Back to Journals » ClinicoEconomics and Outcomes Research » Volume 10
Does knowledge of patient non-compliance change prescribing behavior in the real world? A claims-based analysis of patients with serious mental illness
Authors Shafrin J, Bognar K, Everson K, Brauer M, Lakdawalla DN, Forma FM
Received 31 May 2018
Accepted for publication 16 August 2018
Published 2 October 2018 Volume 2018:10 Pages 573—585
DOI https://doi.org/10.2147/CEOR.S175877
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Dean Smith
Jason Shafrin,1 Katalin Bognar,1 Katie Everson,1 Michelle Brauer,2 Darius N Lakdawalla,3 Felicia M Forma4
1Policy and Economics, Precision Health Economics, Los Angeles, CA, USA; 2Policy and Economics, Precision Health Economics, Boston, MA, USA; 3School of Pharmacy, Sol Price School of Public Policy, Leonard D. Schaeffer Center for Health Policy and Economics, University of Southern California, Los Angeles, CA, USA; 4Health Economics and Outcomes Management, Otsuka Pharmaceutical Development & Commercialization, Inc., Princeton, NJ, USA
Background: New digital technologies offer providers the promise of more accurately tracking patients’ medication adherence. It is unclear, however, whether access to such information will affect provider treatment decisions in the real world.
Methods: Using prescriber-reported information on patient non-compliance from health insurance claims data between 2008 and 2014, we examined whether prescribers’ knowledge of non-compliance was associated with different prescribing patterns for patients with serious mental illness (SMI). We examined patients who initiated an oral atypical antipsychotic, but were later objectively non-adherent to this treatment, defined as proportion of days covered (PDC) <0.8. We examined how a physician’s awareness of patient non-compliance (ICD-9 diagnosis code: V15.81) was correlated with the physician’s real-world treatment decisions for that patient. Treatment decisions studied included the share of patients who increased antipsychotic dose, augmented treatment, switched their antipsychotic, or used a long-acting injectable (LAI).
Results: Among the 286,249 patients with SMI who initiated an antipsychotic and had PDC <0.8, 4,033 (1.4%) had documented non-compliance. When prescribers documented non-compliance, patients were more likely to be switched to another antipsychotic (32.8% vs 24.7%, P<0.001), have their dose increased (24.4% vs 22.1%, P=0.004), or receive an LAI (0.09% vs 0.04%, P=0.008), but were less likely to have augmented therapy with another antipsychotic (1.1% vs 1.3%, P=0.035) than patients without documented non-compliance.
Conclusion: Among SMI patients with documented non-compliance, the frequency of dose, medication switches, and LAI use were higher and augmentation was lower compared to patients without documented non-compliance. Access to adherence information may help prescribers more rapidly switch ineffective medications as well as avoid unnecessary medication augmentation.
Keywords: adherence, prescribing patterns, serious mental illness
Introduction
Serious mental illnesses (SMIs) impose a large burden on the US health care system and economy. About 9.8 million adults or 4.1% of all adults in the US have an SMI, including schizophrenia, bipolar disorder, and major depressive disorder.1 The total economic burden of SMI is $446.5 billion in 2018 US dollars.2
One factor contributing to this high economic burden is medication non-adherence, which can have a serious and costly impact on health outcomes. Three out of every four patients with an SMI diagnosis were non-adherent to their atypical antipsychotic medication.3 Non-adherence for patients with SMI can impede recovery4–6 and increase the risk for, and length of, hospitalizations.6–12 Additionally, medication non-adherence among patients with SMI increases expenditures on inpatient hospitalizations.8,9,13–18
Despite the demonstrated need to address medication non-adherence among patients with SMI, psychiatrists and other health care providers often have difficulty determining whether or not patients adhere to their medication regimen.19–23 Provider perceptions of adherence frequently do not align with objective measures of medication adherence.20–24 Instead, providers typically use their own clinical judgment or rely on patient- or caregiver-reported information. Rarely do they seek objective measures of adherence. Self-reported medication adherence by patients is often unreliable, however, as patients with SMI are often reluctant to reveal information about their non-adherence.19,23,25
Recent technological innovations offer the promise of improved monitoring of patient adherence, allowing prescribers to more precisely calibrate treatment options.26 One example is a digital medicine innovation that uses an ingestible sensor to measure medication ingestion.27 This technology allows for real-time monitoring of adherence, and provides valuable feedback to providers given the difficulties of obtaining reliable self-reported measures from patients with SMI. A previous study surveyed prescribers who routinely treat patients with schizophrenia and found that access to information on non-adherence significantly influenced treatment decisions.28 Digital technologies are also being used to measure adherence in real time for other diseases, such as COPD.29 The question remains, however, whether prescribers in the real world would act on information about patient non-adherence and appropriately adjust patterns of treatment.
