Back to Journals » Journal of Asthma and Allergy » Volume 16
Assessment of Anxiety, Depression, and Sleep Quality in Mothers of Children with Atopic Dermatitis: A Qualitative Questionnaire Study
Authors Song J , Gao Y, Wang Y, Dai H, Jia X, Xiang Q, Zhang H, Zheng R, Zhang W
Received 23 May 2023
Accepted for publication 5 August 2023
Published 22 August 2023 Volume 2023:16 Pages 879—887
DOI https://doi.org/10.2147/JAA.S422534
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amrita Dosanjh
Jingjing Song,* Yuyan Gao,* Yufei Wang, Huan Dai, Xiaoxiao Jia, Qiangwei Xiang, Hui Zhang, Rongying Zheng, Weixi Zhang
Department of Pediatric Allergy and Immunology, The Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University, Wenzhou, 325027, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Weixi Zhang, Email [email protected]
Purpose: To compare the anxiety, depression and sleep quality of mothers of healthy control children and mothers of children with atopic dermatitis (AD) of varying severity, both before and after treatment.
Methods: A total of 120 parent–child dyads participated in the study. These dyads were divided into four subgroups of 30 patients each: mild AD, moderate AD, severe AD, and control groups. The children’s symptoms, their mothers’ psychological status, and their mothers’ sleep quality were evaluated using the Scoring of Atopic Dermatitis (SCORAD), the Hospital Anxiety and Depression Scale (HADS), and the Pittsburgh Sleep Quality Index (PSQI), respectively, before and after a one-month comprehensive treatment.
Results: SCORAD, representing differences in severity of children’s AD, decreased significantly after one month’s treatment (p < 0.001). Anxiety in mothers significantly decreased in all AD severity groups after treatment (p < 0.05). However, for depression, only the mothers in the mild and moderate AD groups showed a decrease after treatment (p < 0.05). The PSQI total score also decreased in the mild AD group after treatment (p < 0.05).
Conclusion: The most severe effect was seen in the psychology and sleep quality of mothers of children with severe AD. After one month of treatment, the psychological health and sleep quality of the mothers in the mild AD group significantly improved, while those of mothers in the moderate and severe AD groups showed partial improvement.
Keywords: atopic dermatitis, mothers, sleep quality, psychological health, questionnaire
Introduction
Atopic dermatitis (AD), also known as “atopic eczema”, is a recurring inflammatory skin disease that is among the most common chronic diseases worldwide. In Chinese cities, AD is the most common skin disease among preschool children, with most cases being mild (74.6%).1,2 The main clinical manifestations of AD are skin lesions and severe itching that can critically influence quality of life.3 AD tends to occur in children, mainly in infancy, and is often complicated by bronchial asthma (asthma), allergic rhinitis, and other allergic diseases.4 AD has a high incidence and is a long-term, comprehensive disease for which basic treatment, drug treatment, and health education are the most common and standard treatment options worldwide.5 Improving symptoms and establishing long-term disease control are the main goals of treatment, which is usually based on the specified condition of the individual.4 Studies have focused on the physical and mental symptoms in children with moderate-to-severe AD before and after treatment, often to the exclusion of patients with mild AD and their parents.6–8 Short-term improvement in the disease condition is often easily achieved, but long-term management can be frustrating for the child and their parents.
AD has significantly negative effects on several quality-of-life indicators in affected individuals and their families, including behavioral, physical, and psychosocial health; sleep disturbances; and family functioning.9–11 The quality of life of parents of children with AD is lower than that of parents with healthy children—the more severe the disease, the worse the quality of life of parents with AD12,13—and parents of children with moderate-to-severe AD suffer from anxiety and depression to varying degrees. Sleep disturbance may also increase anxiety, depression, and other psychological problems in mothers.14,15 Research has shown that both the health and growth of children with AD are strongly influenced by the physical and psychosocial situations of their parents.16 Therefore, while focusing on children with AD, we must also consider the quality of life of their mothers. “Positive medicine” not only relieves symptoms but also strengthens the positive aspects of life.17 As main caregivers, mothers have the most frequent interactions with their children, take on most parenting responsibilities, and display the most significant influence on their children’s development. Using the Impact on Family Scale (IOF), a study in Germany has explored changes in the quality of life of parents of children with AD of varying severity.13 However, little attention has been paid to the mental state and sleep quality of mothers of children with AD of varying severity in China.
