Back to Journals » International Journal of Women's Health » Volume 15
Assessment of Cardiovascular Risk in Women: Progress so Far and Progress to Come
Authors Tschiderer L , Seekircher L, Willeit P, Peters SA
Received 1 December 2022
Accepted for publication 3 February 2023
Published 10 February 2023 Volume 2023:15 Pages 191—212
DOI https://doi.org/10.2147/IJWH.S364012
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Elie Al-Chaer
Lena Tschiderer,1,2 Lisa Seekircher,1 Peter Willeit,1,3 Sanne AE Peters2,4,5
1Institute of Health Economics, Medical University of Innsbruck, Innsbruck, Austria; 2Julius Center for Health Sciences and Primary Care, University Medical Center Utrecht, Utrecht, the Netherlands; 3Department of Public Health and Primary Care, University of Cambridge, Cambridge, UK; 4The George Institute for Global Health, School of Public Health, Imperial College London, London, UK; 5The George Institute for Global Health, University of New South Wales, Sydney, New South Wales, Australia
Correspondence: Lena Tschiderer, Institute of Health Economics, Medical University of Innsbruck, Innsbruck, Austria, Tel +43 50 504 26272, Email [email protected]
Abstract: Cardiovascular disease is the leading cause of death in women worldwide. Nonetheless, there exist several uncertainties in the prediction, diagnosis, and treatment of cardiovascular disease in women. A cornerstone in the prediction of cardiovascular disease is the implementation of risk scores. A variety of pregnancy- and reproductive-factors have been associated with lower or higher risk of cardiovascular disease. Consequently, the question has been raised, whether these female-specific factors also provide added value to cardiovascular risk prediction. In this review, we provide an overview of the existing literature on sex differences in the association of established cardiovascular risk factors with cardiovascular disease and the relation between female-specific factors and cardiovascular risk. Furthermore, we systematically reviewed the literature for studies that assessed the added value of female-specific factors beyond already established cardiovascular risk factors. Adding female-specific factors to models containing established cardiovascular risk factors has led to little or no significant improvement in the prediction of cardiovascular events. However, analyses primarily relied on data from women aged ≥ 40 years. Future investigations are needed to quantify whether pregnancy-related factors improve cardiovascular risk prediction in young women in order to support adequate treatment of risk factors and enhance prevention of cardiovascular disease in women.
Keywords: sex differences, female-specific factors, cardiovascular disease, risk prediction, added value
Introduction
Cardiovascular disease (CVD) has long been considered a disease that predominantly affects men. However, CVD is also a serious health concern in women and the number of deaths from CVD is higher in women than in men. In 2019, CVD was responsible for 31.4% of deaths in men and 34.6% in women.1
Despite the broadly similar burden of CVD in women and men, it is only over the last decades that medical guidelines have started to recognize the importance of CVD in women. In 1993, the American Heart Association published the first statement on CVD in women.2 Six years later, in 1999, the first clinical guidelines on the prevention of CVD in women were published by the American Heart Association,3 which were updated in 2004,4 2007,5 and finally in 2011.6 Recently, in 2022, the American Heart Association published a “Call to Action” on reducing burden and risk for CVD in women.7 The European Society of Cardiology initiated the “Women at Heart” program in 2005, in order to promote research and education of CVD in women.8 Furthermore, at the same time, they published their first statement on CVD in women.8
Prevention of CVD in women requires an effective multi-level approach including raising awareness, effective communication, and appropriate risk prediction.7 There is still much room for improvement in raising awareness of CVD in women. A recent survey of US women reported a decline in the awareness of heart disease being the leading cause of death in women between 2009 and 2019.9 Awareness of CVD risk goes hand in hand with effective communication of cardiovascular risk factors. Communicating factors leading to higher risk of CVD is crucial as many of these factors can be affected and modified by patients themselves. Finally, a cornerstone in the prevention of CVD is a reasonable approach to estimate an individual’s risk for a future CVD event, which may also guide decision-making and help to implement adequate treatment.7
Although research on CVD in women is increasingly conducted, medical guidelines do not include sex-specific recommendations.7 Moreover, substantial sex differences still exist in the prediction, diagnosis, and treatment of CVD that predominantly disadvantages women.10 In this review, we will provide an overview of sex differences in risk factors for CVD, cardiovascular risk prediction, and how prediction and prevention of CVD in women could be improved in the future.
CVD Epidemiology
In the United States, every 40 seconds a person experiences a myocardial infarction and every 3.5 minutes someone dies of stroke.11 Also, at a global level, CVD has a significant impact on people’s health. Since the first Global Burden of Disease data had been released in 1990, CVD remained the leading cause of death worldwide.1 This is true for both women and men.1 Earlier data from the United States showed that CVD was already among the top five causes of death in women back in 1900.12 Although, in 2019, the global age-standardized incidence rate of CVD was higher in men (730 per 100,000) than in women (643 per 100,000), there exist significant differences in the development and the phenotype of the disease.1 For instance, in the Rotterdam Study, lifetime risk for CVD has been demonstrated to be similar in women and men.13 Nevertheless, women had a higher risk to experience cerebrovascular disease or heart failure and men a higher risk to develop coronary heart disease as their first CVD event.13 Furthermore, subarachnoid hemorrhage affects women by a higher frequency than men.1 In 2019, the global age-standardized incidence rate of subarachnoid hemorrhage was 15.7 per 100,000 in women versus 13.0 per 100,000 in men.1 Contrarily, the global age-standardized incidence rate of ischemic heart disease was 333.5 per 100,000 in men compared to 198.5 per 100,000 in women.1
Sex Differences in Associations of Established Risk Factors with Cardiovascular Disease
The main modifiable risk factors related to the risk to develop CVD are overweight and obesity, high blood pressure, diabetes mellitus, smoking, and lipid abnormalities, among others.14 Table 1 summarizes large-scale studies and meta-analyses on sex differences in risk factor associations with various CVD outcomes including women-to-men ratios of relative risks. A statistically significant women-to-men ratio of relative risks >1 indicates a higher risk for women with the risk factor compared to women without the risk factor than for men with the risk factor compared to men without the risk factor. If the ratio is <1, the relative risk is greater in men than in women.
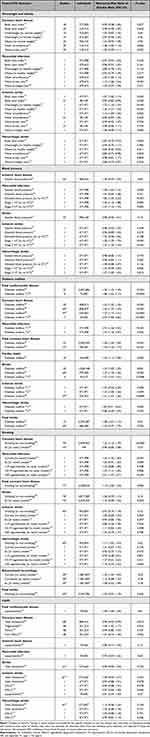 |
Table 1 Sex Differences in Associations Between Cardiovascular Risk Factors and Cardiovascular Disease Outcomes |
Overweight and Obesity
Women and men have different body anthropometries and compositions, which can be assessed by various measures including body mass index, waist circumference, and waist-to-hip ratio.
