Back to Journals » Journal of Asthma and Allergy » Volume 15
Treatment of Allergic Rhinitis and Asthma in Primary Care: Dispensations Do Not Align with Prescriptions
Authors Belhassen M , Bérard M, Devouassoux G, Dalon F, Bousquet J, Van Ganse E
Received 31 May 2022
Accepted for publication 20 November 2022
Published 25 November 2022 Volume 2022:15 Pages 1721—1729
DOI https://doi.org/10.2147/JAA.S376786
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Amrita Dosanjh
Manon Belhassen,1 Marjorie Bérard,1 Gilles Devouassoux,2 Faustine Dalon,1 Jean Bousquet,3,4 Eric Van Ganse1,2,5
1Pelyon, Pharmacoepidemiology Lyon, Lyon, France; 2Respiratory Medicine, Croix Rousse Hospital, Lyon, France; 3MACVIA-France, Fondation Partenariale FMC VIA-LR, Montpellier, France; 4INSERM U 1168, VIMA, Ageing and Chronic Diseases Epidemiological and Public Health Approaches, Villejuif, France; 5Research on Health Care Performance RESHAPE, INSERM U1290, University Claude Bernard Lyon 1, Lyon, France
Correspondence: Eric Van Ganse, Respiratory Medicine, Croix Rousse Hospital, Lyon, France, Tel +33 472 071 730, Email [email protected]
Background: Appropriate use of effective treatments is required for satisfactory control of allergic symptoms. Coherent medical care -regular prescribing by the same Health Care Professionals- is a preliminary need.
Objective: We investigated the numbers of distinct prescribers, the regularity of medical visits, and the agreement between prescriptions and associated dispensations in individual patients with perennial allergic rhinitis (PAR) and asthma.
Methods: In primary care electronic health records (EHRs), a cohort of patients with PAR and asthma was identified. Individual EHRs were linked to corresponding claims recording all dispensations. Prescribing patterns were analyzed for the major treatment classes, and the dispensations linked to individual prescriptions were retrieved to compute the proportions of days covered (PDCs) for asthma and PAR therapy.
Results: A total of 3654 patients were included, with 62% being female (mean age, 46.1 years). At inclusion, asthma control was not optimal in 51% of the patients and 48% had received oral corticosteroids. The mean interval between successive prescriptions varied between 93 (leukotriene receptor antagonists, LTRAs) and 103 (inhaled corticosteroids, ICS) days, and 97 (antihistamines, AHs) and 103 days (nasal corticosteroids, NCS). On average, individual prescriptions lead to 1.2, 1.5, 1.7 and 1.8 dispensations of ICS, ICS/Long-Acting Beta-Agonist (LABA) fixed-dose combinations, LABAs, and LTRAs, respectively, and to 1.3 and 1.6 dispensations of NCS and AHs, respectively. PDCs then varied between 37.8% for ICS and 58.6% for LTRAs, and between 39.7% for NCS and 50.4% for AHs. Care was nonetheless coherent, with > 90% of all dispensations related to prescriptions issued by single General Practitioners (GPs).
Conclusion: Despite regular healthcare visits and medication prescriptions, allergic patients only partly and selectively refilled their treatments, preferring the less effective therapy, in a context of poor control of asthma symptoms.
Keywords: asthma, rhinitis, care, control, therapy, adherence, misuse
Introduction
Allergic rhinitis (AR) is a common chronic condition, with a prevalence of up to 40% in some countries.1 Asthma is also a common condition, affecting 5–16% of people worldwide.1 In parallel, it has been shown that 19–38% of patients with AR have concomitant asthma, while 30–80% of asthmatic patients have AR.2
Untreated asthma and rhinitis may seriously impact patients’ quality of life, but the appropriate use of effective therapy alleviates symptoms for most patients.2,3 Population surveys, however, concur to document the overall poor levels of disease control among rhinitis and asthmatic patients.4,5 The irregular use of therapy is often identified as the reason for the poor overall control of symptoms.6 It may originate from health care providers who do not regularly prescribe or from patients who do not regularly fill their prescriptions at community pharmacies.