In the present study, we chose preschool children with AD of varying severity and their mothers as the research objects, including mild cases, and sought to explore the psychological changes in the mothers using scales to assess specific anxiety and depression levels and sleep disorders before and after the standard treatment described below. Focusing on the psychological status of mothers provides further support for the long-term management and comprehensive treatment of AD.
Materials and Methods
Study Design
This study was conducted from July 2019 to January 2021 at the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University. Children and their mothers were randomly contacted to participate in the study via the Department of Pediatric Allergy and Immunology and the Department of Children’s Health Care. A total of 120 parent–child dyads were enrolled in the study. Of these, 90 children had mild-to-severe AD according to diagnostic criteria. These children were divided into three groups according to severity, each consisting of 30 cases; the remaining 30 children did not have AD or a history of personal or family atopic diseases. Psychological changes were explored using scales to assess mothers’ specific levels of anxiety and depression as well as sleep disturbances before and after standard treatment.
Participating Parent–Child Dyads
The key eligibility criteria were that children should be between one month and five years of age, and their mothers should be the primary caregivers who accompanied them during their sleep. Other criteria were as follows: (1) children had existing AD based on diagnostic criteria;18 two pediatricians with more than five years of diagnostic experience jointly conducted an analysis, and after harmonizing their opinions, the severity of atopic dermatitis and SCORAD scores was analyzed; (2) children had stable vital signs from discovery to participation in this study; (3) children did not have a history of other personal or family atopy, such as allergic rhinitis or bronchial asthma; (4) the child’s symptoms, signs, and examination results did not suggest other systemic or psychological diseases; (5) mothers had no chronic diseases or psychological diseases; and (6) mothers did not use any functional drugs, particularly neuropsychiatric drugs.
This study used SCORAD, which was the most widely recognized because of its high sensitivity and specificity as a diagnostic and evaluation standard for AD.18–20 The extent refers to the area of affected skin. The intensity consists of six items: erythema, edema/papulation, excoriation, lichenification, oozing/crusts, and dryness. Each item is graded from 0 to 3, which is determined by using the total scores of the items (with a moderate score of > 25 and a severe score of > 50).21
AD Treatment
The most recognized treatment plan for childhood AD worldwide aims at improving symptoms and establishing long-term control, including avoidance of individual triggers, use of moisturizers to restore the skin barrier, and approaches to gradually reduce inflammation based on disease severity.4 We administered routine basic treatment, including education for self-managing atopic dermatitis, long-term therapy with emollient, and avoidance of individual trigger factors.4 We provided standard medical treatment using topical corticosteroids (TCS) as the first-line treatment for AD flare-ups.4 We used an anti-staphylococcal antibiotic cream to treat secondary skin infections.22–24 Children over six months of age with noticeable itching or sleep disorders were also treated with oral antihistamines.25 Regarding treatment duration, in this study, we considered the therapeutic effect of topical medication as well as patient compliance, finally choosing one month as the appropriate treatment duration.
Mother-Related Psychological Criteria
To evaluate the psychological symptoms of the mothers, including anxiety and depression, we used the Hospital Anxiety and Depression Scale (HADS) as our measurement.26 HADS is sensitive to psychological changes during the disease process and has presented meaningful results in clinical studies related to several aspects.27 The scale comprises two subscales with a total of 14 items, seven of which assess depression, forming the depression subscale (HAD-D); the other seven evaluate anxiety, comprising the anxiety subscale (HAD-A). A higher score indicates higher anxiety and depression. In addition, we assessed sleep quality using the Pittsburgh Sleep Quality Index (PSQI), a well-established and widely recognized self-report assessment of sleep disturbances among respondents.28 The PSQI scale contains 18 self-assessment items, which addressed subjective sleep quality, time needed to fall asleep, sleep duration, sleep efficiency, sleep disturbance, use of hypnotics, and daytime dysfunction. As the score increases, sleep quality decreases.29 All participating mothers completed these two questionnaires before and after their children finished treatment, as required by our study.
