Back to Journals » Clinical, Cosmetic and Investigational Dentistry » Volume 9
Aseptic-avascular osteonecrosis: local “silent inflammation” in the jawbone and RANTES/CCL5 overexpression
Authors Lechner J , Schuett S, von Baehr V
Received 19 August 2017
Accepted for publication 3 October 2017
Published 9 November 2017 Volume 2017:9 Pages 99—109
DOI https://doi.org/10.2147/CCIDE.S149545
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Christopher E. Okunseri
Johann Lechner,1 Sabine Schuett,2 Volker von Baehr2
1Clinic for Integrative Dentistry, Munich, 2Department of Immunology and Allergology, Institute for Medical Diagnostics in MVZ GbR, Berlin, Germany
Abstract: Of the definitions listed in the International Statistical Classification of Diseases and Related Health Problems, tenth revision (ICD-10), two disease descriptions can be found together: "idiopathic aseptic bone necrosis" and "avascular bone necrosis." The relevant literature on both the conditions abbreviates both as "aseptic ischemic osteonecrosis in the jawbone" (AIOJ). To shed light on the clinical details of this condition, osteolytic jawbone samples of 24 patients with different systemic immunological diseases were examined using four steps: presurgical dental X-ray, postsurgical histology, polymerase chain reaction DNA analysis (PCR DNA) of bacteria, and RANTES/CCL5 (R/C) expression. These four steps showed that neither X-ray nor histology delivered unambiguous results with respect to inflammatory processes; furthermore, the PCR results did not show evidence of any microbial load within the jaw samples. However, there is a striking, coherent overexpression of chemokine R/C in the AIOJ samples. This study proved the aseptic existence of "silent inflammation" within the jawbone. The ICD-10 (AIOJ) definition, which is hard to interpret, can now be substantiated with clinical evidence, while the cytokine expressions described in this report can explain the systemic immunological effects observed within the group of examined patients.
Keywords: jawbone, osteonecrosis, RANTES, CCL5, silent inflammation, aseptic inflammation, PCR, ICD-10
Background
Chronic diseases with unknown triggers have been on the rise for years. Simultaneously, while medical science has been focusing on immunology, experts in the field are starting to realize that chronic inflammatory processes cause a vast number of diseases without the involvement of acute bacterial inflammation. Underlying these phenomena is an immune system that is being constantly triggered by overexpressed cytokines. These triggers lead to the stimulation of different signaling pathways, which are instrumental in the development of chronic disease.1 At the same time, no other organ is exposed to such a large number of surgically invasive procedures than the teeth and jawbone area; for instance, tooth extractions during the mixed-dentition period, the surgical extraction of wisdom teeth, root canal filling on inflamed teeth, and dental implantations can all be accompanied by distorted wound healing. The persistent defects that result after healing, as well as insufficient bone regeneration as a form of “silent inflammation” in the dental area, are thus predestined.2 These issues occur without the typical signs that usually accompany inflammation, and they thus often remain undetected. One must ask if it is possible that these cryptic processes can lead to immunological maladaptation, thereby causing erroneous signals and disease development.
Study objectives
Code M87.0 of the International Statistical Classification of Diseases and Related Health Problems, tenth revision (ICD-10) presents the terms “idiopathic aseptic osteonecrosis” with the inclusion of “avascular osteonecrosis.”3 Code M87.0 includes cranial bones and the jawbone as well. The conceptual analysis of “idiopathic” depicts that a clear cause or infectious trigger is usually either not given or remains undetected. Within orthopedic practice and the relevant literature, those terms are often merged and referred to as “aseptic avascular osteonecrosis.” The term “avascular” is physiologically connected to a metabolic disorder accompanying ischemia and oxygen deficiency. The resulting decreased vascularization is a known possible cause of osteonecrosis. The guiding principles of the present analysis are to determine how the jawbone area is affected by bacterial ischemic and osteolytic processes. Furthermore, whether further chronic inflammatory phenomena are involved next to common acute inflammations in the jawbone such as chronic suppurative osteomyelitis, suppurative ostitits, or bisphosphonate-induced osteonecrosis must be clarified. The present study aimed to elucidate these matters by using aseptic and thereby sterile clinical material and a model of inflammation without bacterial involvement.