In France, as a rule, individual prescriptions are issued for multiple refills, ie, patients may visit community pharmacies several times with the same prescription to obtain a new treatment supply—usually, for patients with chronic conditions, a one-month supply every month for 3 months. However, patients may skip refills, for instance, when their symptoms temporarily improve, when they experience adverse events or low effectiveness, or when they fear dependence on therapy.
The French SNDS (Système National des Données de Santé), ie, the national claims data system, has recorded the dispensations of prescribed and reimbursed therapies to the national population since 2007, with a linkage between individual medical prescriptions and their associated refills obtained from community pharmacies.7
This analysis reports the behaviours of prescribers and patients for the use of specific therapy in a subgroup of a previously described cohort of patients suffering from allergic rhinitis and asthma, where we assessed the burden of allergic conditions in routine care.8 The present analysis was performed on the subset of the initial cohort that suffered from both perennial allergic rhinitis (PAR) and asthma, to investigate the regularity of successive prescriptions written by general practitioners (GPs) affiliated with Disease Analyzer (DA), a network of electronic health records (EHRs), and the regularity of treatment acquisitions by patients for the major therapeutic classes of PAR and asthma. As patients could have visited several GPs, we also studied the origins of the prescriptions, the usual GP (DA) vs other GPs (non-DA).
Methods
Study Design and Data Sources
Using the IMS Lifelink Disease Analyzer (DA) panel, a dataset of individual EHRs, we identified a cohort of patients with PAR and concomitant asthma between 2005 and 2010. Each patient’s EHR was linked to the corresponding claims record with exhaustive medical resource utilization (MRU) between 2011 and 2013. The probabilistic linkage between the two anonymized datasets (DA and the SNDS) was based on the year of birth, sex, dates of visits to GPs (>75% of visits needed to coincide), and GP identifier.8
DA is a French database of longitudinal EHRs of approximately 5 million patients collected from a panel of 1200 GPs since 2000. DA records no information on care provided by specialists; its validity and representativeness have been analyzed and published previously.4 Diagnoses made by GPs were recorded using the International Classification of Diseases 10th version (ICD-10 codes).
The Système National des Données de Santé [SNDS, National System of Health Data] is a real-world dataset of French MRU. It contains comprehensive, anonymous, and individual information on sociodemographic characteristics, the date of death, out-of-hospital reimbursed health care expenditures, and hospital discharge summaries with International Classification of Diseases (ICD)-10 codes.5 In addition, the SNDS contains information on medical diagnoses for patients who receive full reimbursement for MRU associated with the care of specific chronic conditions (“chronic disease”, CD status), and indicates whether patients benefit from free-access-to-care status (CMU-C), an index of socioeconomic deprivation. CD and CMU-C status allow patients to collect refills free-of-charge.
Patient Selection, Criteria and Selection Period
The study population consisted of patients aged 18 to 65 years in 2010 with PAR and asthma identified between 2005 and 2010 in DA.
PAR was identified in DA using GP visits with J30.2, J30.3, or J30.4 ICD-10 diagnosis codes between 2005 and 2010 or from any prescription of antihistamines (R06A ATC code) or nasal corticosteroids (R01AD ATC code) at two adjacent six-month periods in 2010.
Asthma was initially identified in the DA dataset using GP visits with J45.0, J45.8, J45.9, or J46 ICD-10 diagnosis codes between 2005 and 2010 or when a GP prescribed at least one asthma controller or one asthma reliever in 2010.
Using information from DA, patients were excluded if a diagnosis of cystic fibrosis (E84 ICD-10 code) or chronic obstructive pulmonary disease (COPD) (J41, J42, J44, J96.1, or J96.0 ICD-10 codes) was recorded in 2010. Patients were also excluded when a prescription of omalizumab (R03DX05 ATC code) was recorded between 2008 and 2010.
Using information from the SNDS, patients who died between 2010 and 2013 were excluded, as were patients with hospitalization or CD status for cystic fibrosis or COPD between 2010 and 2013, patients without any medical resource use from 2011 to 2013, and patients with any prescription for omalizumab between 2010 and 2013 (the care of these patients was supervised by specialists and is not recorded in DA).