Statistical Analysis
All data were recorded, analyzed, and reported by one person before and after treatment throughout the study. Statistical analysis was performed using SPSS 26.0 statistical software. Normally distributed data were expressed as 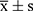 . The LSD-t test was used to compare multiple groups of normally distributed data. The paired t-test was used to compare normally distributed data before and after the treatment. For non–normally distributed data, the results are presented as the median and interquartile range. The Kruskal–Wallis test was used to compare multiple groups of non–normally distributed data. The Wilcoxon rank-sum test was used for non-normally distributed data before and after the treatment. Statistical significance was set to a p-value of less than 0.05.
. The LSD-t test was used to compare multiple groups of normally distributed data. The paired t-test was used to compare normally distributed data before and after the treatment. For non–normally distributed data, the results are presented as the median and interquartile range. The Kruskal–Wallis test was used to compare multiple groups of non–normally distributed data. The Wilcoxon rank-sum test was used for non-normally distributed data before and after the treatment. Statistical significance was set to a p-value of less than 0.05.
Ethical Statement
All parent–child dyads provided written informed consent, which was obtained from parents. Participation in the study was voluntary. This study complies with the Declaration of Helsinki. The ethical approval for this study was granted by the Ethics Committee of the Second Affiliated Hospital and Yuying Children’s Hospital of Wenzhou Medical University (2021-K-79-01).
Results
Participants
A total of 120 parent–child dyads participated in the study. Of these, 111 dyads (92.5%) completed the whole study. The numbers of AD patients diagnosed with AD and control patients who completed follow-up and the main workflow are shown in Figure 1. There were 30 cases in the normal control group, including 19 males and 11 females, aged one month to five years, with a median age of one year. Before treatment, there were 20 male and 10 female children in the mild AD group, 17 male and 13 female children in the moderate AD group, 18 male and 12 female children in the severe AD group, and 19 male and 11 female children in the control group. Each group’s age ranged between one month and five years, with a median age of one year. The demographic characteristics of the children and their mothers are shown in Table 1. There were no statistically significant differences in age and gender (p > 0.05) among the children. Similarly, the mothers did not differ significantly in their age, knowledge level, income, or family environment. Therefore, the results are comparable.
 |
Table 1 Baseline Characteristics of Trial Participants (n=120) |
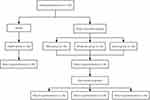 |
Figure 1 The workflow of this study. |
Severity of Atopic Dermatitis Among Children
The children with AD who participated in this experiment were diagnosed with AD, and the severity of their AD was assessed using their SCORAD scores before and after one-month treatment by the same doctor. The SCORAD scores of children at all levels of AD severity decreased significantly after treatment (p < 0.001, Table 2).
 |
Table 2 Comparison of SCORAD Before and After Treatment |
Anxiety and Depression Scores in Mothers
Before treatment, there was a statistically significant difference in the anxiety and depression subscale scores among the four groups (p < 0.05). Further pairwise comparisons revealed that the anxiety scores of mothers in the three AD groups were significantly higher than those of mothers in the healthy control group (p < 0.05), and the anxiety scores of mothers in each AD group were not statistically different (p > 0.05). Only the depression scores of mothers in the moderate and severe AD groups were significantly higher than those of the healthy control group (p < 0.05), and there was no significant difference among the other groups (p > 0.05, Table 3).
 |
Table 3 HADS Score of Mothers in the Various AD Groups and Control Group |
After one month of treatment, the HAD-A scores decreased significantly in all three AD groups (p < 0.05). However, the HAD-D scores only decreased significantly in the mild and moderate AD groups after treatment (p < 0.05, Table 4).
