Back to Journals » Medical Devices: Evidence and Research » Volume 11
Preclinical quantification of air leaks in a physiologic lung model: effects of ventilation modality and staple design
Authors Eckert CE, Harris JL, Wong JB , Thompson S , Kassis ES, Tsuboi M , Ott HC, Force S
Received 28 August 2018
Accepted for publication 15 November 2018
Published 14 December 2018 Volume 2018:11 Pages 433—442
DOI https://doi.org/10.2147/MDER.S184851
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Chad E Eckert,1 Jason L Harris,1 Jordan B Wong,1 Suzanne Thompson,2 Edmund S Kassis,3 Masahiro Tsuboi,4 Harald C Ott,5 Seth Force6
1Ethicon Inc., Research and Development, Cincinnati, OH 45242, USA; 2Ethicon Inc., Preclinical Center of Excellence, Cincinnati, OH 45242, USA; 3Ethicon Inc., Medical Affairs, Cincinnati, OH 45242, USA; 4Division of Thoracic Surgery and Oncology, National Cancer Center Hospital East, Kashiwanoha, Kashiwa, Chiba 277-8577, Japan; 5Department of Surgery, Division of Thoracic Surgery, Massachusetts General Hospital, Boston, MA 02114, USA; 6Department of Surgery, Division of Cardiothoracic Surgery, Emory University Hospital, Atlanta, GA 30322, USA
Purpose: Thoracic air leaks are a common complication following pulmonary resections. Limitations in clinical studies and preclinical models have hindered efforts to understand the pathophysiology of air leaks. With an emphasis on staple-line specific air leaks, we hypothesize that ventilation modality – intraoperative positive pressure vs postoperative negative pressure – and stapler design may play a role in air leaks.
Methods: Using a novel physiologic lung model, air leaks associated with graduated and uniform staple designs were evaluated under positive and negative pressure ventilation, simulating perioperative breathing in porcine lungs. Air leak incidence, air leak volume, and air leak rate were captured along with ventilation pressure and tidal volume.
Results: In all cases, negative pressure ventilation was associated with a higher occurrence of leaks when compared to positive pressure ventilation. Lungs leaked more air and at a faster rate under negative pressure ventilation compared to positive pressure ventilation. Graduated staple designs were associated with higher occurrence of leaks as well as larger leak rates when compared to uniform staples. Tissue thickness was not associated with differences in air leaks when tested with appropriate staple heights.
Conclusion: Using a novel lung model to investigate the pathophysiology of air leaks, we have identified breathing modality and staple design as two important variables that may impact air leaks. This work will help guide device design and drive future studies in human tissue, and it may help inform clinical practice to ultimately improve patient outcomes.
Keywords: prolonged air leak, lung physiology, preclinical model, surgical stapler, ventilation mechanics
Introduction
Air leaks are relatively common after thoracic procedures and range in duration and severity. Acute air leaks have a reported incidence of 28%–60%,1 while leaks that persist past five postoperative days are classified as prolonged air leaks (PALs) and have a reported incidence of 8%–26%.2,3 While several studies have associated PALs with increased length of stay, hospital costs, and patient morbidity and mortality,2–5 recent studies have also shown a similar impact of acute air leaks.1,4,6 Despite the investigation of numerous possible predictive factors of air leaks, the prognostic nature of these factors has been limited.7–10 Likewise, intraoperative to postoperative correlations of air leaks have proven unsuccessful.11 This is compounded by the very low rate of reoperation for PALs and the difficulty in tracking leaks perioperatively2,11; as a result, a need exists to improve the understanding of air leak pathophysiology, specifically regarding leak origination sites, such as from areas of trauma from manipulation or dissection during the procedure or from staple lines created during the resection, and the impact of devices and surgical techniques on leak etiology and their perioperative evolution.