Study Periods
Two study periods were distinguished, a baseline period to assess the demographic and medical characteristics of the patients and an exposure period to assess the actual use of the therapy:
- Baseline period, GP prescriptions issued between July 2011 and June 2012 and the associated dispensations by community pharmacies were analyzed between July 2011 and December 2012 to allow enough time for treatment acquisitions.
- Exposure period, GP prescriptions issued between July 2012 and June 2013 and the associated dispensations by community pharmacies were analyzed between July 2012 and December 2013 to assess the actual use of therapy during a 12-month period of primary care management of PAR and asthma using proportions of days covered (PDCs) as the summary metrics.
Outcome Variables
The variables assessed during the baseline period included sociodemographic characteristics (age, sex, CD status, CMU-C) and medical characteristics (ie, the level of asthma control estimated from therapeutic ratios and the use of SABAs and specific therapy).
- Level of asthma control (from the SNDS): Patients with a therapeutic ratio >0.5 in 2010 and 0, 1, or 2 dispensings of SABAs in 2010 (baseline period) were classified as having “well-controlled asthma”; other patients were considered to have “not well-controlled asthma”.8–11 As an additional marker of poor control (rhinitis and asthma), the use of oral corticosteroids (OCS) was assessed (% of users and the numbers of refills by users) during the baseline period.
- DA-GP prescriptions: All prescriptions of asthma controller therapy (ICS, Fixed-Dose Combination (FDCs), Long-Acting Beta Agonist (LABAs), and Leukotriene Receptor Antagonist (LTRAs)) and PAR therapy (NCS and AHs) issued to the patients and recorded in the SNDS during the exposure period were considered. The time intervals between successive prescriptions were described for patients receiving 2 or more prescriptions during the exposure period.
- Fills and refills: All SNDS-recorded dispensations of asthma and PAR therapy acquired by individual patients and associated with prescriptions provided by a DA-GP during the exposure period were considered. The time intervals between successive refills associated with exposure-period prescriptions were described.
- Proportions of days covered (PDCs) for asthma and PAR therapy: PDCs were computed for the period between July 2012 and June 2013 (with 95% confidence intervals), between the first and the last prescriptions recorded within this period. The PDCs were calculated using the numbers of refills associated with individual prescriptions and the intervals between successive prescriptions.12 Any dispensing of asthma controller therapy (ICS, FDCs, LABAs, LTRAs) or PAR therapy was considered to cover 30 days of use, regardless of the dosage. Then, we standardized PDCs, taking FDCs and nasal corticosteroids as references.
- Medical care for asthma and PAR: The percentages of dispensations associated with prescriptions provided by “non-DA-GPs” were assessed, separately for each treatment class.
Data Analysis
All analyses were performed using SAS software, version 9.3 (SAS Institute Inc, Cary, NC, USA).
Quantitative variables were described using descriptive statistics (means and standard deviations), and qualitative variables were described using counts and percentages.
Regulatory Procedures
The study was conducted with anonymized data after the French data protection committee (commission nationale de l’informatique et des libertés, CNIL) issued an authorization to use SNDS data for this project (Approval no. °1763241). This study was performed after approval was received from the French Institute for Health Data (Institut des données de santé, Approval no. °87, September 9, 2014).
Results
Patient Selection
After the application of successive selection criteria, a total of 3654 patients suffering from asthma and perennial allergic rhinitis (PAR) were included (Figure 1).
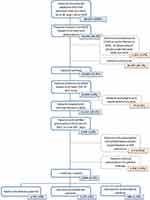 |
Figure 1 Patients’ flow chart. |
Patients’ Sociodemographic and Medical Characteristics
The mean age was 48.1 years (±12.3), and 62% of the patients were female. Altogether, 6.2% of the patients had CD status for asthma, and 8.5% benefited from CMU-C. In this population, 15.5% of the patients had spirometry performed during the baseline period. Asthma was well controlled in 49.0% of the patients, while it was not well controlled or very poorly controlled in 51.0% of the patients. Almost half of the patients (48.3%) were dispensed OCS in the baseline period, with a mean dispensing of 2.5 packs over 12 months, while 16.1% of patients had one or more ER visits or unscheduled contacts with their GP (Table 1).