 |
Table 4 Comparison of HADS Scores Before and After Treatment |
Sleep Quality Scores in Mothers
As the PSQI score increased, sleep quality decreased.29 All participating mothers completed these two questionnaires before and after their children finished treatment. Before treatment, there was a statistically significant difference in the total PSQI scores among the four groups of mothers (p < 0.05). Further pairwise comparison revealed that the total PSQI scores of the mothers in the three AD groups were higher than those in the normal control group (p < 0.05), but no statistical difference was found in the total PSQI scores of the mothers among the three AD groups (p > 0.05, Table 5). Only the mild AD group showed decreased PSQI scores after treatment (p < 0.05, Table 6).
 |
Table 5 PSQI Scores of Mothers in the Various AD Groups and Control Group |
 |
Table 6 Comparison of PSQI Score Before and After Treatment |
Discussion
Our results indicate that mothers of children with different degrees of AD show varying degrees of improvement in anxiety levels, depression levels, and sleep quality after their children receive standard treatment. AD is a burdensome disorder, and caregivers in particular have higher stress levels than children not affected by the disorder.30,31 Mothers are usually the primary caregivers of children with AD, and their quality of life generally suffers more than that of fathers.32 This study is important in the long run for families and children with varying severity of atopic dermatitis.
Many reports have examined parenting stress and decreased quality of life for caregivers of children with allergic diseases, represented by parents of children with AD. One study found that parents of children with AD exhibited sleep disturbances as well as increased anxiety levels and depression scores.14 However, most studies have focused on families of children with moderate-to-severe AD. A 2004 study of mothers of children with AD found no statistically significant correlation between mothers’ quality of life and the severity of their children’s AD.13 Our study further explored this issue regarding anxiety and depression using the HADS and found that mothers in the AD group had significantly higher anxiety than controls, while mothers in the moderate and severe AD groups showed significant depression. Another related study using the HADS found no differences in anxiety and only found similar results in depression for mothers of children with moderate-to-severe AD. These findings are, of course, related to the location, time, and social factors of the experiment. Mothers’ fears of using hormonal drugs and increased worries about subsequent recurrence of this chronic disease may explain these differences in recent years.33–35
Sleep disorders are among the most troubling symptoms in more than 50% of children with AD.36 A US study of 270 patients with AD and parents found that most of these parents (66%) were bothered by the cosleeping.37 It has been proven that as children suffer from AD, sleep deprivation negatively impacts parents’ emotional state, level of coping with stress, happiness, and sleep.38 Sleep problems are manifestations of anxiety and depression in mothers. A recent prospective Chinese study of sleep disorders among mothers of children with AD noted significant differences between mothers in the standard group in terms of difficulty falling asleep, lack of subjective sleep, and daytime fatigue. However, no significant differences were found in the duration of sleep and early awakening.16 Another study found that the total PSQI scores of parents to children with AD were higher than those of the control group, but the study failed to compare the severity of AD.39 To better understand mothers’ sleep quality, our study comprehensively assessed the sleep quality of mothers with AD using the PSQI. We found that the PSQI scores of mothers to children with varying levels of AD severity were higher than those in the control group before treatment. However, we found that only the mild group had clinical differences before and after treatment, and there were no significant differences among the other groups. This may be because feeding young children with moderate-to-severe AD, sleep mood disorders, behavioral problems, and school and work performance issues40 would have required a lot of energy, making it difficult for mothers to be aware of the nuanced effects of the disease at the time of the questionnaire.
As atopic dermatitis is a long-term condition, improvements in children’s rashes and health may mediate anxiety and depression in mothers. In Chinese society, mothers need more attention as primary caregivers. The findings have important implications for clinical practice, and a better understanding of the mental health and sleep of mothers of children with AD can help develop interventions that can allow them to maintain a healthy and stable mindset during their child’s long-term treatment to improve the quality of life of affected children and their families. Professionals also need to provide parents with adequate information about the prevalence of AD; assess, manage, and support the needs of caregivers of children with AD; and provide mental health services when necessary.
