Back to Journals » Patient Preference and Adherence » Volume 14
Assessing Preferences for Rare Disease Treatment: Qualitative Development of the Paroxysmal Nocturnal Hemoglobinuria Patient Preference Questionnaire (PNH-PPQ©)
Authors Kaiser K , Yount SE , Martens CE, Webster KA , Shaunfield S, Sparling A, Peipert JD , Cella D , Rottinghaus ST, Donato BMK , Wells R, Tomazos I
Received 8 October 2019
Accepted for publication 5 February 2020
Published 5 April 2020 Volume 2020:14 Pages 705—715
DOI https://doi.org/10.2147/PPA.S233830
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Karen Kaiser,1 Susan E Yount,1 Christa E Martens,1 Kimberly A Webster,1 Sara Shaunfield,1 Amy Sparling,1 John Devin Peipert,1 David Cella,1 Scott T Rottinghaus,2 Bonnie MK Donato,2 Richard Wells,3 Ioannis Tomazos2
1Department of Medical Social Sciences, Northwestern University Feinberg School of Medicine, Chicago, IL, USA; 2Alexion Pharmaceuticals Inc, Boston, MA, USA; 3Sunnybrook Health Sciences Centre, Toronto, ON, Canada
Correspondence: Karen Kaiser
Department of Medical Social Sciences, Northwestern University Feinberg School of Medicine, 625 N. Michigan Ave. Suite 2700, Chicago, IL 60611, USA
Tel +1 312-503-3521
Fax +1 312-503-4800
Email [email protected]
Purpose: To develop a patient preference questionnaire (PPQ) assessing eculizumab and ravulizumab treatment for paroxysmal nocturnal hemoglobinuria (PNH).
Patients and Methods: The development of the PNH-PPQ© was consistent with Food and Drug Administration guidelines for patient-reported outcome measure development, and included 1) a targeted literature review; 2) PNH expert clinician input on treatment preferences; 3) review of existing qualitative data on the PNH treatment and disease experience; 4) concept elicitation interviews with 8 PNH patients who received eculizumab and/or ravulizumab; 5) translatability review; and 6) cognitive debriefing with 5 patients. Interview participants were recruited through a United Kingdom PNH patient advocacy group and a Canadian clinical site involved in clinical trial ALXN1210-PNH-302.
Results: Six themes were identified as most relevant to the PNH treatment experience from the concept elicitation interviews: disease symptoms (n=8/8); treatment frequency (n=7/8); quality of life impact of treatment/disease (n=7/8); treatment burden (n=7/8); treatment efficacy (n=5/8); and treatment side effects (n=5/8). An initial list of 88 preference questions was reduced to 11 highly relevant and non-redundant questions reflecting the 6 themes. Cognitive interview participants unanimously agreed that the PNH-PPQ instructions were clear; response options were understandable, easy to use, and provided enough choices; and the questions captured the factors that inform treatment preferences.
Discussion: When new drugs have similar efficacy to existing medications, documenting patient preferences is important for confirming patient benefit from the new medication. Understanding what matters most to patients is essential for delivering patient-centered care and may play a particularly significant role in treatment decision making. The availability of such a tool may be especially important as new orphan drugs are developed and patients with rare diseases have more than one treatment option to consider.
Conclusion: The PNH-PPQ provides a patient-centered approach for evaluating preferences for the treatment of PNH. The PNH-PPQ has subsequently assessed patient preference in the clinical trial sub-study ALXN1210-PNH-302s.
Keywords: paroxysmal nocturnal hemoglobinuria, questionnaire development, ravulizumab, eculizumab, treatment experience
Introduction
Paroxysmal nocturnal hemoglobinuria (PNH) is an acquired hemolytic anemia resulting from a somatic mutation in the PIG-A gene in the hematopoietic stem cells.1 Manifestations include hemolytic anemia, bone marrow failure, and thrombosis.2–5 PNH is a very rare disease with prevalence of about 16 per million people,6 and it can present at any age but mainly occurs in young adults, with the median age at diagnosis being 34.5–7
Prior to 2007, treatment options for PNH were primarily supportive, including blood transfusion, erythropoiesis-stimulating agents, corticosteroids, anabolic steroids, iron therapy, thrombosis prophylaxis, and sometimes thrombolytic therapy or allogenic bone marrow transplantation.8,9 In 2007, eculizumab was approved for the treatment of patients with PNH by the United States Food and Drug Administration (FDA) and by the European Medicines Agency.8,10 Eculizumab is a humanized monoclonal antibody directed against the terminal complement protein C5, which blocks the formation of the membrane attack complex (C5–9) and protects red blood cells from complement-mediated intravascular hemolysis in patients with PNH.9,11 It has been shown to reduce or eliminate the need for blood transfusion, decrease the incidence of thrombosis, improve anemia, reduce fatigue, and improve quality of life (QOL).1,10,12 Before the development of eculizumab, the median survival in patients with PNH was 10 to 15 years from the time of diagnosis;6 with the use of eculizumab, the overall survival of PNH patients has improved significantly, approaching that of the general population. However, eculizumab dosing may be burdensome to patients. Eculizumab is administered intravenously every 7 days for the first 5 weeks and then biweekly thereafter.1,8,13 Given that PNH is a chronic disease, eculizumab’s frequent dosing regimen may impact patients’ quality of life and influence treatment compliance.