 |
Table 1 Patients’ Characteristics at Baseline (N =3654) |
Time Intervals Between Successive Prescriptions
In the 2814 patients with at least two prescriptions of asthma or rhinitis treatments, the mean time intervals were similar for all classes of asthma therapy, ranging from 93 days for LTRAs to 103 days for LABAs, while for rhinitis therapy, the mean time intervals were 97 days for AHs and 100 days for NCS (Table 2).
 |
Table 2 Intervals Between Successive DA-GP Prescriptions, in Days (in Patients with 2 or More Prescriptions for Asthma or Rhinitis Treatments, n=2814) |
Number of Dispensations Associated with Successive Prescriptions
During the 12-month exposure period, for asthma therapy, the mean number of dispensations associated with a specific prescription were 1.2 for ICS, 1.5 for FDCs, and 1.8 for LTRAs. For rhinitis, the mean number of dispensations was 1.3 for NCS and 1.6 for antihistamines (Table 3).
 |
Table 3 Numbers of Refills Associated with Individual Prescriptions Issued by DA-GPs |
PDCs for Asthma Controller and Rhinitis Therapy
In the 2814 patients with at least two prescriptions within the 12-month exposure period, the PDCs for asthma treatments varied from 37.8% for ICS to 44.9%, 50.3% and 58.6% for ICS/LABA FDCs, LABAs and LTRAs, respectively. The PDCs for rhinitis treatments were 39.7% for NCS and 50.4% for AHs (Table 4). Taking ICS/LABAS FDCs as a reference, standardized estimates were 1.12, 0.84 and 1.31 for LABAs, ICS and LTRAs, respectively.
 |
Table 4 Proportion of Days Covered (PDC, in %) by Asthma and Rhinitis Treatments (in Patients with 2 or More Prescriptions, n=2814) |
Origin of GPs’ Prescriptions, DA-GPs vs Non-DA-GPs
Altogether, 14.8% of the patients obtained one or more prescriptions for asthma therapy from a non-DA-GP; this percentage varied from 1.3% of the prescriptions of LABAs to 3.4% of prescriptions of ICS, 3.6% of the prescriptions of LTRAs, 7.6% of prescriptions of ICS/LABA FDCs, and 8.4% of the prescriptions of SABAs (data not shown). For rhinitis therapy, 9.3% of all prescriptions originated from non-DA-GPs (9.0% for AHs and 9.8% for NCS) (data not shown).
Discussion
In our population of allergic patients suffering from inappropriately controlled asthma and rhinitis, general practitioners’ prescriptions for both conditions were regular and comparable, with approximately 3 months between successive prescriptions, both for asthma and rhinitis therapy. In contrast, the dispensing rates associated with individual prescriptions were low and varied to a large extent with treatment classes, suggesting selective patient acquisition and limited compliance with GP prescriptions, with a marked preference for less effective treatments. As a result, the patients were undertreated for their allergic conditions, as evidenced by the coverage rates of 0.38 and 0.45 for ICS and ICS/LABA FDCs, respectively, and 0.50 and 0.40 for AHs and NCS, respectively. Asthma and rhinitis care were nonetheless coherent, with >90% of all dispensations related to individual patients’ prescriptions issued by the same GPs.
PAR and asthma were identified from GP records with specific diagnoses, from repeated use of AHs or NCS (PAR), or from the use of asthma therapy in 2010. During the one-year pre-study period, 16% of the patients had spirometry performed, suggesting similar rates of contact with respiratory physicians. In France, as a rule, GPs do not perform spirometry while they care for a large majority of asthma cases.6 In parallel, 16% of the patients had an unscheduled visit to the GP or an emergency room visit, and 6.2% of the patients benefited from a CD status for asthma. Altogether, when compared to recent data, these figures suggest that our population likely included all levels of asthma severity.6
Our findings illustrate that patients visited their GPs regularly, while they irregularly acquired the prescribed therapy. This is in line with other studies, such as the Bosnic-Anticevich study that investigated the use of ICS/LABA FDCs in an Australian population suffering from both asthma and rhinitis and showed that only 41% of the patients used their therapy daily.7 Irregular use was also observed for other treatment classes, as illustrated by Yegit et al’s13 recent study on the use of subcutaneous immunotherapy that showed irregular use in a third of their cohort. Of note, the PDCs were computed on the assumption that each refill covered a 30-day treatment period, which is the rule for most commercial units in France for the treatment of asthma and rhinitis.