In individuals with a body mass index ≥20 kg/m2, higher body mass index is significantly associated with an increased risk for coronary heart disease in both women and men.15 However, analyses suggest no sex differences in the relation of body mass index and risk of coronary heart disease or myocardial infarction.15–17 Similarly, no difference in the association of waist circumference with risk of coronary heart disease has been reported between women and men.15 However, in the UK Biobank, waist-to-hip ratio was stronger associated with myocardial infarction in women than in men.18
In contrast, significant sex differences have been reported in the association between measures of overweight and obesity and the risk of stroke.15,19 When comparing participants of the UK Biobank with obesity and overweight to those with normal weight, the women-to-men ratios of hazard ratios for ischemic stroke were 1.36 (95% confidence interval [CI] 1.21, 1.54) and 1.22 (1.10, 1.35), respectively.19 Also, waist circumference was more strongly associated with the risk of ischemic stroke in women than in men.19 In contrast, in the Emerging Risk Factors Collaboration (ERFC) the hazard ratio for ischemic stroke per standard deviation higher body mass index was higher in men (1.33 [1.21, 1.46]) than in women (1.20 [1.05, 1.37]) with body mass index ≥20 kg/m2 (P-value for interaction 0.030).15 Contrarily to ischemic stroke, in the UK Biobank, body mass index, waist circumference, and waist-to-height ratio were more strongly related to the risk for hemorrhagic stroke in men than in women.19
Blood Pressure
Steeper increases in blood pressure parameters have been reported for women compared to men during their course of life.20 In a large-scale meta-analysis, however, the association between higher systolic blood pressure and risk of ischemic heart disease was similar in women and men.21 Contrarily, an analysis in the UK Biobank found a stronger association for the risk of myocardial infarction for increased levels of systolic blood pressure in women compared to men, while there were no sex differences for diastolic blood pressure.16
The association between systolic blood pressure and stroke was similar in women and men in a meta-analysis of almost 1 million participants.21 These results were also confirmed for ischemic and hemorrhagic stroke in the UK Biobank.19 However, in the UK Biobank, having stage 1 or stage 2 hypertension compared to having no hypertension was a greater risk factor for ischemic stroke for women compared to men.19 Having stage 2 hypertension was also related to a higher risk for hemorrhagic stroke in women compared to men.19 No sex differences have been reported in the association between diastolic blood pressure and risk of ischemic and hemorrhagic stroke.19
Diabetes Mellitus
Sex differences have also been reported in the relation of diabetes mellitus and risk of CVD.22 A meta-analysis including more than 2 million individuals reported stronger association for women compared to men when assessing the relation between diabetes mellitus and risk for fatal CVD.23
Moreover, three large-scale meta-analyses reported a higher risk for coronary heart disease associated with diabetes in women than in men.24,25 Another meta-analysis confirmed the stronger relation with the risk of coronary heart disease for women than for men associated with type 1 diabetes mellitus.26
Similar results were found for the risk of stroke. In two meta-analyses, the risk of stroke was more strongly associated with diabetes in women than in men.25,27 Also, in the ERFC, the risk for ischemic stroke related to diabetes was stronger in women than in men (P-value for interaction 0.0089).28 In contrast, the UK Biobank investigated the sex-specific association of diabetes with risk of hemorrhagic stroke and found similar results for women and men.19
Smoking
Smoking has also been shown to be a stronger risk factor for coronary heart disease in women than in men. In a meta-analysis of 75 cohorts and more than 2 million participants, the women-to-men ratio of relative risks of smoking associated with the risk to develop coronary heart disease was 1.25 (1.12, 1.39).29 An investigation in the UK Biobank also found a stronger excess risk of myocardial infarction related to current smoking in women compared to men, and the women-to-men ratio of hazard ratios increased with higher smoking intensity.16
Sex differences in the association between smoking and risk of stroke have also been investigated. A large-scale meta-analysis including almost 4 million individuals reported a significant association between smoking and higher risk of stroke in both women and men.30 However, no statistically significant sex differences in these associations were reported when analyzing the whole sample of studies.30 Notably, the meta-analysis found significant sex differences in the relation between smoking and risk of stroke when restricting the analysis to Western populations.30 Another meta-analysis found a women-to-men ratio of relative risks for subarachnoid hemorrhage of 1.39 (1.05, 1.83) related to smoking.31
Lipids
In a meta-analysis of more than 1 million individuals, total cholesterol was a stronger risk factor for coronary heart disease in men compared to women with a pooled women-to-men relative risk ratio of 0.96 (0.93, 0.99).32 In the ERFC, the association between triglycerides and coronary heart disease was stronger in women than in men, while no sex differences have been reported for high-density lipoprotein cholesterol and non-high-density lipoprotein cholesterol.33
No significant sex differences were identified for the association of various lipid parameters, including total cholesterol, low-density lipoprotein cholesterol, high-density lipoprotein cholesterol, and lipoprotein(a) with risk of different types of stroke.19,32,34
Female-Specific Factors
A variety of female-specific factors have been associated with lower or higher risk to develop CVD.35–37
Age at Menarche
Both women at younger and older age at menarche have been demonstrated to be at higher risk for CVD later in life.38 In the Nurses’ Health Study (NHS), women with early age at menarche (≤10 years) had a higher risk for CVD, coronary heart disease, and stroke, compared to women who had menarche at an age of 13 years.39 In the Women’s Ischemia Syndrome Evaluation Study, hazard ratios for CVD were 4.21 (1.90, 9.30) and 2.27 (1.09, 4.75) for women aged ≤10 or 11 years at menarche compared to women aged 12 years at menarche.40 Also, women who were 14 and ≥15 years old at menarche had an elevated risk for CVD compared to women aged 12 years at menarche with hazard ratios of 2.74 (1.21, 6.20) and 2.52 (1.14, 5.57), respectively.40 In a large-scale analysis of the Million Women Study including over 1.2 million women, again, a U-shaped association between age at menarche and risk of coronary heart disease and cerebrovascular disease was reported.41 Compared to women aged 13 years at menopause, women aged ≤10, 11, and 12 years at menopause had a relative risk for coronary heart disease of 1.27 (1.22, 1.31), 1.12 (1.10, 1.14), and 1.02 (1.01, 1.04), respectively.41 Similarly, women who were older than 13 years at menopause also had a higher risk for coronary heart disease with hazard ratios of 1.04 (1.02, 1.06), 1.06 (1.04, 1.08), 1.10 (1.07, 1.14), and 1.23 (1.16, 1.29) for women aged 14, 15, 16, and ≥17 years at menarche.41 A similar but less pronounced shape of association has been reported for risk of cerebrovascular disease.41
Gestational Hypertension and Pre-Eclampsia
In the Million Women Study, women with hypertension during pregnancy had a significantly higher risk for a variety of future CVD events compared to women without hypertension during pregnancy.42 The relative risk for coronary heart disease was 1.29 (1.27, 1.31) and the relative risk for cerebrovascular disease was 1.23 (1.20, 1.27).42 In the Swedish Medical Birth Register, the adjusted hazard ratio for cardiovascular mortality was 1.79 (1.20, 2.66) comparing women with versus women without gestational hypertension.43 In a large-scale meta-analysis the pooled odds ratio for women with gestational hypertension was 1.67 (1.28, 2.19) for CVD and 1.83 (0.79, 4.22) for cerebrovascular disease compared to those without.44 In addition, pre-eclampsia, a pregnancy-related disease that induces hypertension, has been demonstrated to be associated with a significantly increased risk of future CVD. Compared to women without pre-eclampsia, women with pre-eclampsia had a significantly higher risk for cardiovascular mortality (adjusted hazard ratio 2.10 [1.47, 2.99]) in the Swedish Medical Birth Register.43 A meta-analysis found relative risks of 4.19 (2.09, 8.38) for heart failure, 2.50 (1.43, 4.37) for coronary heart disease, 1.81 (1.29, 2.55) for stroke, and 2.21 (1.83, 2.66) for fatal CVD comparing women with pre-eclampsia to those without.45 In another more recently published meta-analysis, similar findings were reported with pooled odds ratios for CVD of 2.24 (1.72, 2.93) and 2.74 (2.48, 3.04) for women with moderate and severe pre-eclampsia, respectively, and odds ratios of 1.73 (1.46, 2.06) for ischemic heart disease, 2.95 (1.10, 7.90) for cerebrovascular disease, and 1.73 (1.46, 2.06) for cardiovascular mortality, comparing women with pre-eclampsia with women without pre-eclampsia.44