Ventilation modality is one possible contributor to the manifestation and progression of leaks that warrants further investigation. Suggestive of pressure-mediated effects on air leak etiology, clinical studies comparing postoperative chest tube management in lung volume reduction surgeries have reported that larger suction pressures were associated with increased air leak duration and may have created new leaks12,13; similar outcomes have been observed in pulmonary resection procedures.2 Although intriguing, these studies were not able to precisely control ventilation parameters, did not explore perioperative ventilation conditions, and lacked the ability to visualize, isolate, and track leak sites, such as staple lines, from intraoperative through postoperative conditions. Thus, an important question on the impact of perioperative ventilation remains: how are air leaks influenced by ventilator-controlled breathing (ie, positive pressure mechanical ventilation) and natural breathing (ie, negative pressure in the pleural space)? Further, the role of surgical stapling devices – instruments ubiquitous in pulmonary resections – and design differences around staple-line configuration have not been investigated through perioperative ventilation conditions. Few studies have sought to isolate the role of stapler design in air leak outcomes, but a recent publication by Imhoff and Monnet suggests that differences in staple-line configurations such as uniform staple heights (UNI) vs graduated staple heights (GRD) may influence air leaks, but the study was limited to positive pressure benchtop testing.14
The limitations of available thoracic models have been a significant impediment to pursuing this area of research. In vivo models do not permit direct observation of air leaks or the isolation of leak sites under negative pressure (closed chest) conditions. Though new digital chest tube devices enable the quantification of air leaks, the collection and quantification of site-specific air leaks are not possible in such models.15–17 Further, ventilation parameters under negative pressure are difficult to monitor or control. Ex vivo models can address some of the concerns, but current models rely solely on positive pressure ventilation (PPV) or require small animal lungs not suitable for surgical device studies. To address these limitations, a novel physiologic lung model was developed that enables direct visualization, isolation, collection, and quantification of site-specific air leaks while accommodating a range of intraoperative and postoperative breathing modalities.18 An initial study with pinhole pleural defects in porcine lungs showed a substantial increase in leak rate upon transition from positive to negative pressure ventilation (NPV), but how staple-line-related air leaks behave under such conditions remains in question.18 In this work, we sought to evaluate the impact of breathing modality and stapler design on staple-line-specific air leaks using a physiologic lung model.
Methods
Model overview
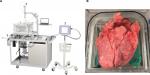
A previously described ex vivo porcine lung model was used to investigate differences in air leak occurrence and air leak rates between two different breathing modalities: PPV meant to mimic intraoperative ventilation and NPV meant to mimic postoperative/natural breathing conditions.18 In the context of this model, NPV refers to the transpulmonary pressure approximating what occurs under natural breathing generated by subatmospheric intrapleural pressure, not negative pressure ventilators (eg, “iron lungs”). The system consists of a saline-filled polycarbonate chamber with a domed lid, shown in Figure 1(A). PPV is achieved by cannulating the trachea and connecting it to a ventilator (Respironics, Murrysville, PA, USA). With the ventilator, PPV can be achieved via a pressure-control mode. Motion of a computer-controlled piston results in NPV, which simulates physiologic breathing. With the control algorithm of the piston, NPV is achieved via a volume-control mode. By measuring tidal volume during PPV, the piston volume for NPV can be specified to replicate the tidal volume. This results in similar tidal volumes between ventilation modalities. Pressures in the chamber and trachea are measured using commercial transducers (PendoTech, Princeton, NJ, USA). A real-time leak quantification system is used to assess leak volumes and leak rates during both ventilation phases, shown in Figure 1(A).
Study groups
Pulmonary wedge resections (eg, nonanatomical resections) were performed on excised porcine lungs using GRD and UNI staplers. To better enable comparison of outcomes and to mimic perioperative ventilation, each set of stapled lungs was exposed to PPV (simulating intraoperative conditions) followed by NPV (simulating postoperative conditions). The PPV-to-NPV testing workflow was conducted to mimic what occurs clinically: PPV during surgery as the patient is under anesthesia and NPV following surgery as the patient recovers. Stapler reloads commonly used in lung wedge resections were utilized; as a result, locations on the left cranial lobe of the lungs were selected based on tissue thickness. GRD staple lines were created with Endo GIATM Ultra Universal Staplers with 45 mm reloads utilizing Tri-StapleTM Technology (Medtronic, Dublin, Ireland), and UNI staple lines were created with the Echelon FlexTM GST System with 45 mm reloads (Ethicon Endo-Surgery Inc., Cincinnati, OH, USA). In thinner tissue (approximately 2.5–4.0 mm thick when compressed at 8 g/mm2), EGIA45AMT (GRD) and GST45B (UNI) reloads were used, while in thicker tissue (approximately 4.0–6.5 mm thick when compressed at 8 g/mm2), EGIA45AXT (GRD) and GST45G (UNI) reloads were used. Stapler firing techniques and reload selection were based on manufacturer recommendations as found on their associated websites, and all staplers were fired per their Instructions for Use.19,20 Previous pilot studies were performed using both reload groups from GRD and UNI staplers, which revealed nonparametric distributions of the leak rate data. This guided power analyses leading to estimated sample sizes of 25–30 specimens per group. Each set of lungs was resected only one time, and full nonanatomical wedge resections were completed with the same stapler type and reload size. Only staple-line leaks were evaluated; therefore, if a non-staple line leak was present or if a non-staple line leak manifested during testing, the set of lungs were excluded from the study because leak quantification would be affected by additional leak sites.