To better understand the potential real-world impacts of digital adherence measurement technologies, this study measured the real-world association between prescriber awareness of non-adherence and prescribing patterns. Although the importance of medication adherence in patients with SMI has been well established,30 there is limited claims-based evidence that prescribers optimally respond to knowledge of non-adherence in a real-world setting. This study aimed to address this gap in the literature and provide insights into the influence of adherence information on prescribing decisions.
Materials and methods
Data
Our study uses the proprietary Truven MarketScan Commercial Claims, Medicare Supplement, and Medicaid claims data from 2008 to 2014. The Truven MarketScan database is a large convenience sample, covering about 66 million lives in each year. The MarketScan Commercial database includes enrollment data and medical and pharmacy claims for individuals and their dependants covered by employer-sponsored private health insurance. The MarketScan Medicare database includes similar information for Medicare-eligible retirees with employer-sponsored supplemental plans, incorporating both Medicare-covered and employer-paid portions of the health care encounter in these data. Finally, the Medicaid database contains similar information for Medicaid enrollees from eleven geographically dispersed states. No institutional review board oversight was required for this study as no protected health information was included in any of the data sets nor was there any risk to patient safety or privacy.
Sample definition
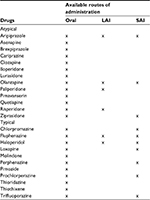
Our analysis sample was restricted to non-adherent adults aged ≥18 years with a diagnosis of at least one of the following SMIs: schizophrenia (ICD-9: 295.x), major depressive disorder (ICD-9: 296.2x, 296.3x, 311.x), or bipolar disorder (ICD-296.0x-296.1x, 296.4x-296.8x). Included patients were required to have filled a prescription for oral atypical antipsychotic medications (aripiprazole, asenapine, brexpiprazole, cariprazine, clozapine, iloperidone, lurasidone, olanzapine, paliperidone, pimavanserin, quetiapine, risperidone, ziprasidone; Table S1) and to have been continuously enrolled during the 6 months prior and 12 months after initiation. Patients with claims for oral, short, or long-acting injectable (LAI) atypical antipsychotics during the 6 months prior to this claim were excluded.
We further restricted our sample to patients who were non-adherent to the antipsychotic initiated. We defined non-adherence as having a proportion of days covered (PDC) <0.8 for the antipsychotic initiated, during the year after the first fill. To compute the number of days covered, we summed days supplied for fills of the first antipsychotic during the year and adjusted for overlapping periods. Then we calculated PDC as the days covered during the year divided by 365, top-coded at a maximum value of 1.0. The use of PDC and the 0.8 cut-off to determine non-adherence is consistent with specification of the quality measure “Adherence to Antipsychotic Medications for People with Schizophrenia” which is part of The Healthcare Effectiveness Data and Information Set31 and also included in The Merit-Based Incentive Payment System.32
Outcomes
We measured differences in treatment decisions between prescribers who were and were not aware of patient medication non-adherence. The treatment decisions of interest for this study were: dose increases, switches to another antipsychotic drug, switches to a LAI, and augmenting current therapy with another antipsychotic or another drug treating SMI. For details, see Tables 1 and Table S2. Patients could have multiple treatment changes during the year (eg, both a dose increase and medication switch).