Gestational Diabetes
In the Swedish Medical Birth Register, women with gestational diabetes had a significantly increased risk for cardiovascular mortality compared to those without with an adjusted hazard ratio of 3.03 (1.49, 6.16).43 A meta-analysis reported an odds ratio for cardiovascular morbidity and mortality of 1.68 (1.11, 2.52) for women with gestational diabetes mellitus versus those without.44 Another large-scale meta-analysis reported the association between history of gestational diabetes mellitus and a variety of cardiovascular outcomes.46 They found risk ratios of 1.45 (1.36, 1.53) for cardiovascular or cerebrovascular disease, 1.72 (1.40, 2.11) for CVD, 1.40 (1.18, 1.65) for coronary artery disease, 1.74 (1.37, 2.20) for myocardial infarction 2.27 (1.79, 2.87) for angina pectoris, 1.62 (1.29, 2.05) for heart failure, 1.87 (1.34, 2.62) for cardiovascular procedures, 1.40 (1.29, 1.51) for cerebrovascular disease, 1.45 (1.29, 1.63) for overall stroke, 1.49 (1.29, 1.71) for ischemic stroke, 1.44 (1.16, 1.78) for hemorrhagic stroke, and 1.28 (1.13, 1.46) for venous thromboembolism comparing women with a history of gestational diabetes mellitus to those without.46
Ectopic Pregnancy
In a large-scale cohort study in Canada, ectopic pregnancy has been related to an increased risk for cardiovascular mortality (hazard ratio 2.18 [1.39, 3.42]).47 Furthermore, an analysis in the NHS II showed that women with ectopic pregnancy are at a higher risk for incident hypertension (hazard ratio 1.21 [1.04, 1.40]).48
Stillbirths
As demonstrated in a meta-analysis, pooled odds ratios were 1.49 (1.08, 2.06) for CVD and 2.23 (1.90, 2.62) for cardiovascular mortality comparing women with a history of stillbirth to women without a history of stillbirth.44 The Swedish Medical Birth Register reported an adjusted hazard ratio for cardiovascular mortality of 3.14 (1.81, 5.44) comparing women with history of stillbirth to women without a history of stillbirth.43 In the China Kadoorie Biobank, women with a history of stillbirth had an increased risk for stroke (hazard ratio 1.06 [1.01, 1.12]) but not for coronary heart disease.49 Similar results were found in the UK Biobank, in which the risk for CVD and stroke was significantly increased when comparing women with a history of stillbirth to those without (hazard ratios of 1.22 [1.01, 1.46] and 1.44 [1.12, 1.85], respectively) and no significant association was found between history of stillbirth and risk of coronary heart disease.50
Preterm Delivery
Several studies reported a significant association between a history of preterm delivery and cardiovascular risk.43,44,51 In an analysis of data from the Swedish Medical Birth Register, preterm birth was related to a higher risk for cardiovascular mortality with an adjusted hazard ratio of 1.84 (1.38, 2.44).43 In a meta-analysis, women with a history of preterm delivery were at higher risk for CVD (pooled odds ratio 1.63 [1.39, 1.93]) and cardiovascular mortality (pooled odds ratio 1.93 [1.83, 2.03]).44 Another meta-analysis including over 5.8 million women reported preterm delivery to be related to a higher risk of future CVD.51 Risk ratios were 1.43 (1.18, 1.72) for CVD, 1.78 (1.42, 2.21) for fatal CVD, 1.49 (1.38, 1.60) for coronary heart disease, 2.10 (1.87, 2.36) for fatal coronary heart disease, 1.65 (1.51, 1.79) and for stroke comparing women with to women without preterm delivery.51
Breastfeeding
Breastfeeding was one of few factors that had been related to a lower risk for CVD. A large-scale meta-analysis of more than 1 million parous women investigated the association of breastfeeding behavior with risk to develop CVD.52 Compared to parous women who never breastfed, women who breastfed during their lifetime had a significantly lower risk for CVD (hazard ratio 0.89 [0.83, 0.95]).52 In addition, in outcome-specific analysis, a lower risk for coronary heart disease, stroke, and fatal CVD has been reported.52
Parity and Parenthood
In the Tehran Lipid and Glucose Study (TLGS), a J-shaped association was found between the number of live births and risk of CVD.53 The hazard ratio for CVD comparing women with ≥4 live births to women with one live birth was 2.17 (1.18, 4.00) and attenuated to 1.72 (0.92, 3.21) after multivariable adjustment.53 Furthermore, in the UK Biobank, compared to non-parous women, parous women were at significantly higher risk for coronary heart disease but not for stroke.50 Recent data from the US National Health and Nutrition Examination Survey from 2007 to 2018 showed that women with parity 1–2, 3–4, and ≥5 were at higher risk for CVD with odds ratios of 1.85 (1.29, 2.64), 1.70 (1.15, 2.50), and 1.92 (1.28, 2.88) compared to nulliparous women.54 In women with children from the China Kadoorie Biobank the hazard ratios per additional child for coronary heart disease and stroke were 1.02 (1.01, 1.04) and 1.02 (1.01, 1.03), respectively.55
Several studies have also shown associations between parenthood and CVD in men.50,53,55
Age at Menopause
Early age at menopause has been related to a higher risk to develop CVD. In the NHS, women aged <40, 40–44, and 45–49 years had a higher risk to develop CVD compared to women who were 50–54 years at menopause.39 Similarly, women in these age at menopause categories were at elevated risk for future coronary heart disease.39 Furthermore, compared to women aged 50–54 years at menopause, women at <40 and 40–44 years also had a higher risk to develop stroke.39 An individual-participant data meta-analysis of more than 300,000 women found early menopause to be associated with a significantly higher risk of CVD. Women aged 40, 40–44, and 45–49 years at menopause had a significantly higher risk for CVD compared to women aged 50–51 years at menopause with corresponding hazard ratios of 1.55 (1.38, 1.73), 1.30 (1.22, 1.39), and 1.12 (1.07, 1.18), respectively.56 Compared to women aged 50–51 years at menopause, the hazard ratio for CVD in women who were 55 or older was 0.88 (0.83, 0.93).56 Similar results were found when analyzing the outcomes coronary heart disease and stroke separately.56 However, although observational studies reported a significant association between early age at menopause and risk of CVD, a recent Mendelian Randomization analysis on age at menopause and the risk of coronary heart disease demonstrated that this relation is unlikely to be causal.57
Risk Scores
A cornerstone in the prevention of CVD is early identification of individuals at high risk. In this context, risk scores are helpful tools as they aim to provide guidance on cardiovascular risk identification and initiation of treatment. Risk prediction scores have been developed for various population groups including individuals with diabetes mellitus,58 prior CVD,59 or apparently healthy individuals.60,61 Sex differences in the association between cardiovascular risk factors and the risk to experience CVD are important to be included in these risk scores. An overview of frequently used CVD risk scores is provided in Table 2.
 |
Table 2 Overview of Frequently Used Risk Scores to Predict 10-Year Cardiovascular Risk |
One of the most popular and oldest cardiovascular risk scores is the Framingham Risk Score. It has been developed for various cardiovascular outcomes using data of the population-based Framingham Heart Study. The Framingham Risk Score to predict 10-year CVD risk includes information on age, sex, total cholesterol, high-density lipoprotein cholesterol, blood pressure, diabetes mellitus, and smoking.62 It accounts for sex differences in risk factor associations as separate scores are available for women and men.