Tissue preparation and testing
Heart–lung blocs from 110 to 120 kg pigs were harvested, flushed with heparinized saline (Midwest Research Swine, Gibbon, MN, USA), and shipped overnight on wet ice. Upon delivery, tissue was slowly warmed in a water bath prior to positive pressure recruitment. A trachea cannula was attached proximal to the cranial lobe airway bifurcation, shown in Figure 1(B), and the lungs were gently inflated under 20–25 cm H2O until 1000 mL of tidal volume was reached. The target tidal volume was based on published ranges of 5–10 mL/breath/kg21,22 and was simplified to a fixed value (1000 mL) for all lung sets to simplify the protocol; under pressure-controlled PPV, tidal volumes were dynamic and thus changed on a per-breath basis but stayed within the published ranges. Following recruitment, lungs were removed from the ventilator and deflated. A location on the left cranial lobe was chosen, and thickness was measured while under a uniform tissue compression of 8 g/mm2. The lobe was transected with subsequent staple lines. Lungs were placed in the saline-filled chamber maintained at approximately 37°C and recruited to 1000 mL tidal volume. The chamber was closed and filled with saline, and then excess air was purged from the system. Lungs were ventilated using positive pressure at 5 breaths a minute, 5 cm H2O positive end-expiratory pressure, and 40 cm H2O max inspiratory pressure. The leak collection system was activated, and lungs were evaluated for 10 minutes under PPV. During the last 2 minutes of testing, the tidal volume was recorded and averaged so that the same tidal volume under PPV could be prescribed for the given set of lungs under NPV. Maintaining the same tidal volume enabled the study of pressure modality without potentially confounding leak rate with different volumes of air being delivered (changes in tidal volume).
After PPV, the ventilator was changed to continuous positive airway pressure mode at 5 cm H2O, and the chamber piston was activated at 200 mL displacement with inhale/exhale durations of 6 seconds. Displacement was increased in 200 mL increments to the recorded average tidal volume, after which the leak collection system was activated, and lungs were evaluated for 10 minutes under NPV. After testing, ventilation was stopped, lungs were removed from the chamber, and staple lines were excised from the tissue. Individual staples were measured, and their formation quality graded on a five-point scale ranging from a perfectly formed “B” to a completely unformed staple.
Statistical analysis
Statistics were performed using Minitab 17 (Minitab Inc., State College, PA, USA). For comparisons of proportions, two-sample proportion tests were used. Pilot work revealed the highly nonparametric nature of the leak volume data; therefore, to compare negative and positive pressure groups, a one-sample sign test was used on the difference in leak volumes (negative leak volume – positive leak volume) for those specimens that exhibited leaks during at least one breathing phase. Mann–Whitney tests were used to compare leak volume and leak rate between GRD and UNI groups. In the case of the UNI group on thinner tissue with no PPV leaks, a one-sample sign test was used to compare leak volumes of the comparable GRD group to 0. For tissue thickness, trachea pressure, and chamber pressure, ANOVA tests were performed. Tukey post hoc comparison tests were executed if warranted. Differences were considered statistically significant if P ≤0.05.
Results
A total of 110 lung sets were tested to completion (55 per stapler type) across 2 thickness groups. A statistical difference in the tissue thickness between thick and thin groups was observed; however, no differences in tissue thickness were observed between staple groups at each thickness (Table 1). Tidal volume (Table 1), trachea pressure (Table 2), and chamber pressure (Table 3) were not statistically different between staple groups at either thickness. An additional 44 lung sets were tested but were excluded from analysis because they displayed ancillary leaks (non-staple line leaks) that may have interfered with staple-line leak collection. Most often, the ancillary leaks occurred in the cranial lobes due to abrasion during harvesting and flushing of the vasculature.
