomas PA. Spectrum of bacterial keratitis at a tertiary eye care centre in India. Biomed Res Int. 2013;2013:1–8. doi:10.1155/2013/181564
28. Jongkhajornpong P, Nimworaphan J, Lekhanont K, Chuckpaiwong V, Rattanasiri S. Predicting factors and prediction model for discriminating between fungal infection and bacterial infection in severe microbial keratitis. PLoS One. 2019;14(3):e0214076. doi:10.1371/journal.pone.0214076
29. Günaydin NT, Kandemir B, Gokce GD, Özen MC, Gün RD, Haciseyitoğlu D. Comparison of culture-positive and culture-negative severe infectious keratitis leading to hospitalization: a tertiary referral center experience. J Heal Sci Med. 2022;5(6):1612–1618.
30. Duarte MCB, Becker GN, Muller GG, Tuon FF. Infectious keratitis in southern Brazil: a comparison culture negative and culture positive patients. Rev Bras Oftalmol. 2020;79(1):46–52.
31. Bhadange Y, Das S, Kasav MK, Sahu SK, Sharma S. Comparison of culture-negative and culture-positive microbial keratitis: cause of culture negativity, clinical features and final outcome. Br J Ophthalmol. 2015;99(11):1498–1502. doi:10.1136/bjophthalmol-2014-306414
32. Sharma S, Taneja M, Gupta R, et al. Comparison of clinical and microbiological profiles in smear-positive and smear-negative cases of suspected microbial keratitis. Indian J Ophthalmol. 2007;55(1):21–25. doi:10.4103/0301-4738.29490
33. Yarımada S, Selver ÖB, Palamar M, et al. Comparison of culture-positive and -negative microbial keratitis. Turkish J Ophthalmol. 2022;52(1):1–5. doi:10.4274/tjo.galenos.2021.98046
34. Fanselow N, Sirajuddin N, Yin XT, Huang AJW, Stuart PM. Acanthamoeba keratitis, pathology, diagnosis and treatment. Pathog. 2021;10(3):1–11. doi:10.3390/pathogens10030323
35. Lorenzo-Morales J, Khan NA, Walochnik J. An update on Acanthamoeba keratitis: diagnosis, pathogenesis and treatment. Parasite. 2015;22:10. doi:10.1051/parasite/2015010
36. Radhakrishnan N, Pathak N, Subramanian KR, et al. Comparative study on costs incurred for treatment of patients with bacterial and fungal keratitis - A retrospective analysis. Indian J Ophthalmol. 2022;70(4):1191. doi:10.4103/ijo.IJO_2176_21
37. Owens DK, Qaseem A, Chou R, Shekelle P. Clinical guideline high-value, cost-conscious health care: concepts for clinicians to evaluate the benefits, harms, and costs of medical interventions. Ann Intern Med. 2011;154(3):174–180. doi:10.7326/0003-4819-154-3-201102010-00007
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