Materials and methods
Patient collective
The present study was executed as a retrospective case control study and was deemed to be retrospective in nature and waived approval by IMD-Berlin (forensic accredited Institute DIN EN 15189/DIN EN 17025). All patients gave their written informed consent. This investigation is patient centered, as samples and data originated directly from daily practice. A total of 24 patients were included in this study. All patients were diagnosed with or treated for systemic immunological diseases or chronic pain in the trigeminus area to exclude the involvement of fatty degenerative osteonecrosis in the jawbone (FDOJ).4 The patient population (n=24) was subdivided into four subgroups: atypical pain within the face and trigeminus area (n=4); neurodegenerative diseases (n=5, multiple sclerosis and amyotrophic lateral sclerosis); tumors (n=32, breast cancer and prostate cancer); rheumatism (n=11, fibromyalgia and Lyme disease); and chronic fatigue syndrome (CFS, n=5). Grounds for exclusion were acute osteonecrotic inflammatory reactions, the application of bisphosphonates due to their known impact on bone density, or a clinically proven diagnosis of osteoporosis. In addition to the presence of the aforementioned systemic diseases, participants were included in the study if they had a preoperative two-dimensional orthopantomogram (OPG), postoperative histology of FDOJ, or a polymerase chain reaction (PCR) pool sample of material taken intra-surgically from the FDOJ area, where the cytokine profile had additionally been examined with the help of multiplex analysis.
Morphology and diagnostic criteria of FDOJ
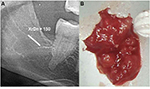
In the FDOJ area, the bone material is irregular with thinned and hollowed out cancellous bone and there is also a medullary cavity. FDOJ is merely a medullary process; the cortex is usually undamaged and can clearly be distinguished from the hollowed and softened cancellous bone. The softening of the FDOJ medulla is so pronounced that the bone marrow space can indeed be sucked or spooned out. Therefore, from a morphological perspective, FDOJ are fatty lumps that can be extracted easily and without excessive bleeding from the bone marrow space. Trabecular structures of the cancellous bone have vanished almost entirely. The medullary bone lesion that arises and is microscopically most similar to FDOJ is ischemic aseptic osteonecrosis in the jawbone (IAOJ).5,6 All the 24 FDOJ samples presented themselves clinically and macroscopically as fatty lumps. Figure 1A shows a sample of a medical preparation that features predominantly fatty alterations of the jawbone. Various types of FDOJ lesions are displayed using a contrast agent in Figure 2B. Several studies have shown that cases of FDOJ are similar to those lesions that occur in the long bones and they stand out primarily due to the presence of ischemia, accompanying bone marrow edemas, and chronic non-suppurative osteomyelitis.7 Light osteonecrosis does not tend to heal without surgical removal or curettage. FDOJ seems to mark the transition from an acute inflammation that arises following dental extraction to a chronically inflamed jawbone area that occurs instead of unimpeded wound healing.
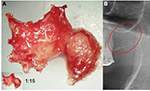  | Figure 1 (A) Morphology of fatty degenerative osteonecrosis in the jawbone. (B) Red circle indicates FDOJ area. Abbreviation: FDOJ, fatty degenerative osteonecrosis in the jawbone. |
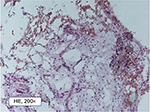  | Figure 2 Pathohistological structure of typical fatty degenerative osteonecrosis in the jawbone tissue (200 fold). |
Sample collection and isolation of necrotic tissue samples
In this study, 24 FDOJ patients underwent jaw surgery to one of the affected jaw areas. Following the administration of local anesthesia and the folding-down of one mucoperiosteal flap, the healthy cortical bone layer was removed to form a bone window. Within the bone marrow space, FDOJ was found in all patients. This form of osteonecrosis was similar to those samples described in the previous literature and is shown in Figure 1.8,9 In all cases, the procedure was executed in edentulous jawbone areas and adjacent retromolar fields. FDOJ samples with a volume of up to 0.5 cm3 (1/2 cc) were extracted and immediately stored in a dry, sterile, airtight, 2 mL bottle (Sarstedt AG & Co, Nümbrecht, Germany) as a pea-sized tissue lump, which was then frozen at –20°C until being further transported.