Air leak incidence rate
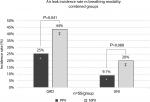
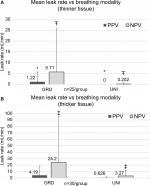
The overall incidence rate of staple-line leaks – those lungs that exhibited a staple-line air leak during testing – was higher under NPV than PPV (Figure 2; both thickness group combined). In both UNI and GRD groups, a larger air leak incidence rate was observed under NPV than PPV, although statistically significant differences were only observed in the GRD group.
In both thickness groups, leaks were observed more under NPV than PPV, shown in Figure 3(A–B), though results were not statistically significant. Separated by stapler type, the UNI groups had significantly fewer leaks than the GRD groups under NPV. Under PPV, the UNI group had significantly fewer leaks than the GRD group in thinner tissue. While a trend was observed toward the same result in thicker tissue, the results were not statistically significant. Qualitatively, leaks were observed under both PPV and NPV originating from either end of the resection line where the tissue was thinnest. These leaks appeared around individual staple legs from the staple row furthest from the cut line (outer row of the stapler and medial to the lungs).
Air leak volume and rate
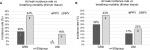
In lung sets that exhibited staple-line leaks, NPV exhibited an increase in air leak rate magnitude compared to PPV. Figure 4(A–B) shows boxplots of air leak rates for both tissue thickness groups. Data are paired, and only lungs that exhibited a leak in either breathing phase were included. In both tissue groups, the median air leak rate was significantly larger in the NPV group when compared to the PPV group.
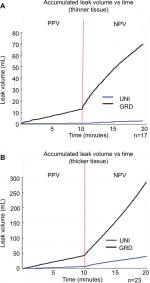
NPV was associated with an increased volume of air leaks in lungs that exhibited a staple-line leak during either/both breathing modalities. Figure 5(A–B) presents the accumulated volume of leaked air for both thickness groups. Leak volumes for the GRD and UNI groups are only shown for specimens that displayed leaks. A dotted line distinguishes the ten-minute durations for each breathing phase. A sharp increase in leak volume was evident at the transition point between breathing phases.
Figure 6(A–B) shows leak rate data for all specimens in both thickness tissue groups. Higher leak rates were observed under NPV when compared to PPV in those lungs that leaked. On thinner tissue, the UNI configurations had statistically smaller leak rates than the GRD configurations under both breathing phases (Tables 2 and 3). A similar statistical difference was observed under NPV between UNI and GRD configurations on thicker tissue (Tables 2 and 3).
Staple-line analysis
Each staple line was analyzed to assess the quality of the staple formation along the length of the staple line. Staple lines were divided into two groups: isolated staple lines and overlapping staple lines. For each tissue thickness, no measurable differences were observed between the GRD and UNI groups when comparing the quality of the staple form in either the isolated group or the overlapping group for each reload (Table 4).
Discussion
Model overview
Air leaks have a significant impact on clinical outcomes in patients following thoracic surgical procedures,1–6,11 but limitations in current preclinical models and clinical studies hinder the understanding of the impact of breathing modality and stapler design on these leaks.6 Our study utilizes a previously described model capable of isolating and quantifying air leaks under simulated perioperative ventilation modalities to evaluate site-specific air leaks in excised lungs.18 To the best of our knowledge, this is the first known effort toward understanding the impact of ventilation modality on air leaks attributed specifically to staple lines.