This irregular use had an impact on the symptoms experienced by the patients, as evidenced by the high percentage of inappropriately controlled asthma (49%) and the common use (48%) of oral corticosteroids in the baseline period, with the understanding that this treatment can be used for both asthma and PAR. In parallel to asthma, PAR is a condition requiring regular therapy, and the level of control of PAR was likely inappropriate, notwithstanding that this outcome was not directly assessed in our study.
The reasons for the irregular use of therapy were not investigated in our study, but prior studies may provide some explanations, such as the perceived ability to self-care14 or beliefs about inhaled controllers. For instance, a study of 200 asthmatic patients showed that one-third of the patients intentionally interrupted ICS therapy when they felt better, a quarter forgot to use the ICS and a fifth changed the doses.14 Another study highlighted the impact of socioeconomic status (SES) on asthma care, with patients of high/medium SES consulting their GP more regularly than patients of lower SES, with also fewer hospital contacts.15
Of interest, our study identified large differences among treatment classes for the level of coverage. In agreement with prior investigations, ICS in monotherapy had the lowest rates of coverage beyond ICS/LABA FDCs.16 In contrast, LABAs and LTRAs had better coverage rates, despite more limited efficacy or lower safety in asthma patients.17 The difference between LABAs and ICS coverage rates has been identified previously, representing a risky situation, with some patients using LABAs as monotherapy, without the simultaneous use of ICS.16,18 Of importance, this prescribing pattern has now virtually disappeared.19
For their rhinitis care, the patients made more regular use of AHs than of NCS; this could be due to the more convenient use of oral therapy, as already observed.20 It may however have impacted the control of symptoms, in view of the higher efficacy of nasal CS in rhinitis patients.21
Some limitations must be acknowledged, such as the lack of information on the actual use of acquired therapy, that could further decrease PDCs if the patients did not use the treatments at hand, our estimates should be considered conservative, as we have no information about the handling of inhalers by patients, for instance.22 The level of asthma control was considered to be inappropriate in more than 50% of the patients and there was a common use of oral corticosteroids, suggesting that the reality could be worse for patients with the most severe forms of asthma.23 Information on asthma control and the use of oral corticosteroids were obtained during the baseline period, but available data suggest that patients’ behaviours and levels of asthma control, the latter being assessed from available algorithms,9–11 remain largely unchanged over time.24 Last, the assumption was made that antihistamines (AHs) were used to treat rhinitis, but AHs are also used for urticaria that could be a comorbidity in our study.
Our findings illustrate the interest of linking prescribing and dispensing data to disentangle the role of HCPs and patients in the irregular use of therapy, claims data are closer to actual use than prescribing data. Additionally, drug use data could be linked to surveys investigating prescribers’ perceptions of the effectiveness of care and the reasons for patients’ behaviours. This information would identify points of action for future interventions to reduce the heavy burden of allergic conditions.25 Our study points to patients’ behaviours as the primary driver of poor disease control, but GPs are however well positioned to improve patients’ understanding of their conditions and to promote the need for regular therapy.
In conclusion, our data showed that despite regular medical prescriptions, mostly issued by the same medical practice, patients suffering from perennial allergic rhinitis and asthma irregularly acquired the prescribed therapy, with a preference for less effective treatments, in a context of uncontrolled symptoms and significant disease burden. A primary condition for reducing this burden would imply that HCPs be informed of the actual use of treatments by their patients, commencing with feedback on the refill rates of all prescriptions. In parallel, patients should be informed of the need to regularly use effective therapy for their allergic conditions to prevent flare-ups of symptoms, self-care training is a priority for patients suffering from allergic conditions.26
Abbreviations
AHs, antihistamines; AR, allergic rhinitis; COPD, chronic obstructive pulmonary disease; DA, disease analyzer; EHR, electronic health record; FDC, fixed-dose combination; GP, general practitioner; HCP, health care professional; ICD, International Classification of Diseases; ICS, inhaled corticosteroids; LABA, long acting beta agonist; LTRA, leukotriene receptor antagonist; MRU, medical resource utilization; NCS, nasal corticosteroids; OCS, oral corticosteroids; PAR, perennial allergic rhinitis; PDC, proportion of days covered; SABA, short acting beta agonist; SNDS, Système National des Données de Santé (National Health Data System).