Histology of the extracted bone samples
Every FDOJ sample underwent histological examination at the Institute for Pathology & Cytology with Drs. Zwicknagel and Assmus (Freising, Germany). Figure 2 shows typical fatty marrow of FDOJ with mucoid degeneration and interstitial edema. Chronic degenerative changes are intermingled with foci of recent reactive adipocyte necrosis. The amount of fat cells is consistently and strikingly increased. Typical signs of inflammation, especially of an inflammatory cell response are missing. The fatty degenerative and osteolytic aspect overweighs due to insufficient metabolic supply. Marrow shows widened intertrabecular spaces that often contain small necrotic bone fragments, fatty microvesicles and pools of liquefied fat similar to oil cysts, with almost complete loss of adipocyte nuclei and residual fatty degenerated marrow and accumulation of acid mucopolysaccharides staining positively using alcianblue. Small nerve fibers are a striking feature in most biopsies of FDOJ, situated in close contact with degenerated and necrotic fatty tissue.
PCR DNA analysis of the extracted bone samples
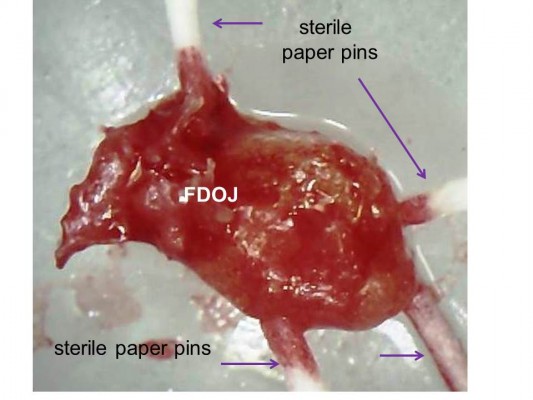
PCR is an artificial procedure that reproduces DNA. To detect possible bacterial colonization of the medullary osteolyses within the FDOJ area, we use a PCR procedure that is known from periodontology: obtaining the DNA proof of periodontopathogenic marker bacteria Aggregatibacter actinomycetemcomitans, Prevotella intermedia, Porphyromonas gingivalis, Treponema denticola, Tannerella forsythia, Campylobacter rectus/showae, Eikenella corrodens, Capnocytophaga gingivalis/ochracea, Parvimonas micra, Eubacterium nodatum, and Fusobacterium spp. Since only one single FDOJ area is usually affected, we extract the characteristic medullary fatty lumps – as described in the “Sample collection and isolation of necrotic tissue samples” section – and insert four sterile paper sticks side by side (Figure 3). Those sticks remained in the area for 20 seconds and were then pooled and dispatched to the laboratory in a designated container.
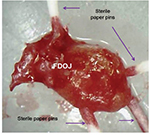  | Figure 3 Paper sticks serving as a pooled sample within a fatty degenerative osteonecrosis in the jawbone lump. Abbreviation: FDOJ, fatty degenerative osteonecrosis in the jawbone. |
Analysis of presurgical radiodensity of the FDOJ area in OPG
In normal dental practice, OPG is used to diagnose inflammation within the jawbone. To achieve an objective X-ray diagnosis of osteolytic FDOJ areas (X-ray typically being a visually performed method), we used OPG X-ray density (OPG-XrDn), which was executed using a KODAK 9000 Extraoral Imaging System X-ray unit in connection with the KODAK Dental Imaging Software 6.12.11.0; this method presented the results using a numerical quantity.10 In the present study, only OPG-XrDn results achieved with OPG units from the author’s practice are compared. At the jawbone areas that underwent surgery, an OPG-XrDn region of 1 cm was measured with a calibrated ruler. In Figure 4A, a normal retromolar jawbone area with an OPG-XrDn of 150 is shown, which corresponds with a normal jawbone in the XrDn.10 Conversely, Figure 4B shows an extracted FDOJ sample, which is compared in size with a ceramic ball mill (ɸ=0.6 mm).