Effects of ventilation modality on air leaks
We observed that ventilation modality had an impact on the incidence and magnitude of staple-line air leaks: negative pressure increased the number and rate of staple-line leaks. The ability to directly observe staple-line air leaks while tracking and quantifying these leaks through intraoperative and postoperative simulated breathing phases made this observation possible and is a unique aspect of our study. Other studies support these findings on negative pressure. Intraoperatively, Brunelli et al found leak rates of 37.5%–44%, but after repairing all leaks before closing, postop leaks were still observed from 1 to 9 days postoperatively23; leaks not previously observed under positive pressure manifested under negative pressure. In comparing postoperative chest tube management strategies, Cooper et al avoided postoperative chest tube suction, as they hypothesized that the typical –20 cm H2O wall pressure may prolong or generate new air leaks based on their observations.12,13 Singhal et al expanded on this by highlighting four randomized prospective studies comparing water seal and either –10 or –20 cm H2O wall suction in which improved leak sealing, reduced leak durations, and decreased leak incidence rates were observed in the group having the least suction (water seal).2 Although these studies did not directly compare ventilation modalities, they support our finding that negative pressure can impact air leaks.
We further observed that, with the same tidal volume being delivered in both ventilation modes for a given set of lungs, the magnitude of air pressure required to expand the lungs was larger under positive pressure than negative pressure (trachea pressure for PPV; chamber pressure for NPV). We suspect two plausible explanations for this observation. First, airway resistance could be larger under PPV, which would affect the alveolar pressures and ultimately the transalveolar pressure driving lung expansion. Pushing air into the lungs in this manner may reduce air that reaches the peripheral portions of the lobes due to resistance in the airways and the propensity of increased nondependent lung ventilation.24,25 Under positive pressure, air may preferentially flow into the larger airways/medial lobes and alveoli. Such effects have been described when full mechanical support has been applied to paralyzed patients, showing a decrease in functional residual capacity and an increase in dead-space-ventilation-to-tidal-volume ratios.26 Likewise, Synder et al showed an increase in dead space ventilation under mechanical support (PPV).27
Second, the effect of negative pressure acting on the outside of the lung may have a different impact than positive pressure acting on the inside of the lung (bronchioles/alveoli). As the forces from negative pressure would be applied across all areas of the lung, including the periphery, regions that under positive pressure would get poor ventilation may see increased ventilation as the alveoli are being pulled open from the pleural surface. Regardless of the causative mechanism, negative pressure appears to more efficiently expand the lungs and aid in the removal of local atelectasis, leading to lungs that inflate more fully, at least in the upper lobe regions and peripherally near the staple lines in our model. In a study investigating deep breathing protocols following cardiac surgery, Westerdahl et al described a reduction in atelectasis with deep breathing, suggesting that increased negative pressure from deep breathing impacts lung inflation.28 A more uniform and complete inflation of the lung may lead to increased stretching in the lungs. More tension may be introduced on the staple line, through the increased inflation of the lung from its collapsed state and this stretching may result in local tissue deformation or damage around each staple leg leading to new air leaks or exacerbating existing leaks.
Effects of stapler design on air leaks
We observed a difference in both the occurrence and magnitude of staple-line air leaks between staple lines with GRDs vs UNI despite no measurable differences in tidal volume, pressures, or the quality of staple formation. Because most leaks were observed to originate in the thinner tissue regions, we hypothesize that insufficient compression of the lung parenchyma at the row of staples closest to the patient is a potential cause of leaks. In a similar experiment under positive pressure, Imhoff and Monnet measured leak pressure in excised canine lungs and found that UNI staples exhibited higher leak pressures than GRD staples14 (thus GRD staple lines leaked at lower pressures). Similarly, they observed leaks in the thinner regions of the tissue and hypothesized that taller outer row staples did not sufficiently compress the tissue to prevent leaks. In contrast, the UNI staples likely resulted in a tighter outer row than equivalent reloads on GRD devices. Because the staple heights in the interior rows of a GRD device are tighter than the outer row, selecting reloads with shorter outer row staples may result in overcompression of tissue within the interior rows. Properly pairing the formed height of the outer row staple to the thinnest tissue within the staple line while ensuring tissue is not overcompressed by interior row staples may improve air leak outcomes.