Our study has several limitations that must be acknowledged. First, the sample size was somewhat small; thus, the statistical difference in data difference was not significant. Second, although this study considers the severity of AD before and after the treatment, the results cannot be causally interpreted because we did not compare the control group across time. In addition, this study mainly explored the psychological situation of mothers of children with AD, which may be affected by other factors such as social environment and family situation. Third, we were unable to confirm participant compliance during the treatment. Finally, this study was subjective; thus, respondents may have had some bias in the questionnaires.
Conclusions
This is the first study to compare the changes in psychological health and sleep quality between mothers of children with varying levels of AD severity and mothers of healthy children, both before and after treatment. Our findings show that mothers of children with severe AD were the most affected. Through primary treatment combined with drug treatment, the severity of the disease lessened, and the mental health and sleep quality of the mothers in the mild AD group improved. The psychological health and sleep quality of mothers in the moderate and severe AD groups also showed some improvement. This study reminds clinicians to pay attention to the psychology and sleep quality of caregivers while treating children with AD.
Acknowledgments
We thank the participants and their families for their time and effort. The authors have no competing interests to report. The research reported in this publication was supported by the Wenzhou Science and Technology Plan Project (Y20180242) and Zhejiang Provincial Clinical Research Center for Pediatric Disease.
Author Contributions
All authors made a significant contribution to the work reported, whether in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; agreed on the journal to which the article has been submitted; and agreed to be accountable for all aspects of the work.
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Guo Y, Li P, Tang J, et al. Prevalence of atopic dermatitis in Chinese children aged 1–7 years. Sci Rep. 2016;6:29751. doi:10.1038/srep29751
2. Guo Y, Zhang H, Liu Q, et al. Phenotypic analysis of atopic dermatitis in children aged 1–12 months: elaboration of novel diagnostic criteria for infants in China and estimation of prevalence. J Eur Acad Dermatol Venereol. 2019;33(8):1569–1576. doi:10.1111/jdv.15618
3. Ramirez FD, Chen S, Langan SM, et al. Association of atopic dermatitis with sleep quality in children. JAMA Pediatr. 2019;173(5):e190025. doi:10.1001/jamapediatrics.2019.0025
4. Langan SM, Irvine AD, Weidinger S. Atopic dermatitis. Lancet. 2020;396(10247):345–360. doi:10.1016/S0140-6736(20)31286-1
5. Yao TC, Wang IJ, Sun HL, et al. Taiwan guidelines for the diagnosis and management of pediatric atopic dermatitis: consensus statement of the Taiwan Academy of Pediatric Allergy, Asthma and Immunology. J Microbiol Immunol Infect. 2022;55:561–572. doi:10.1016/j.jmii.2022.03.004
6. He H, Del Duca E, Diaz A, et al. Mild atopic dermatitis lacks systemic inflammation and shows reduced nonlesional skin abnormalities. J Allergy Clin Immunol. 2021;147(4):1369–1380. doi:10.1016/j.jaci.2020.08.041
7. Paller AS, Siegfried EC, Thaçi D, et al. Efficacy and safety of dupilumab with concomitant topical corticosteroids in children 6 to 11 years old with severe atopic dermatitis: a randomized, double-blinded, placebo-controlled Phase 3 trial. J Am Acad Dermatol. 2020;83(5):1282–1293. doi:10.1016/j.jaad.2020.06.054
8. Simpson EL, Sinclair R, Forman S, et al. Efficacy and safety of abrocitinib in adults and adolescents with moderate-to-severe atopic dermatitis (JADE MONO-1): a multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet. 2020;396(10246):255–266.