Reprocessing and cytokine/chemokine analysis of extracted FDOJ bone samples
The parameter RANTES/CCL5 (R/C) in pg/mL was determined in the tissue homogenate supernatant on a Luminex® 200TM analysis unit with the help of xPonent® Software (Luminex, Austin, TX, USA) at the Institut für Medizinische Diagnostik Nicolaistr (Berlin, Germany).
Statistical methods
The quantitative data obtained from the FDOJ group and the control group were analyzed using descriptive statistics, which were calculated using IBM SPSS, version 19 (IBM Corporation, Armonk, NY, USA). The median, the arithmetic mean value, and data distribution were calculated. Differences between cohorts were computed with Student’s t-test or Spearman’s rho. The two-sided unpaired t-test was used to determine the differences within groups, whereas Spearman’s coefficient was used to analyze correlations among the examined cytokines. The significance level was set at P<0.05.
Results
Histology
Based on the findings of those 24 FDOJ samples, a pathohistological definition for FDOJ can be developed that includes five distinct characteristics: “Undersupply in the form of a chronic trophic dysfunction”; “necrotically changed adipocytes”; “routinely significantly increased changed fat cells”; “myoxide degeneration of fatty tissue”; and “inflammatory cells.” For the statistical analysis, we defined those five aforementioned terms. If an FDOJ sample contained all the five terms, it was rated as a “4”; if only three characteristics were evident, the sample received a score of “3”, and so forth. With respect to the histological results, none of these four characteristics would yield a score of “0”, which would indicate that a presurgical diagnosis of FDOJ is incorrect. In addition, the existence and intensity of inflammatory cells are taken into account. Histologically, three samples (12%) showed evidence of inflammatory processes, but in an inactive form, and they were thus not immediately inflammatory. There were no normal (healthy) samples, as each showed at least two of the characteristics described above. Fibrosis and ischemia – here described as “chronic trophic dysfunction” – were the most common results (Figure 5).
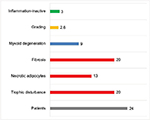  | Figure 5 Histological characteristics of the 24 FDOJ samples, of which only 12% showed “Inflammation-inactive cells.” Abbreviation: FDOJ, fatty degenerative osteonecrosis in the jawbone. |
PCR analysis of FDOJ in terms of bacterial DNA
Table 1 shows the results of the analysis of the 24 FDOJ samples. With the exception of samples 1 and 10, there was no evidence of bacterial colonization in any of the other samples. Table 2 shows the statistical results of periodontal pathogenic marker germs present in the samples.
Cytokine/chemokine analysis of FDOJ samples
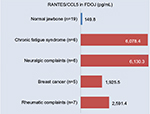
As we have shown in earlier studies, FDOJ areas are characterized by high levels of inflammatory messenger substance R/C, which is unlike normal jawbones.4 A multiplex analysis of 19 comparable samples extracted from normal jawbones yielded R/C levels of 149 pg/mL (SD ±127) for these three cytokines. A search of the relevant literature did not yield corresponding results for these mediators in normal jawbone.4 Remarkably high divergences within the FDOJ collective stemmed from the hyper-activated signal transduction of R/C in 24 FDOJ samples. Figure 6 shows this inflammatory overexpression of the median R/C level (4,184.4 pg/mL, SD ±3,825.7); when comparing the four disease groups with the 19 normal jawbone samples (149.9 pg/mL of R/C), the following results were obtained: CFS, 6,078.4 pg/mL of R/C (n=6); neuralgiform complications, 6,130.3 pg/mL of R/C (n=6); breast cancer, 1,925.5 pg/mL of R/C (n=5); and rheumatoid complications, 2,591.4 pg/mL of R/C (n=7).
XrDn analysis of FDOJ areas
The mean value of the presurgical OPG-XrDn within the FDOJ areas of the analyzed collective (n=24) was 140.6 (SD ±28.83).