Limitations
With our ex vivo model, we sought to address many of the limitations observed in benchtop testing, animal models, and clinical studies, namely the inability to prescribe perioperative ventilation conditions while observing, collecting, and quantifying air leaks from specific locations (such as staple lines). To achieve our goal and begin exploring the etiology of air leaks, we made design decisions to better control testing variables such as the exclusion of a chest wall, lack of tissue coagulation or healing, and use of nondiseased porcine tissue. In our model, a chest wall was omitted to better visualize the staple line and enable the collection of every leak, because apposition provides an unpredictable source of sealing. Similarly, healing and coagulation provide an unpredictable source of leak sealing, and we desired to control for as many variables as possible to better test our hypotheses around ventilation modality and stapler type. We hypothesize that disease state will influence the absolute occurrence of leaks, but not the mechanism of leaks from both a ventilation and stapler design perspective. We recognize that the data presented may be conservative, but we sought to limit unpredictable influences on leak origination and progression in this first study. Future studies will be conducted using cadaveric and transplant-rejected tissue to evaluate the effects of disease state, testing duration, and perfusion on air leaks.
In addition to isolating differences between ventilation modalities, we sought to investigate differences between GRD and UNI designs. Unlike Imhoff and Monnet’s work, which compared GRD and UNI designs utilizing staplers with the same firing and compression mechanisms,14 our study compared GRD and UNI designs that were deployed with fundamentally different staplers. These two manufacturers of staplers describe different mechanisms by which tissue is prepared for stapling by compression. It is possible that differences in stapler design and how forces are exerted on tissue during stapling may contribute to the outcomes observed in this study
Conclusion
Air leaks remain a troublesome complication following thoracic surgical procedures. In this study, we sought to visualize and quantify staple-line-related leaks by utilizing an improved perioperative ventilation model. Similar to our previous efforts on pleural defects, we observed a difference in air leak incidence and air leak rate between the two ventilation modalities. Surprisingly, we observed a difference in GRD staples compared to UNI staples, notably under NPV. Clinically, our observations do not suggest extended ventilator usage (longer positive pressure) or delayed NPV (extubation and natural breathing) but propose that lungs and any associated staple lines behave differently under intraoperative vs postoperative ventilation. This may necessitate different design considerations and more rigorous testing for future staplers. We intend to explore these observations further in diseased human tissue.
Acknowledgment
The authors would like to thank Thomas Jones, III for his assistance in documentation and lab preparation.
Disclosure
This work was supported by Ethicon, Inc. Staplers and reloads were purchased by Ethicon. Dr. Suzanne Thompson is employed by Johnson & Johnson. Dr. Chad E Eckert, Dr. Jason L Harris, Jordan B Wong, and Dr. Edmund S Kassis are employed by Ethicon, Inc. Dr. Seth Force, Dr. Masahiro Tsuboi, and Dr. Harald C Ott were not compensated for their contributions to this work. The authors report no other conflicts of interest in this work.
References
Yoo A, Ghosh SK, Danker W, Kassis E, Kalsekar I. Burden of air leak complications in thoracic surgery estimated using a national hospital billing database. Clinicoecon Outcomes Res. 2017;9:373–383. | ||
Singhal S, Ferraris VA, Bridges CR, et al. Management of alveolar air leaks after pulmonary resection. Ann Thorac Surg. 2010;89(4):1327–1335. | ||
Lackey A, Mitchell JD. The cost of air leak: physicians’ and patients’ perspectives. Thorac Surg Clin. 2010;20(3):407–411. | ||
Brunelli A, Xiume F, Al Refai M, Salati M, Marasco R, Sabbatini A. Air leaks after lobectomy increase the risk of empyema but not of cardiopulmonary complications: a case-matched analysis. Chest. 2006;130(4):1150–1156. | ||