Another frequently used cardiovascular risk score is the Systematic Coronary Risk Evaluation (SCORE) model that has been recommended by the European Society of Cardiology.60 The SCORE model has been developed to assess 10-year risk for mortality from CVD in healthy individuals.60 Factors included in the SCORE model are age, total cholesterol or total cholesterol/high-density lipoprotein cholesterol ratio, systolic blood pressure, and smoking status.60 In 2021, the SCORE model was updated and two new prediction algorithms have been recommended: SCORE2 for predicting the 10-year risk of first-onset of CVD63 and SCORE2-OP for predicting CVD risk in older individuals.64 SCORE2-OP additionally includes information on diabetes mellitus.64 Also, the SCORE2 models consider sex-specific relations of risk factors and have been developed separately for women and men.
The Pooled Cohort Equations published alongside the 2013 American Heart Association and American College of Cardiology guidelines have also been developed to predict 10-year risk for CVD events.61 Again, sex-specific prediction models have been developed including information on age, total cholesterol, high-density lipoprotein cholesterol, systolic blood pressure (treated or untreated), smoking, and diabetes.61
In the UK, the QRISK scores are widely used to estimate 10-year risk of CVD. The latest version is QRISK3, which includes data on a variety of factors and conditions related to CVD such as age, ethnic origin, deprivation, systolic blood pressure, body mass index, total cholesterol/high-density lipoprotein cholesterol ratio, smoking status, family history of premature coronary heart disease, diabetes mellitus, antihypertensive medication, rheumatoid arthritis, atrial fibrillation, chronic kidney disease, migraine, corticosteroid use, systemic lupus erythematosus, antipsychotic use, mental illness, HIV or AIDS, and erectile dysfunction.65 Also, for the QRISK3 score separate risk scores have been developed for women and men.65
For the Reynolds risk score there exists a version that has specifically been developed to assess cardiovascular risk in women.66 This risk score uses information on age, systolic blood pressure, high-sensitivity C-reactive protein, total cholesterol, high-density lipoprotein cholesterol, glycated hemoglobin (HbA1c) in women with diabetes, smoking, and family history of premature myocardial infarction to predict 10-year CVD risk.66
In order to refine cardiovascular risk prediction and incorporate up-to-date evidence, cardiovascular risk scores continuously undergo validation and recalibration. Moreover, new candidate markers that may provide added predictive value to already existing risk scores are constantly evaluated.
Do Cardiovascular Risk Prediction Scores Improve After Adding Female-Specific Factors?
Cardiovascular risk scores have been criticized in the past – especially in cardiovascular risk prediction in women. For instance, it has been shown that the Framingham Risk Score underestimates cardiovascular risk in women with presence of coronary artery calcification.67,68 Furthermore, commonly used risk scores classified the majority of women with ischemia and no obstructive coronary artery disease as being at low cardiovascular risk.69
In general, cardiovascular risk prediction tools primarily rely on traditional cardiovascular risk factors and do not take into account female-specific risk factors. The 2019 American Heart Association and American College of Cardiology guidelines on the primary prevention of CVD suggested a range of risk-enhancing factors that can additionally be taken into account when assessing cardiovascular risk.70 Among those, they also mention history of premature menopause (ie, menopause before the age of 40 years) and history of pregnancy-associated conditions such as pre-eclampsia.70
Implementing female-specific factors in current cardiovascular risk prediction models has recently gained increasing attention. O’Kelly et al reviewed several pregnancy- and reproductive-factors in women and mentioned the role of reproductive factors in cardiovascular risk prediction models as one of the key outstanding questions to be answered.71 Additionally, in a “Call to Action” statement, Wenger et al stated that
Integrating women-specific risk factors in the quantitative risk assessment across the life span is necessary, and the American Heart Association is currently evaluating approaches to do so.7
Although several female-specific factors are known to be significantly associated with the risk to develop CVD, as reported above, this does not automatically translate into added predictive values in risk prediction models.
Methodology of the Evaluation of the Added Predictive Value
Common statistical metrics to assess the improvement of risk prediction models are risk discrimination and reclassification. Risk discrimination quantifies the ability to distinguish correctly between individuals who will likely experience an event of interest from those who will remain event-free.72 Risk reclassification focuses on how well models classify individuals based on predicted risk.73
Measures for Risk Discrimination
One of the most commonly used measures for risk discrimination is the area under the receiver operating characteristic curve, called C-statistic. The C-statistic can be interpreted as the probability that the predicted risk for an individual with an event is higher than the predicted risk for an event-free individual.74 Since the C-statistic only takes the event status into account but not survival times or censoring, several approaches72,75–78 extended the C-statistic for survival analyses. The most commonly used extension is the C-index proposed by Harrell et al.72,78 It assesses whether the model correctly predicts the order of events of randomly selected pairs of individuals.79 The C-statistic and the C-index range from 0.5 to 1 with 0.5 indicating discrimination achieved by chance and 1 indicating perfect discrimination.74,78
Measures for Risk Reclassification
Two measures for risk reclassification of dichotomous outcomes are the net reclassification index (NRI) and the integrated discrimination index (IDI). The NRI categorizes individuals into predefined risk categories and evaluates changes in categories between two prediction models separately for individuals who developed the event of interest (cases) and who remained event-free (non-cases). For non-cases, risk classification improves if they move down into a lower risk category and worsens if they move up into a higher risk category. For cases, it is the opposite way. The overall NRI is the sum of the NRI for cases and non-cases.80 A limitation of the NRI is its dependence on the thresholds of risk categories.81 The IDI overcomes this limitation by comparing average predicted probabilities of cases and non-cases from the reference model to the new model.80 A NRI or IDI higher than zero indicates improved reclassification.
Added Predictive Value of Female-Specific Factors
Multiple studies have investigated the added value of female-specific factors in cardiovascular risk prediction models based on measures of risk discrimination and reclassification.82,83 In order to provide an up-to-date overview of the existing literature, we searched PubMed for articles published until November 2nd 2022. We used the search terms (“future cardiovascular” OR ‘future CVD’ OR ‘future coronary heart disease’ OR ‘future CHD’ OR ‘future stroke’ OR “cardiovascular risk” OR “cardiovascular disease risk” OR “coronary heart disease risk” OR “CHD risk” OR ‘CVD risk’ OR “stroke risk“) AND (“reclassification” OR “discrimination” OR “prediction” OR “added value”) AND (”women” OR “female”) AND (”pregnancy” OR “reproductive” OR “female-specific”). The literature search yielded 90 results. In addition, we screened reference lists of relevant articles to identify additional publications and found one further article. Among the 91 identified articles, nine84–92 examined the added value of pregnancy- or reproductive-factors to already existing cardiovascular risk scores. An overview of the studies identified by our literature search is provided in Table 3. Years of baseline ranged from 1987 to 2008 and studies were conducted in Iran, the Netherlands, Norway, Sweden, and the United States. The majority of studies analyzed 10-year CVD risk. A summary of study-specific results on risk discrimination and reclassification measures is provided in Table 4 and Table 5, respectively.