Ravulizumab, a novel complement inhibitor that provides immediate, complete, and sustained C5 inhibition with 8-week dosing intervals, was approved by the FDA in December 2018 for the treatment of PNH, and has since been approved for use in both Japan and the European Union.14,15 Ravulizumab has demonstrated noninferiority compared with eculizumab on all primary and key secondary efficacy endpoints, including fatigue, in both complement-naive and –experienced patients with PNH.16,17 Given that there are now multiple approved medications for the treatment of PNH in some regions, patient treatment preferences and treatment burden are important to assess. Thus, we aimed to develop a treatment preference questionnaire for patients with PNH suitable for use in clinical studies.
Methods
All study procedures in the 302s sub-study were approved by the Northwestern University Institutional Review Board and were performed in compliance with relevant laws and institutional guidelines. Multiple sources of data were used to develop the PNH treatment preference questionnaire, as shown in Figure 1.
 |
Figure 1 Approach used to develop the PNH-PPQ. |
Literature Review
A targeted literature review was conducted in 2017 to investigate 1) PNH patients’ experiences with eculizumab, 2) factors that influence drug and dosing interval preferences for patients undergoing therapy for chronic conditions, and 3) existing measures of patient treatment preferences.
Delphi Survey of Expert Clinicians
We used a modified Delphi Technique to obtain health care provider PNH treatment preferences, the factors that shape preferences, and provider views of patient preferences.18 Alexion identified 34 English-speaking PNH clinical providers from North America, Europe, and Australia; all had experience treating patients with eculizumab, and the majority also had familiarity with ravulizumab via Alexion clinical trials. After Alexion contacted experts to introduce the Delphi survey to be conducted by Northwestern, the Northwestern team emailed each physician with an invitation to participate. Providers who agreed to participate received follow-up e-mails with survey links and reminders to complete the surveys, as needed. Our target sample size was a minimum of ten expert providers. Each expert completed two surveys and was compensated $400USD for their time and effort.
Review of Existing Qualitative Data from PNH Patients
In 2017, a team of qualitative researchers from Alexion conducted an ethnography (a human experience story) with 10 PNH patients about their eculizumab treatment experiences. The ethnography incorporated patient drawings, photos, quotations, and self-recorded videos in which patients described their experiences with eculizumab treatment. The study team obtained the de-identified ethnography PowerPoint slide deck (148 slides). Two qualitative researchers reviewed the slide deck and independently created a list of themes from the patient experiences, which provided preliminary insights into the eculizumab treatment experience that informed development of the patient interview guide.
Concept Elicitation Interviews
Individual telephone interviews were conducted with PNH patients. PNH patients were eligible to participate if they had previously or were currently receiving ravulizumab or eculizumab, could speak and read English, were at least 18 years old, and were able to provide informed consent. We recruited patients from a patient advocacy organization and a Canadian clinical site involved in Alexion clinical trial, ALXN1210-PNH-302. Our study team representative answered participants’ questions about the study, confirmed eligibility, and scheduled individual phone interviews. Prior to their scheduled interview, eligible participants received the General Data Protection Regulation (GDPR) compliant consent form to review, sign, and return to Northwestern University. Interview participants received a $125USD Visa gift card for their time and effort.
Interviews were conducted using a semi-structured interview guide developed from the literature and modeled after guides used in our prior work to develop patient-reported outcome (PRO) or decision-making tools.19–22 The interviewer first obtained patient demographic information and treatment history, followed by open elicitation of the patient’s views of the benefits and drawbacks of eculizumab and ravulizumab. Patients who had received both drugs were asked to explain which of the drugs they preferred. Finally, patients were asked to explain what aspects of treatment for PNH are most important to them. All interviews were audiotaped and transcribed.
Data Analysis
Data, including field notes and de-identified transcripts, were analyzed systematically to generate a list of patient themes related to eculizumab, ravulizumab, treatment preference, and treatment experiences.23 Next, we combined redundant themes and removed themes deemed irrelevant to PNH treatment preferences. Interview transcripts were reviewed to clarify themes and to extract exemplar patient quotes. We used a saturation grid to track themes as they emerged and determine when no new information was obtained.
Questionnaire Development
Drawing from all data sources, members of the study team who were experienced in PRO assessment and scale development drafted an exhaustive list of questions potentially relevant to PNH treatment preferences. If available, content was drawn from the Functional Assessment of Chronic Illness Therapy (FACIT) item library of approximately 750 items. Draft items were reviewed and refined in a series of team meetings. The draft questionnaire underwent translatability review24 to identify conceptual or linguistic issues that could impede translation or cross-cultural research; any identified issues were addressed prior to cognitive debriefing.