Correlations between radiodensity and RANTES levels in FDOJ samples
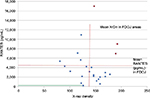
The present data do not suggest that any significant correlations were present between radiodensity and RANTES levels; however, by excluding those three samples that were marked red in the diagram, the calculations resulted in an inverse correlation between radiodensity and R/C expression (r=–0.55; P=0.010). Remarkably, the three marked samples displayed the largest individual numeric differences between high measures of radiodensity (which would indicate a normal jawbone) and exuberant R/C signaling (Figure 7).
Summary of results
Of the four parameters that we used for the diagnosis and clinically pathologic documentation of an intra-osseous inflammatory process, three (OPG, histology, and PCR DNA analysis) did not result in a positive reaction in the sense of a classic inflammatory reaction. Only ~30-times higher overexpression of R/C (pg/mL) in FDOJ samples was striking (Table 3).
Discussion
This discussion centers on four clinical measurements that are used to evaluate the presence of IAOJ.6
Discussion of histological results
The histological results indicate that minimal healing that was evident in FDOJ areas stemmed from local hypoxic, ischemic conditions in degenerative fatty structures. A critical balance between repairing and pro-inflammatory factors determines the clinical picture of incomplete wound healing in the jawbone following tooth or wisdom tooth surgeries, for instance. Incomplete wound healing is associated with four characteristic and reoccurring outcomes in FDOJ samples: 1) undersupply in the form of chronic trophic dysfunction. This serves as the basis for an ischemic metabolic state in FDOJ that – in consequence of a degenerative process – produces. 2) “Necrotically changed adipocytes” or “routinely significantly increased changed fat cells.” The intimate interaction between inflammatory cells and adipocytes is important, as it facilitates the secretion of inflammatory cytokines, which mediate the systemic effects associated with adipose tissue inflammation, which is described further in the “Discussion of PCR analysis of FDOJ’s bacterial DNA” section.11 3) As sign of malfunction within the FDOJ area’s micro-metabolism, a “characteristic myoxide degeneration of fat tissue” occurs, which is the histological equivalent of osseous structure disintegration in the FDOJ area. Finally, 4) only a few of the present inflammatory processes are inactive. Massively acutely produced inflammatory cells were not found in any of the 24 samples.
Radiodensity within the FDOJ area in OPG
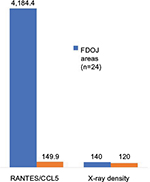
There is a need to discuss the extent to which the diagnostic instrument used most frequently by dentists – the two-dimensional panoramic X-ray – is suitable for the detection of aseptic ischemic osteonecrosis in the jawbone (AIOJ)/FDOJ. Any radiological tests are performed using the tester’s optic visual evaluation, which is a nonscientific, nonobjective procedure. The dangers associated with subjective misinterpretation are high. In radiological diagnostics, FDOJ areas seem to pose significant problems for the dentist, which is why these conditions often remain unrecognized, in terms of both etiology and pathogenesis, as they take on the form of a “silent inflammation.”10 Considerably, high limitations in the assessment of cancellous bone have to be taken into account.12,13 Despite the recent progress that has been made in digital X-ray techniques and in improved medical imaging, problems regarding the display of cancellous bone remain.14 Since FDOJ must not be confused with classic osteomyelitis and given that FDOJ has a largely unknown etiology, the literature has described problems with an “invisible” osteomyelitis in terms of its radiological appearance.15 This form can be so subtle that a radiologist’s ability to detect it is close to impossible, unless the specialist has significant experience in diagnostics.16 In the present study, the mean value of presurgical OPG-XrDn in the FDOJ areas of the analyzed sample (n=24) was 140.6 (SD ±28.83) with an average R/C expression of 4,184.4 pg/mL. In comparison, a normal jawbone expresses 120 KODAK units of RöDi and has an R/C expression of 149.9 pg/mL10 (Figure 8). It can thus be concluded that optically evaluated OPG-XrDn does not indicate the presence of AIOJ/FDOJ.