 |
Table 3 Studies Investigating Risk Discrimination or Reclassification of Female-Specific Factors Upon Established Cardiovascular Risk Factors |
 |
Table 4 Cardiovascular Risk Discrimination After Adding Female-Specific Factors to Established Cardiovascular Risk Factors |
 |
Table 5 Risk Reclassification After Adding Female-Specific Factors to Established Cardiovascular Risk Factors |
Results from analyses regarding risk discrimination based on different concordance measures (C-index, Uno’s C-statistic) are summarized in Table 4. In general, risk discrimination did not improve significantly or improved only modestly after adding information on pregnancy- or reproductive-factors to a reference model on established cardiovascular risk factors. The C-index improved slightly after adding information on pre-eclampsia (difference in C-indexes 0.003 [95% CI 0.001, 0.005]),85 age at first birth (0.0019 [0.0010, 0.0032]),92 number of stillbirths (0.0005 [0.0001, 0.0013]),92 and number of miscarriages (0.0010 [0.0004, 0.0020]).92 Moreover, the C-index improved modestly, when adding data on preterm delivery (0.0002 [0.0001, 0.0002])85 in women aged ≥40 years or both preterm delivery and parity in women aged ≥30 years (0.004 [0.001, 0.008]).88 A slight but statistically significant improvement has also been found when adding a combination of pregnancy-related factors92 or pregnancy complications.85,89
Table 5 outlines risk reclassification assessed with the NRI or the IDI. For the majority of female-specific risk factors, risk reclassification did not improve significantly when adding them to a reference model including established cardiovascular risk factors. However, a slight improvement in risk reclassification of non-events was found after adding pre-eclampsia (NRI 0.002 [0.0006, 0.004])85 or a number of pregnancy complications (NRI 0.004 [0.002, 0.006] in HUNT85 and 0.02 [0.001, 0.04] in TLGS89). Adding data on low birth weight offspring improved the NRI for events in women aged 50 years (0.038 [0.003, 0.074]).91 A significant improvement in the overall NRI (0.02 [0.002, 0.05]) was found after accounting for several pregnancy complications.85 Adding pregnancy-related conditions improved the NRI for non-events (0.002 [0.0001, 0.005] for risk cut-offs at 5% and 10% and 0.002 [0.0003, 0.003] for a risk cut-off at 7.5%) and events (0.009 [0.002, 0.017] for risk cut-off at 7.5%) and led to a significant IDI of 0.0013 (0.0008, 0.0017).92 When adding both preterm delivery and parity, reclassification assessed with the IDI improved slightly.88 Furthermore, after adding preterm delivery and parity, the NRI for non-events worsened, while it improved for events (0.01 [0.003, 0.02]) and overall (0.01 [0.002, 0.02]), but only when analyzing 20-year CVD risk in NHS II participants aged ≥30 years.88
As described above, cardiovascular risk prediction either did not improve statistically significantly after including pregnancy- and/or reproductive-factors or the addition of female-specific factors only led to little improvement. However, measures investigating the added predictive values need to be interpreted with caution. In case the C-index of the reference model is already high, it is more difficult to achieve additional improvement93 and the NRI and IDI are strongly affected by the event rate94 and sensitive to miscalibration.94,95 Moreover, lack of finding a significant improvement after adding female-specific factors may be due to the fact that the added predictive value is not studied in adequate age groups or time frames. The risk to experience CVD increases significantly with older age. In women, the global incidence of CVD in 2019 was 201 per 100,000 at 15–49 years, 1589 per 100,000 at 50–69 years, and 4497 per 100,000 at 70 years of age or older.96 While the incidence of CVD is in general low in younger women, specific CVD risk factors may play an important role at younger age. Grandi et al, for instance, outlined the issue that pregnancy-related factors may have the most significant effect on cardiovascular risk in women at reproductive age, ie, between 15 and 45 years.97 Indeed, the studies that had been identified by our systematic literature search included almost exclusively data on women ≥40 years of age and none of the studies included women below 30 years of age. At younger age, the added value of pregnancy-related conditions may be outdone by other, already established, cardiovascular risk factors. Remarkably, in the NHS II, 20-year CVD risk reclassification and discrimination improved statistically significantly after adding data on preterm delivery and parity, when analyzing women aged ≥30 years but not in women ≥40 years of age.88 Another issue is that commonly used cardiovascular risk prediction scores have not been developed for young women.98 For instance, the SCORE2 risk prediction algorithm was developed for individuals aged 40–69 years,63 the Framingham Risk Score included women and men between 30 and 74 years of age at baseline,99 and the Pooled Cohort Equations were developed for individuals between 40 and 79 years of age.61 Consequently, estimating cardiovascular risk in younger women based on variables used in conventional risk prediction scores may be challenging. Future investigations on the added predictive value of pregnancy-related factors in young women are needed. Such investigations could help to identify women at high cardiovascular risk early and provide adequate and accelerated treatment.
Conclusion
Sex differences in risk factors associated with CVD events have been identified and included in risk prediction tools as they are usually developed separately for women and men. Furthermore, various female-specific reproductive- and pregnancy-related factors have been identified that are related to higher or lower cardiovascular risk. Adding female-specific factors to models containing established cardiovascular risk factors has led to little or no significant improvement in the prediction of cardiovascular events. However, prior analysis on the added value of female-specific factors relied primarily on data from women aged ≥40 years. Especially for pregnancy-related factors, it may be crucial to study their incremental value beyond established cardiovascular risk factors in younger women. Consequently, future investigations are needed to quantify whether pregnancy-related factors improve cardiovascular risk prediction in young women in order to support adequate treatment of risk factors and enhance prevention of CVD in women.