Cognitive Debriefing and Finalization of the Paroxysmal Nocturnal Hemoglobinuria Patient Preference Questionnaire (PNH-PPQ)
A subset of concept elicitation participants was invited to complete a cognitive interview of the draft measure. Our cognitive interviewing protocol, based on the work of Willis,25 ascertained comprehension of the question and response processes. For example, patients were asked to: 1) describe how they arrived at their answer; 2) restate items in their own words; 3) discuss the clarity of the item; and 4) indicate whether the question accurately reflected their experience. The cognitive interview also assessed clarity of instructions and response options. Interviews were audiotaped to ensure capture of all relevant information, and interviewers entered detailed interview notes into an Excel spreadsheet. We summarized patients’ reports on comprehension and appropriateness of instructions, response options, and item content. Cognitive interview transcripts were reviewed for clarification. Items for which there was low comprehension were revised or removed, as appropriate.
Results
Literature Review
Eleven patient preference questionnaires that most closely aligned with the objectives of this project were identified from the literature;26–36 however, none of them evaluated patient preference and dosing schedules in a manner suitable for our aims. After reviewing the literature and the 11 questionnaires, the following concepts were identified as potentially relevant to our measure: symptom relief; effectiveness; ease of use/convenience/bother; impact on daily life; and medication preference.
Delphi Interviews
We invited 34 clinical experts to participate. From March to May 2018, 9 of 34 individuals from treatment centers across 3 continents participated in the Modified Delphi process. Of the 25 who did not participate, 22 did not respond to the invitation, 2 declined, and 1 was not responsive after initially agreeing to participate. The 9 participating clinical experts were hematologists with between 6 and 42 years of experience seeing PNH patients, and an average of 9 years of experience treating PNH patients with eculizumab. We identified the following 6 themes from clinician comments about factors driving PNH patient treatment preferences: efficacy, convenience, side effects, quality of life, cost, and medication safety. Efficacy and convenience received the highest total endorsements (n=9, 100% each), and efficacy and quality of life were the only themes identified as “Highest Priority” by clinicians.
Review of Existing Data
Ethnography participants (N=10) ranged in age from 29 to 55, were diagnosed 4 months to 12 years prior, and 40% received in-home infusions. The following themes were identified in the ethnography data slide deck:
- Treatment efficacy/symptom relief. Eculizumab gave patients their lives back and controlled their disease. The medication allowed some freedom from the mental burden of PNH. However, they desired the medication to be efficacious for a longer duration.
- Treatment frequency. Patients felt trapped in a never-ending 2-week cycle of treatment, which led to frustration and impacts on school, work, leisure time, and ability to travel.
- Fatigue. Fatigue was a significant part of their treatment cycle, with several patients describing feeling fatigue prior to and following infusion.
- Missing out. Because of treatment frequency and the limited number of days each treatment cycle when they felt good, many patients felt as if they were “missing out” on life. They felt guilty for not participating in social events and anxiety about missed work.
- Treatment burden. Patients described the burden of treatment, including planning for treatment, waiting for treatment, needle pricks, exposure to germs, loss of privacy, and anxiety.
Concept Elicitation Interviews
Interviews were completed with 8 patients. Of these, 2 patients had experience with eculizumab only, 4 patients had received eculizumab and ravulizumab as part of a clinical trial, and 2 patients had received ravulizumab only. Patient sociodemographic information is shown in Table 1.
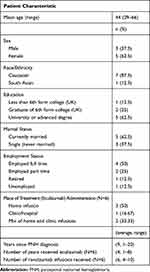 |
Table 1 Patient Concept Elicitation Sample (N=8) |
When asked, “What aspects of treatment for PNH are most important to you?” Patients noted a variety of factors including treatment efficacy, treatment frequency, quality of life, and symptom control (Table 2). Quality of life and symptom control were mentioned most frequently as the most important aspect of treatment for patients. Although access and cost were mentioned by 2 patients as the most important aspects of treatment, they are not included as key treatment preference themes because these factors are equivalent across eculizumab and ravulizumab within a clinical trial setting. We extracted an initial list of 27 themes related to the PNH treatment experience from the concept elicitation interviews. After merging redundant categories, 6 themes were identified as most relevant to PNH patient treatment preference according to the frequency of mention and significance to patients. These themes are summarized in Table 3 and discussed in more detail below.
 |
Table 2 Aspects of Treatment Most Important to PNH Patients (N=8) |
 |
Table 3 Key Themes from the PNH Concept Elicitation Interviews |
Disease Symptoms
Every patient in our sample spontaneously described PNH symptoms such as dark urine, kidney failure, erectile dysfunction, jaundice, trouble breathing, fatigue, and headaches. Symptoms were often debilitating and negatively impacted patients’ quality of life. Symptom control was mentioned by 2 patients as the most important aspect of treatment for PNH (Table 2). Our data on symptom burden complement the ethnography data, in which patients emphasized the debilitating impact of PNH symptoms, particularly fatigue prior to treatment.