Acknowledgments
We thank the French National Health Service (Caisse Nationale de l’Assurance Maladie) and the National Institute of Health Data (Institut national des Données de Santé) for providing the data.
Author Contributions
All authors made a significant contribution to the work reported, be it in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by PELyon (Pharmacoepidemiology Lyon).
Disclosure
MBel, FD and MBer are full-time employees of PELyon. EVG is the Scientific advisor of PELyon. GD reports grants and personal fees for consultancy from Novartis Pharma, Boehringer Ingelheim, Chiesi, AstraZeneca, GlaxoSmithKline, ALK, TEVA, MundiPharma, Vivisol, Sanofi, ALK and Menarini, personal fees for meeting participation from AGIR adom, ALK, Astra-Zeneca, Boehringer Ingelheim, Chiesi, GSK, Meda, MSD, Novartis Pharma, Orkyn, Takeda, TEVA, Sanofi, CIPLA. JB reports personal fees from Chiesi, Cipla, Hikma, Menarini, Mundipharma, Mylan, Novartis, Purina, Sanofi‐Aventis, Takeda, Teva, Uriach. Shareholder of Kyomed Innov and MASK-air-SAS. The authors report no other conflicts of interest in his work.
References
1. Martinez FD, Vercelli D. Asthma. Lancet. 2013;382(9901):1360–1372. doi:10.1016/S0140-6736(13)61536-6
2. Global Initiative for Asthma. Global strategy for asthma management and prevention; 2021. Available from: www.ginasthma.org.
3. Brozek JL, Bousquet J, Agache I, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) guidelines-2016 revision. J Allergy Clin Immunol. 2017;140(4):950–958. doi:10.1016/j.jaci.2017.03.050
4. Becher H, Kostev K, Schroder-Bernhardi D. Validity and representativeness of the “Disease Analyzer” patient database for use in pharmacoepidemiological and pharmacoeconomic studies. Int J Clin Pharmacol Ther. 2009;47(10):617–626. doi:10.5414/CPP47617
5. Tuppin P, Rudant J, Constantinou P, et al. Value of a national administrative database to guide public decisions, From the systeme national d’information interregimes de l’Assurance Maladie (SNIIRAM) to the systeme national des donnees de sante (SNDS) in France. Rev Epidemiol Sante Publique. 2017;65(Suppl 4):S149–S67. doi:10.1016/j.respe.2017.05.004
6. Belhassen M, Nolin M, Nibber A, Ginoux M, Devouassoux G, Van Ganse E. Changes in persistent asthma care and outcomes from 2006 to 2016 in France. J Allergy Clin Immunol Pract. 2019;7(6):1858–1867. doi:10.1016/j.jaip.2019.02.025
7. Bosnic-Anticevich S, Kritikos V, Carter V, et al. Lack of asthma and rhinitis control in general practitioner-managed patients prescribed fixed-dose combination therapy in Australia. J Asthma. 2018;55(6):684–694. doi:10.1080/02770903.2017.1353611
8. Belhassen M, Demoly P, Bloch-Morot E, et al. Costs of perennial allergic rhinitis and allergic asthma increase with severity and poor disease control. Allergy. 2017;72(6):948–958. doi:10.1111/all.13098
9. Laforest L, Licaj I, Devouassoux G, et al. Prescribed therapy for asthma, therapeutic ratios and outcomes. BMC Fam Pract. 2015;16:49. doi:10.1186/s12875-015-0265-2
10. Laforest L, Licaj I, Devouassoux G, Chatte G, Martin J, Van Ganse E. Asthma drug ratios and exacerbations, claims data from universal health coverage systems. Eur Respir J. 2014;43(5):1378–1386. doi:10.1183/09031936.00100113
11. Stempel DA, McLaughin TP, Stanford RH, Fuhlbrigge AL. Patterns of asthma control, a 3-year analysis of patient claims. J Allergy Clin Immunol. 2005;115(5):935–939. doi:10.1016/j.jaci.2005.01.054
12. Hansen RA, Kim MM, Song L, Tu W, Wu J, Murray MD. Comparison of methods to assess medication adherence and classify nonadherence. Ann Pharmacother. 2009;43(3):413–422. doi:10.1345/aph.1L496
13. Yegit OO, Demir S, Unal D, et al. Adherence to subcutaneous immunotherapy with aeroallergens in real-life practice during the COVID-19 pandemic. Allergy. 2021;77(1):197–206.