Funding
LT received funding from the Austrian Science Fund (T-1253). SAEP is supported by a VIDI Fellowship from the Dutch Organisation for Health Research and Development (ZonMW) (09150172010050).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vos T, Lim SS, Abbafati C, et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi:10.1016/S0140-6736(20)30925-9
2. Eaker ED, Chesebro JH, Sacks FM, Wenger NK, Whisnant JP, Winston M. Cardiovascular disease in women. Circulation. 1993;88(4 Pt 1):1999–2009. doi:10.1161/01.cir.88.4.1999
3. Mosca L, Grundy SM, Judelson D, et al. Guide to preventive cardiology for women.AHA/ACC Scientific Statement Consensus panel statement. Circulation. 1999;99(18):2480–2484. doi:10.1161/01.cir.99.18.2480
4. Mosca L, Appel LJ, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women. Circulation. 2004;109(5):672–693. doi:10.1161/01.CIR.0000114834.85476.81
5. Mosca L, Banka CL, Benjamin EJ, et al. Evidence-based guidelines for cardiovascular disease prevention in women: 2007 update. Circulation. 2007;115(11):1481–1501. doi:10.1161/CIRCULATIONAHA.107.181546
6. Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the American Heart Association. J Am Coll Cardiol. 2011;57(12):1404–1423. doi:10.1016/j.jacc.2011.02.005
7. Wenger NK, Lloyd-Jones DM, Elkind MSV, et al. Call to action for cardiovascular disease in women: epidemiology, awareness, access, and delivery of equitable health care: a presidential advisory from the American Heart Association. Circulation. 2022;145(23):e1059–e1071. doi:10.1161/CIR.0000000000001071
8. Stramba-Badiale M, Fox KM, Priori SG, et al. Cardiovascular diseases in women: a statement from the policy conference of the European Society of Cardiology. Eur Heart J. 2006;27(8):994–1005. doi:10.1093/eurheartj/ehi819
9. Cushman M, Shay CM, Howard VJ, et al. Ten-year differences in women’s awareness related to coronary heart disease: results of the 2019 American Heart Association National Survey: a special report from the American Heart Association. Circulation. 2021;143(7):e239–e248. doi:10.1161/CIR.0000000000000907
10. Vogel B, Acevedo M, Appelman Y, et al. The Lancet women and cardiovascular disease commission: reducing the global burden by 2030. Lancet. 2021;397(10292):2385–2438. doi:10.1016/S0140-6736(21)00684-X
11. Tsao CW, Aday AW, Almarzooq ZI, et al. Heart Disease and Stroke Statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–e639. doi:10.1161/CIR.0000000000001052
12. Hahn RA, Chang M-H, Parrish RG, Teutsch SM, Jones WK. Trends in mortality among females in the United States, 1900–2010: progress and challenges. Prev Chronic Dis. 2018;15. doi:10.5888/pcd15.170284
13. Leening MJG, Ferket BS, Steyerberg EW, et al. Sex differences in lifetime risk and first manifestation of cardiovascular disease: prospective population based cohort study. BMJ. 2014;349:g5992. doi:10.1136/bmj.g5992
14. Murray CJL, Aravkin AY, Zheng P, et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. doi:10.1016/S0140-6736(20)30752-2
15. Wormser D, Kaptoge S, Di Angelantonio E, et al. Separate and combined associations of body-mass index and abdominal adiposity with cardiovascular disease: collaborative analysis of 58 prospective studies. Lancet. 2011;377(9771):1085–1095. doi:10.1016/S0140-6736(11)60105-0
16. Millett ERC, Peters SAE, Woodward M. Sex differences in risk factors for myocardial infarction: cohort study of UK Biobank participants. BMJ. 2018;363:k4247. doi:10.1136/bmj.k4247
17. Mongraw-Chaffin ML, Peters SAE, Huxley RR, Woodward M. The sex-specific association between BMI and coronary heart disease: a systematic review and meta-analysis of 95 cohorts with 1·2 million participants. Lancet Diabetes Endocrinol. 2015;3(6):437–449. doi:10.1016/S2213-8587(15)00086-8
18. Peters SAE, Bots SH, Woodward M. Sex differences in the association between measures of general and central adiposity and the risk of myocardial infarction: results from the UK Biobank. J Am Heart Assoc. 2018;7:5. doi:10.1161/JAHA.117.008507
19. Peters SAE, Carcel C, Millett ERC, Woodward M. Sex differences in the association between major risk factors and the risk of stroke in the UK Biobank cohort study. Neurology. 2020;95(20):e2715–e2726. doi:10.1212/WNL.0000000000010982
20. Ji H, Kim A, Ebinger JE, et al. Sex differences in blood pressure trajectories over the life course. JAMA Cardiol. 2020;5(3):19–26. doi:10.1001/jamacardio.2019.5306
21. Peters SAE, Huxley RR, Woodward M. Comparison of the sex-specific associations between systolic blood pressure and the risk of cardiovascular disease: a systematic review and meta-analysis of 124 cohort studies, including 1.2 million individuals. Stroke. 2013;44(9):2394–2401. doi:10.1161/STROKEAHA.113.001624
22. de Ritter R, de Jong M, Vos, RC et al. Sex differences in the risk of vascular disease associated with diabetes. Biol Sex Differ. 2020;11(1):1. doi:10.1186/s13293-019-0277-z
23. Wang Y, O’Neil A, Jiao Y, et al. Sex differences in the association between diabetes and risk of cardiovascular disease, cancer, and all-cause and cause-specific mortality: a systematic review and meta-analysis of 5,162,654 participants. BMC Med. 2019;17(1):136. doi:10.1186/s12916-019-1355-0
24. Peters SAE, Huxley RR, Woodward M. Diabetes as risk factor for incident coronary heart disease in women compared with men: a systematic review and meta-analysis of 64 cohorts including 858,507 individuals and 28,203 coronary events. Diabetologia. 2014;57(8):1542–1551. doi:10.1007/s00125-014-3260-6
25. Wang H, Ba Y, Cai R-C, Xing Q. Association between diabetes mellitus and the risk for major cardiovascular outcomes and all-cause mortality in women compared with men: a meta-analysis of prospective cohort studies. BMJ Open. 2019;9(7):e024935. doi:10.1136/bmjopen-2018-024935
26. Huxley RR, Peters SAE, Mishra GD, Woodward M. Risk of all-cause mortality and vascular events in women versus men with type 1 diabetes: a systematic review and meta-analysis. Lancet Diabetes Endocrinol. 2015;3(3):198–206. doi:10.1016/S2213-8587(14)70248-7
27. Peters SAE, Huxley RR, Woodward M. Diabetes as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 64 cohorts, including 775 385 individuals and 12 539 strokes. Lancet. 2014;383(9933):1973–1980. doi:10.1016/S0140-6736(14)60040-4
28. Sarwar N, Gao P, Seshasai SRK, et al. Diabetes mellitus, fasting blood glucose concentration, and risk of vascular disease: a collaborative meta-analysis of 102 prospective studies. Lancet. 2010;375(9733):2215–2222. doi:10.1016/S0140-6736(10)60484-9
29. Huxley RR, Woodward M. Cigarette smoking as a risk factor for coronary heart disease in women compared with men: a systematic review and meta-analysis of prospective cohort studies. Lancet. 2011;378(9799):1297–1305. doi:10.1016/S0140-6736(11)60781-2
30. Peters SAE, Huxley RR, Woodward M. Smoking as a risk factor for stroke in women compared with men: a systematic review and meta-analysis of 81 cohorts, including 3,980,359 individuals and 42,401 strokes. Stroke. 2013;44(10):2821–2828. doi:10.1161/STROKEAHA.113.002342