  | Table 1 Summary of outcome variables Note: Additional details on drugs tracked for these definitions are listed in Tables S1 and S2. Abbreviations: LAI, long-acting injectable; SMI, serious mental illness. |
To capture differences in treatment approach when a prescriber is or is not aware of patient medication non-adherence, we compared treatment patterns among patients with and without physician-documented non-compliance. Specifically, we identified patients who had the diagnosis code ICD-9: V15.81 within 12 months of the first atypical fill. Our methodology followed the one proposed by Gosmanova et al,33 which used the V15.81 code to identify provider awareness of medication non-adherence among hypertensive veterans in the US. The V15.81 diagnosis code is used in case of “Personal history of non-compliance with medical treatment, presenting hazards to health”34 and was intended to describe non-adherence with medications, refusal of medical procedures, non-adherence, or inability to follow medical plan or dietary recommendations.33 We are not aware of any published reports validating the use of V15.81 diagnosis code. Note that this diagnosis code does not specifically indicate non-adherence to an antipsychotic nor does it even indicate if non-compliance is related to pharmacological treatment.
Statistical analysis
Our statistical analysis followed a two-step approach: 1) evaluate the relationship between documented non-adherence and claims-based non-adherence, and 2) measure the relationship between documented non-adherence and physician treatment choices.
In our first step, to determine face validity of using the V15.81 diagnosis code to measure prescriber awareness of patient adherence, we tested whether documented non-compliance was correlated with measured non-adherence to atypical antipsychotics as captured by PDC. Particularly, we compared adherence (as measured by PDC) for patients with a V15.81 diagnosis code to those who had no such code. A negative association would provide suggestive evidence that the V15.81 diagnosis code was indicative of prescriber awareness of non-compliance to antipsychotic treatment.
Second, we analyzed the differences in treatment patterns among non-adherent SMI patients with documented non-compliance to those without the diagnosis code recorded in their medical history. First, we ran unadjusted analyses and tested for a significant difference in treatment patterns between the two groups using Student’s t-tests.
Then, to account for potential heterogeneity across patients of physicians who did document a history of non-adherence compared to those who did not, we ran multivariate analyses to control for age, gender, comorbidities (Charlson Comorbidity Index [CCI]), SMI diagnosis type, and payer types. CCI was identified based on diagnosis codes on all claims occurring during the 6 months prior to the first atypical fill.35 Specifically, we estimated a logit model for each outcome separately with the indicator for documented non-compliance being the key variable of interest. Then, we predicted the probability of each treatment change for two groups of patients: for the average new starter non-adherent SMI patient whose non-adherence is known and whose non-adherence is not known to the provider.
We reweighted the sample by the share of SMI patients aged 18 years and older in each payer type as reported in the 2015 National Survey on Drug Use and Health36 to improve the generalizability of the analysis. The commercially insured SMI population is overrepresented in the MarketScan data relative to the population insured by public payers. If the effect of adherence information on providers’ treatment recommendations varies by the patient’s insurer, then the unweighted composite results would be biased toward the treatment choices made in the overrepresented commercially insured population.
Sensitivity analysis
To verify the robustness of our results, we conducted several sensitivity analyses. First, we analyzed the adjusted correlation between documented non-compliance separately by the patient’s specific SMI.
Second, we explored whether outcomes varied depending on the time period in which patient non-compliance was documented. Whereas our baseline approach requires the appearance of a V15.81 diagnosis code within 12 months of the initial atypical antipsychotic prescription, in our second sensitivity analysis we allowed either 1) the appearance of V15.81 at any time in patient claims history, or 2) its appearance within 6 months of the first atypical fill.
Third, we ran our multivariate analyses on an unweighted sample and compared the results to our weighted baseline findings. Although the weighted sample better reflects the insured SMI population in the US, we wanted to determine whether the sample reweighting significantly impacted our study findings. We also repeated our analyses separately by payer types to understand variation in treatment patterns that may be due to varying incentives provided by payer formularies.
Fourth, we used propensity score matching as an alternative method to address unobservable differences between patients who did and did not have documented non-compliance. We assembled a 1:3 matched cohort of SMI patients and calculated the difference in frequency of each treatment pattern between patients with and without documented non-compliance. Propensity score matching can reduce selection bias inherent in observational data.37 Particularly, first we estimated the probability of documented non-compliance based on demographic, comorbidity, diagnosis, and payer type variables. Then for each patient with documented non-compliance, we selected three patients without documented non-compliance but with a predicted probability of documented non-compliance very close to that of the patient with actual documented non-compliance.