9. Chernyshov PV, Jirakova A, Ho RC, et al. An international multicenter study on quality of life and family quality of life in children with atopic dermatitis. Indian J Dermatol Venereol Leprol. 2013;79(1):52–58. doi:10.4103/0378-6323.104669
10. Filanovsky MG, Pootongkam S, Tamburro JE, et al. The financial and emotional impact of atopic dermatitis on children and their families. J Pediatr. 2016;169:284–290.e5. doi:10.1016/j.jpeds.2015.10.077
11. Wan J, Takeshita J, Shin DB, et al. Mental health impairment among children with atopic dermatitis: a United States population-based cross-sectional study of the 2013–2017 national health interview survey. J Am Acad Dermatol. 2020;82(6):1368–1375. doi:10.1016/j.jaad.2019.10.019
12. Barbarot S, Silverberg JI, Gadkari A, et al. The family impact of atopic dermatitis in the pediatric population: results from an international cross-sectional study. J Pediatr. 2022;246:220–226.e5. doi:10.1016/j.jpeds.2022.04.027
13. Warschburger P, Buchholz HT, Petermann F. Psychological adjustment in parents of young children with atopic dermatitis: which factors predict parental quality of life? Br J Dermatol. 2004;150(2):304–311. doi:10.1111/j.1365-2133.2004.05743.x
14. Moore K, David TJ, Murray CS, et al. Effect of childhood eczema and asthma on parental sleep and well-being: a prospective comparative study. Br J Dermatol. 2006;154(3):514–518. doi:10.1111/j.1365-2133.2005.07082.x
15. Cho HJ, Eisenberger NI, Olmstead R, et al. Preexisting mild sleep disturbance as a vulnerability factor for inflammation-induced depressed mood: a human experimental study. Transl Psychiatry. 2016;6(3):e750. doi:10.1038/tp.2016.23
16. Ramirez FD, Chen S, Langan SM, et al. Assessment of sleep disturbances and exhaustion in mothers of children with atopic dermatitis. JAMA Dermatol. 2019;155(5):556–563. doi:10.1001/jamadermatol.2018.5641
17. Lou TM, Zhang KL, Slesinger NC, et al. Positive psychology themes in interviews of children With atopic dermatitis: qualitative study. JMIR Pediatr Parent. 2022;5(3):e38725. doi:10.2196/38725
18. Willians HC, Burney PG, Pembroke AC, et al. The U.K. Working Party’s Diagnostic Criteria for Atopic Dermatitis. III. Independent hospital validation. Br J Dermatol. 1994;131(3):406–416. doi:10.1111/j.1365-2133.1994.tb08532.x
19. Brenninkmeijer EE, Schram ME, Leeflang MMG, et al. Diagnostic criteria for atopic dermatitis: a systematic review. Br J Dermatol. 2008;158(4):754–765. doi:10.1111/j.1365-2133.2007.08412.x
20. Schmitt J, Langan S, Deckert S, et al. Assessment of clinical signs of atopic dermatitis: a systematic review and recommendation. J Allergy Clin Immunol. 2013;132(6):1337–1347. doi:10.1016/j.jaci.2013.07.008
21. Oranje AP, Glazenburg EJ, Wolkerstorfer A, et al. Practical issues on interpretation of scoring atopic dermatitis: the SCORAD index, objective SCORAD and the three-item severity score. Br J Dermatol. 2007;157(4):645–648. doi:10.1111/j.1365-2133.2007.08112.x
22. Abeck D, Mempel M. Staphylococcus aureus colonization in atopic dermatitis and its therapeutic implications. Br J Dermatol. 1998;139(53):13–16. doi:10.1046/j.1365-2133.1998.1390s3013.x
23. Gong JQ, Lin L, Lin T, et al. Skin colonization by Staphylococcus aureus in patients with eczema and atopic dermatitis and relevant combined topical therapy: a double-blind multicentre randomized controlled trial. Br J Dermatol. 2006;155(4):680–687. doi:10.1111/j.1365-2133.2006.07410.x