Probably the two days before (treatment), my bones feel empty. I’m exhausted, totally exhausted and fatigued. (Patient 002, eculizumab)
Well, that (symptom reduction) really is the major thing. I mean, obviously my hemoglobin was very, very low. I was jaundiced constantly. I had trouble breathing; I had trouble doing most things. Eculizumab, as I said, it gave me my life back. It was wonderful just to feel normal again. (Patient 003, eculizumab and ravulizumab)
Quality of Life Impact of Treatment/Disease
Quality of life was among the most important aspects of PNH treatment cited by patients (Table 3). Patients expressed gratitude for treatment that positively affected their quality of life, while also noting how the disease or treatment negatively impacted their quality of life.
I’m a mother to a small child and I have parents who are now quite elderly … So, I want to be ideally in a situation where I can forget that I have a chronic illness and function as normally as possible. That’s the ultimate goal—quality of life. (Patient 001, eculizumab and ravulizumab)
(The most important factor for my drug decision) has to be quality of life … that has to be the top, because without quality of life, you can’t really live your life or it makes it difficult to. (Patient 004, eculizumab and ravulizumab)
Treatment Frequency
Five patients reported eculizumab’s two-week treatment schedule was a significant burden. Patients who had received eculizumab prior to receiving ravulizumab unanimously preferred the longer dosing interval of ravulizumab. These patients enthusiastically described the positive impact of ravulizumab dosing upon their lives. For example, ravulizumab allowed them to feel independent, plan future activities, and travel.
That for me is a major plus as well, the fact that it’s now every eight weeks. So, it’s almost like forgetting that I’ve got a condition because it’s much, much better for me. (Patient 003, eculizumab and ravulizumab)
… it was a seamless transition (from eculizumab to ravulizumab) for me … Probably the biggest difference for me is it went from two weeks to once every two months. I’ve got a much greater independence (now). I’m able to, you know, plan things more in advance, whereas with Soliris (eculizumab), you know, I couldn’t go away for more than two weeks because I was essentially chained to the system, to the health system. (Patient 005, eculizumab and ravulizumab)
Treatment Burden
Patients in our sample and in the ethnography sample described multiple ways in which treatment was a burden in their lives. Treatment burden could be physical, such as the exposure to needles, or logistical, such as the need to coordinate work schedules around treatment. Treatment also brought an emotional and psychological burden due to the anxiety some patients experienced leading up to treatment or the ways in which treatment reminded patients that they were living with a serious disease. The impact of frequent treatment on one’s ability to take vacations or plan activities was a particularly salient burden among PNH patients.
… the actual therapy itself, it’s not painful … I think it’s more the psychological idea that going every two weeks, it’s like a constant reminder that you do have a severe illness. Going to the therapy room and seeing other patients, it’s quite, I’d say, psychologically affecting. (Patient 006, eculizumab)
The downsides of it was the fact that obviously I was tied to weekly infusions. I had to almost build my life around having my infusions every two weeks … That came first, so I couldn’t plan times to go out during holiday are things like that. My life was planning around my infusions really. (Patient 003, eculizumab and ravulizumab)
Treatment Efficacy
Patients emphasized the importance of treatment as a means to save their lives.
I think the main positive (of treatment) was that it sort of saved my life really, because I know prior to eculizumab being licensed there was no kind of treatments other than having a bone marrow transplant, and the life expectancy was about ten years. So, I’d say definitely obviously the big pro is that it saved my life … (Patient 004, eculizumab and ravulizumab)
However, while the majority of our sample did not experience decreased symptom control over the course of the treatment cycle, some patients in our concept elicitation sample and the ethnography sample struggled with treatment that seemed to lose efficacy (i.e., symptom control) prior to the next dose. Patient 001 noted that she experienced PNH symptoms, including fatigue, 2–3 days before her next eculizumab dose and about 2 weeks before her next ravulizumab dose. Patient 006 also reported fatigue prior to treatment:
Yeah. I’d say two days really and generally the day before I feel really, really, really exhausted and maybe it’s wearing off. (Patient 006, eculizumab)
In contrast, many patients said they felt completely “normal” on eculizumab and ravulizumab, and Patient 004 reported feeling less fatigued on ravulizumab compared with eculizumab.
Treatment Side Effects
For some patients, side effects occurred with only their first dose of treatment, while a few patients in both our concept elicitation sample and the ethnography sample experienced side effects following each treatment. Other patients noted that they were grateful that they did not experience any side effects from PNH treatment.
The side effects, when I have the treatment, about 15 minutes into the treatment, I suddenly get incredibly tired and my body is just overwhelmed and exhausted, just wiped out, completely wiped out. (Patient 002, eculizumab)
Fortunately I’ve been very lucky. I haven’t had any bad effects with either of the drugs. (Patient 003, eculizumab and ravulizumab)
Development of the Draft Preference Questionnaire
An initial list of 88 patient preference questionnaire items was drafted by the study team to reflect the 6 key themes. Through an iterative process of team discussions, item reduction, and item revisions, 15 items were selected for the draft questionnaire. These items underwent translatability review and revisions, as necessary, to produce Draft Version 1.0 of the PNH-PPQ.