31. Li X, Wang T, Feng D, et al. Sex-specific associations of smoking with spontaneous subarachnoid hemorrhage: findings from observational studies. J Stroke Cerebrovasc Dis. 2020;29(10):105144. doi:10.1016/j.jstrokecerebrovasdis.2020.105144
32. Peters SAE, Singhateh Y, Mackay D, Huxley RR, Woodward M. Total cholesterol as a risk factor for coronary heart disease and stroke in women compared with men: a systematic review and meta-analysis. Atherosclerosis. 2016;248:123–131. doi:10.1016/j.atherosclerosis.2016.03.016
33. Di Angelantonio E, Sarwar N, Perry P, et al. Major lipids, apolipoproteins, and risk of vascular disease. JAMA. 2009;302(18):1993–2000. doi:10.1001/jama.2009.1619
34. Simony SB, Mortensen MB, Langsted A, Afzal S, Kamstrup PR, Nordestgaard BG. Sex differences of lipoprotein(a) levels and associated risk of morbidity and mortality by age: the Copenhagen General Population Study. Atherosclerosis. 2022. doi:10.1016/j.atherosclerosis.2022.06.1023
35. Agarwala A, Michos ED, Samad Z, Ballantyne CM, Virani SS. The use of sex-specific factors in the assessment of women’s cardiovascular risk. Circulation. 2020;141(7):592–599. doi:10.1161/CIRCULATIONAHA.119.043429
36. Maas AHEM, Rosano G, Cifkova R, et al. Cardiovascular health after menopause transition, pregnancy disorders, and other gynaecologic conditions: a consensus document from European cardiologists, gynaecologists, and endocrinologists. Eur Heart J. 2021;42(10):967–984. doi:10.1093/eurheartj/ehaa1044
37. Okoth K, Chandan JS, Marshall T, et al. Association between the reproductive health of young women and cardiovascular disease in later life: umbrella review. BMJ. 2020;371:m3502. doi:10.1136/bmj.m3502
38. Luijken J, van der Schouw YT, Mensink D, Onland-Moret NC. Association between age at menarche and cardiovascular disease: a systematic review on risk and potential mechanisms. Maturitas. 2017;104:96–116. doi:10.1016/j.maturitas.2017.07.009
39. Ley SH, Li Y, Tobias DK, et al. Duration of reproductive life span, age at menarche, and age at menopause are associated with risk of cardiovascular disease in women. J Am Heart Assoc. 2017;6:11. doi:10.1161/JAHA.117.006713
40. Lee JJ, Cook-Wiens G, Johnson BD, et al. Age at menarche and risk of cardiovascular disease outcomes: findings from the National Heart Lung and Blood Institute-Sponsored Women’s Ischemia Syndrome Evaluation. J Am Heart Assoc. 2019;8(12):e012406. doi:10.1161/JAHA.119.012406
41. Canoy D, Beral V, Balkwill A, et al. Age at menarche and risks of coronary heart and other vascular diseases in a large UK cohort. Circulation. 2015;131(3):237–244. doi:10.1161/CIRCULATIONAHA.114.010070
42. Canoy D, Cairns BJ, Balkwill A, et al. Hypertension in pregnancy and risk of coronary heart disease and stroke: a prospective study in a large UK cohort. Int J Cardiol. 2016;222:1012–1018. doi:10.1016/j.ijcard.2016.07.170
43. Täufer Cederlöf E, Lundgren M, Lindahl B, Christersson C. Pregnancy complications and risk of cardiovascular disease later in life: a nationwide cohort study. J Am Heart Assoc. 2022;11(2):e023079. doi:10.1161/JAHA.121.023079
44. Grandi SM, Filion KB, Yoon S, et al. Cardiovascular disease-related morbidity and mortality in women with a history of pregnancy complications. Circulation. 2019;139(8):1069–1079. doi:10.1161/CIRCULATIONAHA.118.036748
45. Wu P, Haththotuwa R, Kwok CS, et al. Preeclampsia and future cardiovascular health: a systematic review and meta-analysis. Circ Cardiovasc Qual Outcomes. 2017;10:2. doi:10.1161/CIRCOUTCOMES.116.003497
46. Xie W, Wang Y, Xiao S, Qiu L, Yu Y, Zhang Z. Association of gestational diabetes mellitus with overall and type specific cardiovascular and cerebrovascular diseases: systematic review and meta-analysis. BMJ. 2022;378:e070244. doi:10.1136/bmj-2022-070244
47. Auger N, Ghadirian M, Low N, Healy-Profitós J, Wei SQ. Premature mortality after pregnancy loss: trends at 1, 5, 10 years, and beyond. Eur J Obstet Gynecol Reprod Biol. 2021;267:155–160. doi:10.1016/j.ejogrb.2021.10.033
48. Levine LD. Pregnancy outcomes identify women at risk for cardiac disease. Now what? BJOG. 2019;126(1):43. doi:10.1111/1471-0528.15478
49. Peters SAE, Yang L, Guo Y, et al. Pregnancy, pregnancy loss, and the risk of cardiovascular disease in Chinese women: findings from the China Kadoorie Biobank. BMC Med. 2017;15(1):148. doi:10.1186/s12916-017-0912-7
50. Peters SA, Woodward M. Women’s reproductive factors and incident cardiovascular disease in the UK Biobank. Heart. 2018;104(13):1069–1075. doi:10.1136/heartjnl-2017-312289
51. Wu P, Gulati M, Kwok CS, et al. Preterm delivery and future risk of maternal cardiovascular disease: a systematic review and meta-analysis. J Am Heart Assoc. 2018;7:2. doi:10.1161/JAHA.117.007809
52. Tschiderer L, Seekircher L, Kunutsor SK, Peters SAE, O’Keeffe LM, Willeit P. Breastfeeding is associated with a reduced maternal cardiovascular risk: systematic review and meta-analysis involving data from 8 studies and 1 192 700 Parous women. J Am Heart Assoc. 2022;11(2):e022746. doi:10.1161/JAHA.121.022746
53. Moazzeni SS, Toreyhi H, Asgari S, Azizi F, Tehrani FR, Hadaegh F. Number of parity/live birth(s) and cardiovascular disease among Iranian women and men: results of over 15 years of follow-up. BMC Pregnancy Childbirth. 2021;21(1):28. doi:10.1186/s12884-020-03499-2
54. Xing Z, Alman AC, Kirby RS. Parity and risk of cardiovascular disease in women over 45 years in the United States: National Health and Nutrition Examination Survey 2007-2018. J Womens Health. 2022. doi:10.1089/jwh.2021.0650
55. Peters SA, Yang L, Guo Y, et al. Parenthood and the risk of cardiovascular diseases among 0.5 million men and women: findings from the China Kadoorie Biobank. Int J Epidemiol. 2017;46(1):180–189. doi:10.1093/ije/dyw144
56. Zhu D, Chung H-F, Dobson AJ, et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health. 2019;4(11):e553–e564. doi:10.1016/S2468-2667(19)30155-0
57. Dam V, Onland-Moret NC, Burgess S, et al. Genetically determined reproductive aging and coronary heart disease: a bidirectional 2-sample mendelian randomization. J Clin Endocrinol Metab. 2022;107(7):e2952–e2961. doi:10.1210/clinem/dgac171
58. Kengne AP, Patel A, Marre M, et al. Contemporary model for cardiovascular risk prediction in people with type 2 diabetes. Eur J Cardiovasc Prev Rehabil. 2011;18(3):393–398. doi:10.1177/1741826710394270
59. Dorresteijn JAN, Visseren FLJ, Wassink AMJ, et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: the SMART risk score. Heart. 2013;99(12):866–872. doi:10.1136/heartjnl-2013-303640
60. Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: the Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315–2381. doi:10.1093/eurheartj/ehw106
61. Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25Suppl 2):S49–73. doi:10.1161/01.cir.0000437741.48606.98
62. D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham Heart Study. Circulation. 2008;117(6):743–753. doi:10.1161/CIRCULATIONAHA.107.699579
63. SCORE2 working group and ESC Cardiovascular risk collaboration. SCORE2 risk prediction algorithms: new models to estimate 10-year risk of cardiovascular disease in Europe. Eur Heart J. 2021;42(25):2439–2454. doi:10.1093/eurheartj/ehab309