Discussion of PCR analysis of bacterial DNA of FDOJ
Since none of the analyzed samples demonstrated evidence of the bacterial colonization associated with periodontal pathogenic marker germs in the PCR pool samples (with the exception of two samples in which we suspected remnants were present in the extraction), then the notion that bacterial involvement as a microbial trigger occurs in FDOJ can be rejected. Apart from that, what else could serve as the inflammation carrier? The answer can be found in the “Discussion of the cytokine/chemokine analysis in FDOJ” section. We will discuss that signaling pathways are instrumental in the development of chronic disease.1
Discussion of the cytokine/chemokine analysis in FDOJ
The messenger substances of immune system and healthy cancellous bone (n=19)
Of the 19 participants included in this study, implant drills from normal jawbone were extracted. In a laboratory setting, those extractions were – as described in “Reprocessing and cytokine/chemokine analysis of extracted FDOJ bone samples” – tested for seven immunomediators. The mean values were as follows: fibroblast growth factor-2, 27.6 pg/mL; interleukin (IL)-1 ra, 196.5 pg/mL; IL-6, 101.0 pg/mL; IL-8, 7.5 pg/mL; MCP-1, 20.3 pg/mL; tumor necrosis factor (TNF)-a, 11.5 pg/mL; and R/C, 149.9 pg/mL.4
Hyperactivated R/C in osteolytic cancellous bone
Since histology, PCR analysis, and OPG were unable to detect pathologic osseous processes, the most prominent contradiction that emerged stemmed from the sample population’s thirtyfold increase in the mean hyperactivated inflammatory R/C signaling within osteolytic areas. This constitutes the single positive parameter of a local inflammatory reaction. R/C is part of the chemotactic cytokine family (known as CC chemokines), which is reflected in the more modern description of CCL5. An early response gene synthesizes R/C; R/C is chemotactic for eosinophil granulocytes and basophilic cells and plays a key role in the recruiting process of leucocytes, which are at the center of inflammation. The importance of R/C with respect to disease occurrence seems to be enormous: R/C impairs the immunological reaction at many levels and therefore plays a key role in pathological pain. R/C’s chemotactic characteristics send T-cells, dendritic cells, eosinophil granulocytes, natural killer cells, mast cells, and basophilic cells to the center of the inflammation.17 In addition, R/C is a powerful leukocyte activator that is associated with many inflammatory complaints.18 The tests performed on 24 FDOJ samples in this study unambiguously identified those areas as jawbones with a significant inflammatory burden, as these samples showed R/C values that were 30 times greater than those of normal jawbones (Figure 6).
Systemic impact of chemokine R/C
Why is local R/C expression characteristic of AIOJ/FDOJ, and why is it of particular importance in the development of systemic immunological diseases? While acute inflammation during the wound-healing process constitutes an adaptive reaction via acute cytokines TNF-a and IL-6 with the aim of overcoming a disease, R/C overexpression takes place outside the normal reaction’s framework and thus leads to a chronically maladaptive response.19 Cytokine imbalances in the jawbone result in internal signal transmission due to the additional paths that stem from excess R/C. In the end, these paths may result in chronic pathologies such as cancer, diabetes, and cardiovascular diseases, as well as neurodegenerative and inflammatory processes – dependent on many other different implications. If a chronic disease is already present, the progressive deterioration that arises from undetected “silent inflammation” in the jawbone leads to a number of pathologic complications that have a negative impact on the overall condition of the jawbone and induce a vicious cycle of ongoing aggravation.20 Furthermore, R/C is a powerful leukocyte activator that is of importance in another field of inflammatory malfunctions.17 It affects several levels of the immune response and is thus substantially involved in infections or pathologic conditions. Most of the time, the defective regulation of R/C expression results in a self-reinforcing effect that can result in critical states in the human body, especially those that are in connection with diseases that affect the central nervous system, such as multiple sclerosis and Hodgkin’s disease.21–23 Immunohistological tests of various tissues showed that R/C is expressed only in small amounts in normal adult tissue. However, the proportion of R/C-positive cells increases dramatically, if inflammatory reactions occur.18 In rheumatoid arthritis, the expression of R/C plays a vital role.24 These findings underline that any of the immunologic systemic diseases that occurred in this sample might be linked to locally overexpressed R/C signaling.