24. Kedzierska A, Kapińska-Mrowiecka M, Czubak-Macugowska M, et al. Susceptibility testing and resistance phenotype detection in Staphylococcus aureus strains isolated from patients with atopic dermatitis, with apparent and recurrent skin colonization. Br J Dermatol. 2008;159(6):1290–1299. doi:10.1111/j.1365-2133.2008.08817.x
25. Imaizumi A, Kawakami T, Murakami F, et al. Effective treatment of pruritus in atopic dermatitis using H1 antihistamines (second-generation antihistamines): changes in blood histamine and tryptase levels. J Dermatol Sci. 2003;33(1):23–29. doi:10.1016/S0923-1811(03)00132-4
26. Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi:10.1111/j.1600-0447.1983.tb09716.x
27. Herrmann C. International experiences with the Hospital Anxiety and Depression Scale--A review of validation data and clinical results. J Psychosom Res. 1997;42(1):17–41. doi:10.1016/S0022-3999(96)00216-4
28. Nicassio PM, Ormseth SR, Kay M, et al. The contribution of pain and depression to self-reported sleep disturbance in patients with rheumatoid arthritis. Pain. 2012;153(1):107–112. doi:10.1016/j.pain.2011.09.024
29. Buysse DJ, Reynolds CF
30. Gieler U, Schoof S, Gieler T, et al. Atopic eczema and stress among single parents and families: an empirical study of 96 mothers. Acta Derm Venereol. 2017;97(1):42–46. doi:10.2340/00015555-2457
31. Kobusiewicz AK, Tarkowski B, Kaszuba A, et al. The relationship between atopic dermatitis and atopic itch in children and the psychosocial functioning of their mothers: a cross-sectional study. Front Med. 2023;10:1066495. doi:10.3389/fmed.2023.1066495
32. Marciniak J, Reich A, Szepietowski JC. Quality of life of parents of children with atopic dermatitis. Acta Derm Venereol. 2017;97(6):711–714. doi:10.2340/00015555-2633
33. El Hachem M, Gesualdo F, Ricci G, et al. Topical corticosteroid phobia in parents of pediatric patients with atopic dermatitis: a multicentre survey. Ital J Pediatr. 2017;43(1):22. doi:10.1186/s13052-017-0330-7
34. Zuberbier T, Orlow SJ, Paller AS, et al. Patient perspectives on the management of atopic dermatitis. J Allergy Clin Immunol. 2006;118(1):226–232. doi:10.1016/j.jaci.2006.02.031
35. Capozza K, Gadd H, Kelley K, et al. Insights from caregivers on the impact of pediatric atopic dermatitis on families: “I’m tired, overwhelmed, and feel like I’m failing as a mother”. Dermatitis. 2020;31(3):223–227. doi:10.1097/DER.0000000000000582
36. Bawany F, Northcott CA, Beck LA, et al. Sleep disturbances and atopic dermatitis: relationships, methods for assessment, and therapies. J Allergy Clin Immunol Pract. 2021;9(4):1488–1500. doi:10.1016/j.jaip.2020.12.007
37. Chamlin SL, Mattson CL, Frieden IJ, et al. The price of pruritus: sleep disturbance and cosleeping in atopic dermatitis. Arch Pediatr Adolesc Med. 2005;159(8):745–750. doi:10.1001/archpedi.159.8.745
38. Angelhoff C, Askenteg H, Wikner U, et al. “To cope with everyday life, I need to sleep” – a phenomenographic study exploring sleep loss in parents of children with atopic dermatitis. J Pediatr Nurs. 2018;43:e59–e65. doi:10.1016/j.pedn.2018.07.005
39. Meltzer LJ, Booster GD. Sleep disturbance in caregivers of children with respiratory and atopic disease. J Pediatr Psychol. 2016;41(6):643–650. doi:10.1093/jpepsy/jsw016
40. Kahn D, Iturriaga C, Bertran K, et al. Sleep quality in children with atopic dermatitis during flares and after treatment. Sleep Sci. 2020;13(2):172–175. doi:10.5935/1984-0063.20190139
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