64. SCORE2-OP working group and ESC Cardiovascular risk collaboration. SCORE2-OP risk prediction algorithms: estimating incident cardiovascular event risk in older persons in four geographical risk regions. Eur Heart J. 2021;42(25):2455–2467. doi:10.1093/eurheartj/ehab312
65. Hippisley-Cox J, Coupland C, Brindle P. Development and validation of QRISK3 risk prediction algorithms to estimate future risk of cardiovascular disease: prospective cohort study. BMJ. 2017;357:j2099. doi:10.1136/bmj.j2099
66. Ridker PM, Buring JE, Rifai N, Cook NR. Development and validation of improved algorithms for the assessment of global cardiovascular risk in women: the Reynolds Risk Score. JAMA. 2007;297(6):611–619. doi:10.1001/jama.297.6.611
67. Michos ED, Nasir K, Braunstein JB, et al. Framingham risk equation underestimates subclinical atherosclerosis risk in asymptomatic women. Atherosclerosis. 2006;184(1):201–206. doi:10.1016/j.atherosclerosis.2005.04.004
68. Lakoski SG, Greenland P, Wong ND, et al. Coronary artery calcium scores and risk for cardiovascular events in women classified as “low risk” based on Framingham risk score: the multi-ethnic study of atherosclerosis (Mesa). Arch Intern Med. 2007;167(22):2437–2442. doi:10.1001/archinte.167.22.2437
69. Sedlak T, Herscovici R, Cook-Wiens G, et al. Predicted versus observed major adverse cardiac event risk in women with evidence of ischemia and no obstructive coronary artery disease: a report from WISE (Women’s Ischemia Syndrome Evaluation). J Am Heart Assoc. 2020;9(7):e013234. doi:10.1161/JAHA.119.013234
70. Arnett DK, Blumenthal RS, Albert MA, et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on clinical practice guidelines. Circulation. 2019;140(11):e563–e595. doi:10.1161/CIR.0000000000000677
71. O’Kelly AC, Michos ED, Shufelt CL, et al. Pregnancy and reproductive risk factors for cardiovascular disease in women. Circ Res. 2022;130(4):652–672. doi:10.1161/CIRCRESAHA.121.319895
72. Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23(13):2109–2123. doi:10.1002/sim.1802
73. Cook NR. Quantifying the added value of new biomarkers: how and how not. Diagn Progn Res. 2018;2:14. doi:10.1186/s41512-018-0037-2
74. Cook NR. Use and misuse of the receiver operating characteristic curve in risk prediction. Circulation. 2007;115(7):928–935. doi:10.1161/CIRCULATIONAHA.106.672402
75. Uno H, Cai T, Pencina MJ, D’Agostino RB, Wei LJ. On the C-statistics for evaluating overall adequacy of risk prediction procedures with censored survival data. Stat Med. 2011;30(10):1105–1117. doi:10.1002/sim.4154
76. Gönen M, Heller G. Concordance probability and discriminatory power in proportional hazards regression. Biometrika. 2005;92(4):965–970. doi:10.1093/biomet/92.4.965
77. Chambless LE, Diao G. Estimation of time-dependent area under the ROC curve for long-term risk prediction. Stat Med. 2006;25(20):3474–3486. doi:10.1002/sim.2299
78. Harrell FE, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Statist Med. 1996;15(4):361–387. doi:10.1002/(SICI)1097-0258(19960229)15:4<361:
79. Harrell FE. Evaluating the yield of medical tests. JAMA. 1982;247(18):2543. doi:10.1001/jama.1982.03320430047030
80. Pencina MJ, D’Agostino RB, Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Statist Med. 2008;27(2):157–172. doi:10.1002/sim.2929
81. Cook NR, Paynter NP. Performance of reclassification statistics in comparing risk prediction models. Biom J. 2011;53(2):237–258. doi:10.1002/bimj.201000078
82. Baart SJ, Dam V, Scheres LJJ, et al. Cardiovascular risk prediction models for women in the general population: a systematic review. PLoS One. 2019;14(1):e0210329. doi:10.1371/journal.pone.0210329
83. Gunnarsson OS, Timpka S. Pregnancy complication history in 10-year cardiovascular disease risk prediction: a review of recent evidence. Curr Epidemiol Rep. 2019;6(3):321–328. doi:10.1007/s40471-019-00208-2
84. Zhou X-H, Wang X, Duncan A, Hu G, Zheng J. Statistical evaluation of adding multiple risk factors improves Framingham stroke risk score. BMC Med Res Methodol. 2017;17(1):58. doi:10.1186/s12874-017-0330-8
85. Markovitz AR, Stuart JJ, Horn J, et al. Does pregnancy complication history improve cardiovascular disease risk prediction? Findings from the HUNT study in Norway. Eur Heart J. 2019;40(14):1113–1120. doi:10.1093/eurheartj/ehy863
86. van der Meer MG, van der Graaf Y, Schuit E, et al. Added value of female-specific factors beyond traditional predictors for future cardiovascular disease. J Am Coll Cardiol. 2016;67(17):2084–2086. doi:10.1016/j.jacc.2016.02.031
87. Stuart JJ, Tanz LJ, Cook NR, et al. Hypertensive disorders of pregnancy and 10-year cardiovascular risk prediction. J Am Coll Cardiol. 2018;72(11):1252–1263. doi:10.1016/j.jacc.2018.05.077
88. Tanz LJ, Stuart JJ, Williams PL, et al. Contributions of preterm delivery to cardiovascular disease risk prediction in women. J Womens Health. 2021;30(10):1431–1439. doi:10.1089/jwh.2021.0166
89. Saei Ghare Naz M, Sheidaei A, Aflatounian A, Azizi F, Ramezani Tehrani F. Does adding adverse pregnancy outcomes improve the Framingham Cardiovascular Risk Score in women? Data from the Tehran Lipid and Glucose Study. J Am Heart Assoc. 2022;11(2):e022349. doi:10.1161/JAHA.121.022349
90. Hadaegh F, Asgari S, Moosaie F, et al. The risk and added values of the atherosclerotic cardiovascular risk enhancers on prediction of cardiovascular events: Tehran lipid and glucose study. J Transl Med. 2021;19(1):25. doi:10.1186/s12967-020-02686-1
91. Timpka S, Fraser A, Schyman T, et al. The value of pregnancy complication history for 10-year cardiovascular disease risk prediction in middle-aged women. Eur J Epidemiol. 2018;33(10):1003–1010. doi:10.1007/s10654-018-0429-1
92. Parikh NI, Jeppson RP, Berger JS, et al. Reproductive risk factors and coronary heart disease in the women’s health initiative observational study. Circulation. 2016;133(22):2149–2158. doi:10.1161/CIRCULATIONAHA.115.017854
93. Pencina MJ, D’Agostino RB, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Statist Med. 2011;30(1):11–21. doi:10.1002/sim.4085
94. Cook NR, Demler OV, Paynter NP. Clinical risk reclassification at 10 years. Statist Med. 2017;36(28):4498–4502. doi:10.1002/sim.7340
95. Leening MJG, Vedder MM, Witteman JCM, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician’s guide. Ann Intern Med. 2014;160(2):122–131. doi:10.7326/M13-1522
96. Institute for Health Metrics and Evaluation. GBD Compare. Available from: http://vizhub.healthdata.org/gbd-compare.
97. Grandi SM, Smith GN, Platt RW. The relative contribution of pregnancy complications to cardiovascular risk prediction: are we getting it wrong? Circulation. 2019;140(24):1965–1967. doi:10.1161/CIRCULATIONAHA.119.040917
98. Arnott C, Patel S, Hyett J, Jennings G, Woodward M, Celermajer DS. Women and cardiovascular disease: pregnancy, the forgotten risk factor. Heart Lung Circ. 2020;29(5):662–667. doi:10.1016/j.hlc.2019.09.011
99. Wilson PW, D’Agostino RB, Levy D, Belanger AM, Silbershatz H, Kannel WB. Prediction of coronary heart disease using risk factor categories. Circulation. 1998;97(18):1837–1847. doi:10.1161/01.CIR.97.18.1837
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