14. Laforest L, El Hasnaoui A, Pribil C, et al. Asthma patients’ self-reported behaviours toward inhaled corticosteroids. Respir Med. 2009;103(9):1366–1375. doi:10.1016/j.rmed.2009.03.010
15. Mazalovic K, Jacoud F, Dima AL, et al. Asthma exacerbations and socio-economic status in French adults with persistent asthma: A prospective cohort study. J Asthma. 2018;55(10):1043–1051.
16. Belhassen M, Langlois C, Laforest L, et al. Level of Asthma Controller Therapy Before Admission to the Hospital. J Allergy Clin Immunol Pract. 2016;4(5):877–883. doi:10.1016/j.jaip.2016.06.012
17. Reddel HK, Bacharier LB, Bateman ED, et al. Global Initiative For Asthma (GINA) strategy 2021 - executive summary and rationale for key changes. Am J Respir Crit Care Med. 2021;27(1):14–35.
18. Van Ganse E, Texier N, Dima AL, et al. Effects of short- and long-acting beta-agonists on asthma exacerbations, a prospective cohort. Ann Allergy Asthma Immunol. 2020;124(3):254–260. doi:10.1016/j.anai.2019.12.012
19. Belhassen M, Nibber A, Van Ganse E, et al. Inappropriate asthma therapy-a tale of two countries, a parallel population-based cohort study. NPJ Prim Care Respir Med. 2016;26:16076. doi:10.1038/npjpcrm.2016.76
20. Kelloway JS, Wyatt RA, Adlis SA. Comparison of patients’ compliance with prescribed oral and inhaled asthma medications. Arch Intern Med. 1994;154(12):1349–1352. doi:10.1001/archinte.1994.00420120066007
21. Juel-Berg N, Darling P, Bolvig J, et al. Intranasal corticosteroids compared with oral antihistamines in allergic rhinitis, A systematic review and meta-analysis. Am J Rhinol Allergy. 2017;31(1):19–28. doi:10.2500/ajra.2016.30.4397
22. Dahlen E, Ekberg S, Lundholm C, et al. Sibship and dispensing patterns of asthma medication in young children-a population-based study. Pharmacoepidemiol Drug Saf. 2019;28(8):1109–1116. doi:10.1002/pds.4802
23. Van Ganse E, Laforest L, Pietri G, et al. Persistent asthma, disease control, resource utilisation and direct costs. Eur Respir J. 2002;20(2):260–267. doi:10.1183/09031936.02.02542001
24. Baptist AP, Hao W, Song PX, Carpenter L, Steinberg J, Cardozo LJ. A behavioral intervention can decrease asthma exacerbations in older adults. Ann Allergy Asthma Immunol. 2020;124(3):248–53 e3. doi:10.1016/j.anai.2019.12.015
25. de Bruin M, Dima AL, Texier N, van Ganse E. Explaining the amount and consistency of medical care and self-management support in asthma, a survey of primary care providers in France and the United Kingdom. J Allergy Clin Immunol Pract. 2018;6(6):1916–25 e7. doi:10.1016/j.jaip.2018.04.039
26. Hodkinson A, Bower P, Grigoroglou C, et al. Self-management interventions to reduce healthcare use and improve quality of life among patients with asthma, systematic review and network meta-analysis. BMJ. 2020;370:m2521. doi:10.1136/bmj.m2521
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