Cognitive Debriefing of the Draft Preference Questionnaire
Two team members completed cognitive debriefing interviews with 5 PNH patients from the concept elicitation sample. Cognitive interviews occurred in an iterative fashion, whereby we analyzed data following each interview and made changes to the questionnaire, as necessary. As such, Draft Version 1.0 underwent cognitive debriefing with 3 patients, revision, and then further debriefing as Draft Version 2.0 with 2 additional patients.
Across the 5 cognitive debriefing interviews, responses were unanimous regarding the following: questionnaire instructions were clear; response options made sense; response options were easy to use; and response options provided enough choices. In response to the cognitive debriefing question, “Do these questions, in your opinion, capture the factors that inform your preferences for PNH treatment?” All patient participants (n=5) said yes. Thus, overall, patients supported the content validity of the questionnaire.
Nearly every item was endorsed as relevant by 100% (n=5) of participants, with the exception of 5 items that referenced side effects or treatment experiences some had not experienced (e.g., nausea). Nonetheless, patients often noted that other PNH patients would likely find those questions relevant. For all but three items on the questionnaire, 100% of patients indicated that the meaning of the question was clear. One patient thought the question, “Which medication did you prefer based on convenience of receiving treatment?” was unclear; she thought about travel for treatment but was not sure if that was the intended meaning of the question. One patient was not sure if the question, “Which medication did you prefer based on pain related to the infusion?” was asking about immediate pain from infusion or about pain due to other side effects. One patient thought the meaning of the item, “Which medication did you prefer based on the effectiveness of the medication until the next infusion?” was somewhat unclear. No additional changes were deemed necessary after the final 2 cognitive interviews. However, the study team made additional revisions to the questionnaire to ensure consistency in item wording and removed 4 questions due to concerns about compliance with adverse event reporting.
Final Questionnaire
The questionnaire contains 11 questions and includes 1 overall preference question, 1 question evaluating preference for eculizumab or ravulizumab according to 9 treatment characteristics (“Controlling fatigue," “Frequency of infusions," etc.) including 1 write-in option, 1 question asking patients to indicate which treatment characteristic was most important for their overall medication preference, 4 questions evaluating aspects of treatment with eculizumab, and 4 questions evaluating those same aspects of treatment with ravulizumab. Table 4 illustrates how the final questionnaire content supports the 6 themes from the patient concept elicitation interviews.
 |
Table 4 Final Questionnaire Content as It Relates to Themes from Concept Elicitation Interviews |
Discussion
Evaluating multiple specific aspects of treatment and using an overall preference question supports current evidence that patients form both types of judgments (multidimensional and summary) when evaluating their satisfaction with medical care.44 Dosing intervals, in particular, have received attention in the patient preference/satisfaction literature. Exploratory research has shown general trends toward preference for less frequent dosing, although there is some variability in these findings depending on population, type of therapy, and the disease and symptoms being treated.30,45-50 Reducing the frequency of dosing has been shown to improve patient adherence to the treatment plan,51 with no compromise on patient QOL. Thus, evaluating PNH patient preference for eculizumab and ravulizumab and their respective dosing frequencies is important for understanding and improving the PNH patient treatment experience. Moreover, when new drugs are developed that have similar efficacy to existing medications, documenting patient preferences is important for confirming patient benefit from the new medication. Likewise, the number of orphan drugs approved by the FDA has increased greatly in recent years;52 patient input is critical for this drug development process.
The main limitation of the study was sample size, which was constrained largely by the rare nature of PNH.31 Moreover, we were limited in the number of patients for concept elicitation interviews because the optimal participant was one who had experience with both eculizumab and ravulizumab, and ravulizumab was only available through participation in the clinical trial. However, there was consistency among our study participants around the key issues for PNH treatment preferences, which were further confirmed by expert and existing patient ethnography data. As such, we do not believe a larger sample would have changed the measure content. Additionally, we acknowledge that the treatment issues that PNH patients face may vary by country and healthcare system. For example, treatment costs are covered by a national health care system in some countries and patients may not be able to obtain their PNH treatment at home in particular locations. We have tried to include the major concerns facing PNH patients while keeping the response burden minimal. Moreover, by including space for patients to write in factors that impact preference, the questionnaire allows additional factors to be recognized.
Finally, the PNH-PPQ has not yet been psychometrically validated due to the time constraints for developing the questionnaire for implementation in the ALXN1210-PNH-302 trial. However, our confidence in the construct validity of the measure is enhanced by the rigorous nature of the development process, which included a literature review, an international sample of expert clinicians, and an international sample of PNH patients. In addition, the data obtained from the literature and clinician and patient interviews all converged to provide a concordant set of themes with which to build the questionnaire.