The problem associated with radiological diagnoses of FDOJ lesions
In the “Radiodensity within the FDOJ area in OPG” section, we demonstrated that FDOJ areas are invisible on X-ray, rendering a precise traditional OPG-based diagnosis impossible. It follows that the existence of an FDOJ area and its meaning for the human organism are neglected in mainstream medicine and dentistry. The only limited possibilities associated with making a radiological diagnosis in connection with osseous edemas in FDOJ lesions necessitate bone density measurement by means of trans-alveolar ultrasound (TAU).25 TAU very accurately and identifiably displays cavitational porosity in the jawbone and is thus more suitable for an FDOJ diagnosis than OPG. Due to these diagnostic inadequacies, FDOJ disorders often remain unrecognized by dentists.
Summary
Here, we describe the overexpression of R/C in the jawbone with known diagnostic tools, including presurgical X-ray, intra-surgical histology, and PCR DNA analysis of bacterial colonization. Despite the negative test results obtained with OPG, the histological inflammatory parameters (no inflammatory cells traceable) and PCR parameters identified that the overexpression of R/C explains the possible systemic impact of “silent inflammation” in the IAOJ/FDOJ area. This led us to provide an overview of four inflammatory types in the jawbone, which were further subdivided based on X-ray visibility, bacterial involvement, pain, the associated R/C chemokine profile, and their systemic impact (Table 4).
Conclusion
Using currently available methods, the present research shows that overactivated signal transduction cascades, especially of chemokine R/C, in osteolytic changes of the jawbone may be associated with the impacts of R/C on complex chronic diseases. The source of this inflammatory signaling is what we refer to as “fatty degenerative osteonecrosis in the jawbone” (FDOJ) due to its morphology, particularly given the presence of medullary cavities. Physiologically, FDOJ resembles asymptomatic AIOJ. By examining five primary factors, we can derive the following clinical conclusions:
- Due to the absence of bacteria in an FDOJ/AIOJ area, a therapeutic prescription of antibiotics is futile.
- OPG is not a sufficient method to exclude FDOJ/AIOJ during presurgical analysis.
- For postsurgical analysis, a focus on histologically absent inflammatory cells is not sufficient; the classic “textbook” parameters of inflammation need to be expanded to include inflammatory mediators in the case of the jawbone, especially with respect to R/C.
- The extension, which involves the chemokine expression of R/C in the intra-osseous definition of inflammation proposed here, may broaden a currently static perspective in dentistry and it may further add integrative dimensions.
- To achieve the successful implementation of these perspectives, dentistry needs an analytic tool that is sufficient enough to display FDOJ/AIOJ and that is a complement to diagnostic radiology. Using clinical experience and research from the relevant literature, TAU is best for quantitatively determining bone density.26
As far as we are aware, this study is the first to clinically define “idiopathic avascular bone necrosis”, which is described in ICD-10 Code M87.0, as a carrier of so-called silent inflammation. Desirably, these subchronic inflammatory phenomena will receive more attention in mainstream medicine and dentistry, and it will be placed in context with systemic immunological diseases. Further studies are needed to fully understand the aseptic mechanisms in the jawbone that underlie chronic inflammation in systemic immune diseases following R/C overexpression in FDOJ.
Acknowledgment
English-language editing of this manuscript was provided by Journal Prep.
Disclosure
The authors report no conflicts of interest in this work.