Varela G, Jiménez MF, Novoa N, Aranda JL. Estimating hospital costs attributable to prolonged air leak in pulmonary lobectomy. Eur J Cardiothorac Surg. 2005;27(2):329–333. | ||
Okereke I, Murthy SC, Alster JM, Blackstone EH, Rice TW. Characterization and importance of air leak after lobectomy. Ann Thorac Surg. 2005;79(4):1167–1173. | ||
Abolhoda A, Liu D, Brooks A, Burt M. Prolonged air leak following radical upper lobectomy: an analysis of incidence and possible risk factors. Chest. 1998;113(6):1507–1510. | ||
Cerfolio RJ, Bass C, Katholi CR. Prospective randomized trial compares suction versus water seal for air leaks. Ann Thorac Surg. 2001;71(5):1613–1617. | ||
Elsayed H, Mcshane J, Shackcloth M. Air leaks following pulmonary resection for lung cancer: is it a patient or surgeon related problem? Ann R Coll Surg Engl. 2012;94(6):422–427. | ||
Okada S, Shimada J, Kato D, Tsunezuka H, Inoue M. Prolonged air leak following lobectomy can be predicted in lung cancer patients. Surg Today. 2017;47(8):973–979. | ||
Wain JC, Kaiser LR, Johnstone DW, et al. Trial of a novel synthetic sealant in preventing air leaks after lung resection. Ann Thorac Surg. 2001;71(5):1623–1629. | ||
Cooper JD, Patterson GA. Lung-volume reduction surgery for severe emphysema. Chest Surg Clini N Am. 1995;5(4):815–831. | ||
Cooper JD, Patterson GA, Sundaresan RS, et al. Results of 150 consecutive bilateral lung volume reduction procedures in patients with severe emphysema. J Thorac Cardiovasc Surg. 1996;112(5):1319–1330. | ||
Imhoff DJ, Monnet E. Inflation pressures for ex vivo lung biopsies after application of graduated compression staples. Vet Surg. 2016;45(1):79–82. | ||
Mori R, Yamazaki K, Shoji F, et al. Assessment of pleural air leakage using digital chest drainage system after surgical pulmonary resection: comparison of visible alveolar air leakage with the digital value measured by a digital chest drainage system. PLoS One. 2017;12(11):e0187705–0187709. | ||
Shoji F, Takamori S, Akamine T, et al. Clinical evaluation and outcomes of digital chest drainage after lung resection. Ann Thorac Cardiovasc Surg. 2016;22(6):354–358. | ||
Cerfolio RJ, Bryant AS. The benefits of continuous and digital air leak assessment after elective pulmonary resection: a prospective study. Ann Thorac Surg. 2008;86(2):396–401. | ||
Klassen C, Eckert CE, Wong J, et al. Ex vivo modeling of perioperative air leaks in porcine lungs. IEEE Trans Biomed Eng. 2018;65(12):2827–2836 | ||
Covidien Endo GIATM. Ultra Universal Stapler [package insert]. Mansfield, MA: Covidien LLC; 2011. | ||
Ethicon Echelon FlexTM. Powered Plus Stapler [package insert]. Cincinnati, OH: Ethicon Endo-Surgery, Inc.; 2016. | ||
Hannon JP, Bossone CA, Wade CE. Normal Physiological Values for Conscious Pigs Used in Biomedical Research [Report]. San Francisco, CA: Letterman Army Institute of Research; 1989: 1–19. | ||
Research Animal Resources and Compliance [homepage on the Internet]. Wisconsin: Research Animal Resources and Compliance, University of Wisconsin-Madison; c1996-2018 [updated 2018 Mar 19; cited 2018 Nov 12]. Available from: www.rarc.wisc.edu/animal_health/normative_data.html. Accessed November 12, 2018. | ||
Brunelli A, Cassivi SD, Salati M, et al. Digital measurements of air leak flow and intrapleural pressures in the immediate postoperative period predict risk of prolonged air leak after pulmonary lobectomy. Eur J Cardiothorac Surg. 2011;39(4):584–588. | ||
Frerichs I, Hahn G, Golisch W, Kurpitz M, Burchardi H, Hellige G. Monitoring perioperative changes in distribution of pulmonary ventilation by functional electrical impedance tomography. Acta Anaesthesiol Scand. 1998;42(6):721–726. | ||
Rehder K, Sessler AD, Rodarte JR. Regional intrapulmonary gas distribution in awake and anesthetized-paralyzed man. J Appl Physiol Respir Environ Exerc Physiol. 1977;42(3):391–402. | ||
Norwood S. Physiologic principles of conventional mechanical ventilation. In: Kirby RR, Banner MJ, Downs JB, editors. Clinical Applications of Ventilatory Support. New York: Churchill Livingstone; 1990:145–172. | ||
Snyder JV, Carroll GC, Schuster DP, Culpepper J, Klain M. Mechanical ventilation: physiology and application. Curr Probl Surg. 1984;21(3):4–87. | ||
Westerdahl E, Lindmark B, Eriksson T, Hedenstierna G, Tenling A. The immediate effects of deep breathing exercises on atelectasis and oxygenation after cardiac surgery. Scand Cardiovasc J. 2003;37(6):363–367. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.