The PNH-PPQ represents a patient-centered tool for assessing patient preference between the only two FDA-approved medications for the rare disease PNH as well as the factors influencing those preferences. The measure was developed using a rigorous process consistent with Food and Drug Administration guidelines for the development of PRO instruments53 and reflects patients’ priorities and has strong face and content validity for PNH patients. Subsequent to its development, the PNH-PPQ was implemented in a substudy of the ALXN1210-PNH-302 (registered at www.clinicaltrials.gov as NCT03056040) clinical trial to assess patient treatment preferences in a sample of clinical trial patients.54 Understanding what matters most to patients is essential for delivering patient-centered care and may play a particularly significant role in treatment decision-making for rare diseases when more than one treatment exists. The availability of such a tool may be especially important as new orphan drugs are developed and patients with these rare diseases have more than one treatment option to consider.
Conclusions
The final 11-item questionnaire provides a patient-centered approach for evaluating preferences for the treatment of PNH. The value of and attention devoted to the patient perspective, including patient preference, experience, and satisfaction, by payers and healthcare providers, has been increasing over the past two decades.37–41 Patients with chronic conditions increasingly prefer to be involved in decisions around their care,38 and younger patients, in particular, are seeking more active roles in their medical decision-making and care.40,42 The increased involvement of younger patients is highly salient for PNH, where the median age at diagnosis is 34.43 Therefore, additional insights into their treatment preferences and satisfaction, which can be gained through the PNH-PPQ, are likely to be valuable.
Acknowledgments
Copyright for the PNH-PPQ questionnaire is held by Alexion Pharmaceuticals, Inc. A copy of the questionnaire can be obtained from the authors upon request. This study was supported by Alexion Pharmaceuticals, Inc (Boston, MA, USA). The involvement of Alexion Pharmaceuticals, Inc. was limited to funding of the study and reviewing the manuscript for scientific accuracy. Alexion Pharmaceuticals, Inc. had no role in the study design; in collection, analysis, and interpretation of the data; in the writing of the report; or in the decision to submit this article for publication. Editorial and submission support were provided by ApotheCom (Yardley, PA, USA).
Disclosure
David Cella is a consultant to, and has received grant support from, Alexion Pharmaceuticals, Inc. Scott T. Rottinghaus is an employee of, and owns stock in, Alexion Pharmaceuticals Inc. Bonnie M. K. Donato was an employee and stockholder of Alexion Pharmaceuticals, Inc. at the time of this analysis and her current affiliation is at Boehringer Ingelheim Pharmaceuticals Inc, Ridgefield, CT, USA. Richard Wells has received honoraria and consulting fees from Alexion Pharmaceuticals, Inc. Ioannis Tomazos is an employee of, and owns stock in, Alexion Pharmaceuticals, Inc. The authors report no other conflicts of interest in this work.
References
1. Loschi M, Porcher R, Barraco F, et al. Impact of eculizumab treatment on paroxysmal nocturnal hemoglobinuria: a treatment versus no-treatment study. Am J Hematol. 2016;91(4):366–370. doi:10.1002/ajh.24278
2. Brodsky RA. Narrative review: paroxysmal nocturnal hemoglobinuria: the physiology of complement-related hemolytic anemia. Ann Intern Med. 2008;148(8):587–595. doi:10.7326/0003-4819-148-8-200804150-00003
3. Parker CJ. Historical aspects of paroxysmal nocturnal haemoglobinuria: ‘defining the disease’. Br J Haematol. 2002;117(1):3–22.
4. Socie G, Mary JY, de Gramont A, et al. Paroxysmal nocturnal haemoglobinuria: long-term follow-up and prognostic factors. French Society of Haematology. Lancet. 1996;348(9027):573–577. doi:10.1016/S0140-6736(95)12360-1
5. Nishimura J, Kanakura Y, Ware RE, et al. Clinical course and flow cytometric analysis of paroxysmal nocturnal hemoglobinuria in the United States and Japan. Medicine (Baltimore). 2004;83(3):193–207. doi:10.1097/01.md.0000126763.68170.46
6. Hillmen P, Lewis SM, Bessler M, Luzzatto L, Dacie JV. Natural history of paroxysmal nocturnal hemoglobinuria. N Engl J Med. 1995;333(19):1253–1258. doi:10.1056/NEJM199511093331904
7. Moyo VM, Mukhina GL, Garrett ES, Brodsky RA. Natural history of paroxysmal nocturnal haemoglobinuria using modern diagnostic assays. Br J Haematol. 2004;126(1):133–138. doi:10.1111/bjh.2004.126.issue-1
8. Dmytrijuk A, Robie-Suh K, Cohen MH, Rieves D, Weiss K, Pazdur R. FDA report: eculizumab (Soliris) for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Oncologist. 2008;13(9):993–1000. doi:10.1634/theoncologist.2008-0086
9. Luzzatto L. Recent advances in the pathogenesis and treatment of paroxysmal nocturnal hemoglobinuria. F1000Res. 2016;5. doi:10.12688/f1000research.7288.1
10. Kelly RJ, Hill A, Arnold LM, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117(25):6786–6792. doi:10.1182/blood-2011-02-333997
11. Thomas TC, Rollins SA, Rother RP, et al. Inhibition of complement activity by humanized anti-C5 antibody and single-chain Fv. Mol Immunol. 1996;33(17–18):1389–1401. doi:10.1016/S0161-5890(96)00078-8
12. Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–1243. doi:10.1056/NEJMoa061648
13. Brodsky RA. Paroxysmal nocturnal hemoglobinuria. Blood. 2014;124(18):2804–2811. doi:10.1182/blood-2014-02-522128
14. FDA approves new treatment for adult patients with rare, life-threatening blood disease. Available from: https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm629022.htm?rel=0”?rel=0”. December 21, 2018.