References
Townsend MJ, McKenzie AN. Unravelling the net? Cytokines and diseases. J Cell Sci. 2000;10(113):3549–3550. | ||
Lechner J, von Baehr V. Chemokine RANTES/CCL5 as an unknown link between wound healing in the jawbone and systemic disease: is prediction and tailored treatments in the horizon? EPMA J. 2015;6:10. | ||
DIMDI, Deutsches Institut für Medizinische Dokumentation und Information. [10th revision of the International Statistical Classification of Diseases and Related Health Problems]. Waisenhausgasse 36-38a 50676 Köln, 2017. | ||
Lechner J, von Baehr V. RANTES and fibroblast growth factor 2 in jawbone cavitations: triggers for systemic disease? Int J Gen Med. 2013;6:277–290. | ||
Ono K. Symposium: recent advances in avascular osteonecrosis. Clin Orthop. 1992;277:2. | ||
Mankin HJ. Nontraumatic necrosis of bone (osteonecrosis). N Engl J Med. 1992;326:1473. | ||
Ratner EJ, Langer B, Evins ML. Alveolar cavitational osteopathosis: manifestations of an infectious process and its implication in the causation of chronic pain. J Periodontol. 1986;57:593. | ||
Graff-Radford SB, Simmons M, Fox L, et al. Are bony cavities exclusively associated with atypical facial pain and trigeminal neuralgia? Proceedings of Annual Meeting, Western USA Pain Society, Santa Fe, New Mexico; May 1988. | ||
Bouquot JE, Roberts AM, Person P, et al. NICO (neuralgia-inducing cavitational osteonecrosis): osteomyelitis in 224 jawbone samples from patients with facial neuralgias. Oral Surg Oral Med Oral Pathol. 1992;73:307. | ||
Lechner, J. Validation of dental X-ray by cytokine RANTES – comparison of X-ray findings with cytokine overexpression in jawbone. Clin Cosmet Investig Dent. 2014;6:71–79. | ||
Stulnig T. Adipositas und die Entzündung des Fettgewebes. [Obesity and the inflammation of adipose tissue]. J Klin Endokrinol Stoffw. 2009;2(3):17–21. German. | ||
Schwartz SF, Foster JK. Roentgenographic interpretation of experimentally produced bony lesions. I. Oral Surg. 1971;32:606. | ||
Ardran CM. Bone destruction not demonstrable by radiograph. Br J Radiol. 1951;24:107. | ||
Patel S, Dawood A, Mannocci F, Wilson R, Pitt Ford T. Detection of periapical bone defects in human jaws using cone beam computed tomography and intraoral radiography. Int Endod J. 2009;42:507–515. | ||
Segall RO, Del Rio CE. Cavitational bone defect: a diagnostic challenge. J Endod. 1991;17:396. | ||
Bouquot JE, Roberts A. OJ (neuralgia-inducing cavitational osteonecrosis): radiographic appearance of the “invisible” osteomyelitis. Oral Surg Oral Med Oral Pathol. 1992;74:600. | ||
Levy JA. The unexpected pleiotropic activities of RANTES. J Immunol. 1999;182(7):3945–3946. | ||
von Luettichau I, Nelson PJ, Pattison JM, et al. RANTES chemokine expression in diseased and normal human tissues. Cytokine. 1996;8:89–98. | ||
Stearns SC. The Evolution of Life Histories. Oxford: Oxford University Press; 1992. | ||
Tilg H, Moschen RA. Adipocytokines: mediators linking adipose tissue, inflammation and immunity. Nat Rev Immunol. 2006;6(10):772–783. | ||
Appay V, Rowland-Jones SL. RANTES: a versatile and controversial chemokine. Trends Immunol. 2001;22:83–87. | ||
Bolin LM, Murray R, Lukacs NW, et al. Primary sensory neurons migrate in response to the chemokine RANTES. J Neuroimmunol. 1998;81(1–2):49–57. | ||
Fischer M, Juremalm M, Olsson N, et al. Expression of CCL5/RANTES by Hodgkin and Reed-Sternberg cells and its possible role in the recruitment of mast cells into lymphomatous tissue. Int J Cancer. 2003; 107(2):197–201. | ||
Rathanaswami P, Hachicha M, Sadick M, Schall TJ, McColl SR. Expression of the cytokine RANTES in human rheumatoid synovial fibroblasts. Differential regulation of RANTES genes by inflammatory cytokines. J Biol Chem.1993;268(8):5834–5839. | ||
Bouquot JE, Margolis M, Shankland W, Imbeau J. Through-transmission alveolar ultrasonography (TAU): new technology for evaluation of medullary diseases. Correlation with histopathology of 285 scanned jaw sites. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;\94:210. | ||
Al-Nawas B, Klein MO, Götz H, et al. Dental implantation: ultrasound transmission velocity to evaluate critical bone quality – an animal model. Ultraschall Med. 2008;29(3):302–307. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.