15. Ravulizumab [Prescribing Information]. Boston, MA: Alexion Pharmaceuticals, Inc.; 2018.
16. Lee JW, Sicre de Fontbrune F, Wong Lee Lee L, et al. Ravulizumab (ALXN1210) vs eculizumab in adult patients with PNH naive to complement inhibitors: the 301 study. Blood. 2019;133(6):530–539. doi:10.1182/blood-2018-09-876136
17. Kulasekararaj AG, Hill A, Rottinghaus ST, et al. Ravulizumab (ALXN1210) vs eculizumab in C5-inhibitor-experienced adult patients with PNH: the 302 study. Blood. 2019;133(6):540–549. doi:10.1182/blood-2018-09-876805
18. RAND Corporation. Delphi method. Available from: http://www.rand.org/topics/delphi-method.html.
19. Cella D, Rosenbloom SK, Beaumont JL, et al. Development and validation of 11 symptom indexes to evaluate response to chemotherapy for advanced cancer. J Natl Compr Canc Netw. 2011;9(3):268–278. doi:10.6004/jnccn.2011.0026
20. Kaiser K, Beaumont JL, Webster K, et al. Development and validation of the functional assessment of cancer therapy–antiangiogenesis subscale. Cancer Med. 2015;4(5):690–698. doi:10.1002/cam4.385
21. Kaiser K, Cheng WY, Jensen S, et al. Development of a shared decision-making tool to assist patients and clinicians with decisions on oral anticoagulant treatment for atrial fibrillation. Curr Med Res Opin. 2015;31(12):2261–2272. doi:10.1185/03007995.2015.1096767
22. Magasi S, Mallick R, Kaiser K, et al. Importance and relevance of pulmonary symptoms among patients receiving second-and third-line treatment for advanced non–small-cell lung cancer: support for the content validity of the 4-item pulmonary symptom index. Clin Lung Cancer. 2013;14(3):245–253. doi:10.1016/j.cllc.2012.07.001
23. Glaser B, Strauss AL. The Discovery of Grounded Theory. Chicago: Aldine; 1967.
24. Correia H. Translatability & cultural harmonization review. In: PROMIS® Instrument Development and Validation Scientific Standards. Vol. 2; 2013.
25. Willis GB. Cognitive Interviewing: A Tool for Improving Questionnaire Design. Thousand Oaks, CA: Sage; 2005.
26. Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2(1):12. doi:10.1186/1477-7525-2-12
27. Flood EM, Beusterien KM, Green H, et al. Psychometric evaluation of the Osteoporosis Patient Treatment Satisfaction Questionnaire (OPSAT-Q), a novel measure to assess satisfaction with bisphosphonate treatment in postmenopausal women. Health Qual Life Outcomes. 2006;4:42. doi:10.1186/1477-7525-4-42
28. Gold DT, Horne R, Coon CD, et al. Development, reliability, and validity of a new preference and satisfaction questionnaire. Value Health. 2011;14(8):1109–1116. doi:10.1016/j.jval.2011.06.010
29. Horne R, Weinman J, Hankins M. The beliefs about medicines questionnaire: the development and evaluation of a new method for assessing the cognitive representation of medication. Psychol Health. 1999;14(1):1–24. doi:10.1080/08870449908407311
30. Iwamoto J, Okano H, Furuya T, et al. Patient preference for monthly bisphosphonate versus weekly bisphosphonate in a cluster-randomized, open-label, crossover trial: Minodroate Alendronate/Risedronate Trial in Osteoporosis (MARTO). J Bone Miner Metab. 2016;34(2):201–208. doi:10.1007/s00774-015-0653-7
31. Kimman ML, Rotteveel AH, Wijsenbeek M, et al. Development and pretesting of a questionnaire to assess patient experiences and satisfaction with medications (PESaM questionnaire). Patient. 2017;10(5):629–642. doi:10.1007/s40271-017-0234-z
32. Meltzer EO, Hadley J, Blaiss M, et al. Development of questionnaires to measure patient preferences for intranasal corticosteroids in patients with allergic rhinitis. Otolaryngol Head Neck Surg. 2005;132(2):197–207. doi:10.1016/j.otohns.2004.10.010
33. Milic M, Foster A, Rihawi K, Anthoney A, Twelves C. ‘Tablet burden’ in patients with metastatic breast cancer. Eur J Cancer. 2016;55:1–6. doi:10.1016/j.ejca.2015.11.015
34. Peipert JD, Beaumont JL, Bode R, Cella D, Garcia SF, Hahn EA. Development and validation of the functional assessment of chronic illness therapy treatment satisfaction (FACIT TS) measures. Qual Life Res. 2014;23(3):815–824. doi:10.1007/s11136-013-0520-8
35. Ruiz MA, Pardo A, Rejas J, Soto J, Villasante F, Aranguren JL. Development and validation of the “Treatment Satisfaction with Medicines Questionnaire” (SATMED-Q)©. Value Health. 2008;11(5):913–926. doi:10.1111/j.1524-4733.2008.00323.x
36. Shikiar R, Rentz AM. Satisfaction with medication: an overview of conceptual, methodologic, and regulatory issues. Value Health. 2004;7(2):204–215. doi:10.1111/j.1524-4733.2004.72252.x
37. Ahmed F, Burt J, Roland M. Measuring patient experience: concepts and methods. Patient. 2014;7(3):235–241. doi:10.1007/s40271-014-0060-5
38. Chewning B, Bylund CL, Shah B, Arora NK, Gueguen JA, Makoul G. Patient preferences for shared decisions: a systematic review. Patient Educ Couns. 2012;86(1):9–18. doi:10.1016/j.pec.2011.02.004
39. Chewning B, Sleath B. Medication decision-making and management: a client-centered model. Soc Sci Med. 1996;42(3):389–398. doi:10.1016/0277-9536(95)00156-5
40. Street RL, Elwyn G, Epstein RM. Patient preferences and healthcare outcomes: an ecological perspective. Expert Rev Pharmacoecon Outcomes Res. 2012;12(2):167–180. doi:10.1586/erp.12.3
41. Institute of Medicine. Crossing the Quality Chasm: A New Health System for the 21st Century. Washington, DC: National Academy Press; 2001.
42. Lindhiem O, Bennett CB, Trentacosta CJ, McLear C. Client preferences affect treatment satisfaction, completion, and clinical outcome: a meta-analysis. Clin Psychol Rev. 2014;34(6):506–517. doi:10.1016/j.cpr.2014.06.002
43. Sharma R, Keyzner A, Liu J, Bradley T, Allen SL. Successful pregnancy outcome in paroxysmal nocturnal hemoglobinuria (PNH) following escalated eculizumab dosing to control breakthrough hemolysis. Leuk Res Rep. 2015;4(1):36–38. doi:10.1016/j.lrr.2015.05.001
44. Strasser S, Aharony L, Greenberger D. The patient satisfaction process: moving toward a comprehensive model. Med Care Rev. 1993;50(2):219–248. doi:10.1177/107755879305000205
45. Chern B, Joseph D, Joshua D, et al. Bisphosphonate infusions: patient preference, safety and clinic use. Support Care Cancer. 2004;12(6):463–466. doi:10.1007/s00520-004-0628-z
46. Hadji P, Ziller V, Gamerdinger D, et al. Quality of life and health status with zoledronic acid and generic alendronate–a secondary analysis of the Rapid Onset and Sustained Efficacy (ROSE) study in postmenopausal women with low bone mass. Osteoporos Int. 2012;23(7):2043–2051. doi:10.1007/s00198-011-1834-4
47. Ijiri S. Further characterization between oral bisphosphonate dosing interval and patient preference–patient preference for daily, weekly and monthly bisphosphonate, based on results of 3052 outpatients questionnaires. Nihon Rinsho. 2013;71(12):2223–2231.
48. Reginster JY, Rabenda V, Neuprez A. Adherence, patient preference and dosing frequency: understanding the relationship. Bone. 2006;38(4 Suppl 1):S2–S6. doi:10.1016/j.bone.2006.01.150
49. Ryzner KL, Burkiewicz JS, Griffin BL, Komperda KE. Survey of bisphosphonate regimen preferences in an urban community health center. Consult Pharm. 2010;25(10):671–675. doi:10.4140/TCP.n.2010.671
50. Willeke P, Becker H, Wassenberg S, Pavenstadt H, Jacobi AM. Patient/rheumatologist evaluation of infusion treatment for rheumatoid arthritis. Z Rheumatol. 2011;70(3):
51. Richter A, Anton SF, Koch P, Dennett SL. The impact of reducing dose frequency on health outcomes. Clin Ther. 2003;25(8):
52. Updated Study Analyzes Use and Cost of Orphan Drugs [press release]. Available from: https://rarediseases.org/updated-study-analyzes-use-and-cost-of-orphan-drugs/. October 18, 2018.
53. US Department of Health and Human Services Food and Drug Administration. Guidance for industry patient-reported outcome measures: use in medical product development to support labeling claims. Available from: https://www.fda.gov/media/77832/download. December 2009.
54. Peipert JD, Kulesekararaj A, Fernandez FAG, et al. Patient preferences for the treatment of paroxysmal nocturnal hemoglobinuria: interim results of a patient survey of ravulizumab (ALXN1210) and eculizumab. Abstract presented at Academy of Managed Care & Specialty Pharmacy Annual Meeting; San Diego, CA. J Manag Care Specialty Pharm. 2019;25(3–a):S37.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