All statistical analyses were performed using Stata 14 (StataCorp LLC, College Station, TX, USA).
Results
Descriptive analysis
The Truven Health MarketScan database contained 11,058,657 patients with commercial, 2,794,056 patients with Medicaid, and 1,230,052 patients with Medicare coverage who had at least one SMI diagnosis code of interest. There were 240,090 patients with commercial, 149,390 patients with Medicaid, and 30,485 patients with Medicare coverage meeting our inclusion criteria. Our final data set included 419,965 patients with SMI that initiated an antipsychotic. Table 2 provides details of the cohort selection.
Among all patients initiating an antipsychotic – both adherent and non-adherent – the average age was 43.8 years and 65.1% were female (Table S3). In our sample, 68.2% (286,249 out of 419,952) of SMI patients initiating an antipsychotic were non-adherent according to the claims-based PDC measure (Table S3).
Among our baseline sample of patients initiating an antipsychotic with PDC <0.8, we found statistically significant differences in patient characteristics across patients with document non-compliance compared to those without. Patients with documented non-compliance were younger (mean age of 38.7 years vs 42.5 years, P<0.001), less likely to be female (53.0% vs 66.0%, P<0.001), and had more comorbidities (average CCI: 0.77 vs 0.42, P<0.001) than those without a documented non-compliance diagnosis code recorded in their claims data (Table 3). Approximately three out of every four non-adherent patients with SMI had a diagnosis of major depressive disorder. Bipolar disorder was the second most prevalent SMI, affecting 54.1% and 40.5% of patients with and without documented non-compliance, respectively. Note that patients could have multiple SMI diagnoses simultaneously. Most patients with documented non-compliance were covered by Medicaid (66.2%), and 31.0% were covered by commercial insurance; however, we observed the opposite for patients without documented non-compliance (29.8% Medicaid, 63.5% commercial insurance). In both cohorts, coverage by Medicare was infrequent (2.8% and 6.7%).
Relationship between documented non-compliance and PDC
Patients with a V15.81 diagnosis code were less likely to be adherent to their atypical antipsychotic. Non-compliance within 12 months of the first atypical fill was documented for 1.4% of non-adherent patients, compared to only 0.9% of adherent patients (P<0.001). Furthermore, approximately twice as many individuals had a documented non-compliance during the first month after the first atypical fill (14% of all V15.81 diagnosis codes within the first year) compared to any of the later months (7%–8%).
Relationship between documented non-compliance and treatment choice
Among all new starter patients, 64.7% experienced a pharmacotherapy change (dose increases, augmentation, and/or switches) in the year after the first atypical fill. Of these treatment courses, augmentation with another SMI medication was observed in 40.2%, dose increases of the initial antipsychotic treatment were observed in 30.4%, and switches to another SMI medication (oral or LAI) were observed in 19.4% of the sample. Augmentation with another atypical drug was less frequent (4.0%) (Table S3).
In our unadjusted analyses, patients with a documented non-compliance were more likely to experience changes in pharmacotherapy during their first year on an oral atypical antipsychotic (Table 3). Specifically, the rate of pharmacotherapy changes was 19.1% higher for patients with documented non-compliance compared to those without (69.7% vs 58.5%, P<0.001). This result was primarily driven by a higher rate of switches to another atypical (43.6% vs 25.6%, P<0.001) and of dose increases in the initial antipsychotic treatment (26.2% vs 22.5%, P<0.001). Switches to an LAI of the same molecule were also more common for this cohort (1.4% vs 0.2%; P<0.001). Augmentation with another SMI drug, however, was not statistically different between the two groups (34.2% vs 33.5%, P=0.176).
Mirroring our unadjusted results, documented non-compliance was associated with an increased probability of treatment changes among non-adherent patients when we controlled for other patient characteristics. Table S4 reports the results of our logistic regressions estimating the probability of various treatment changes during the 12 months after the first atypical fill among patients who were non-adherent to their first atypical drug. Older non-adherent patients were typically less likely to experience treatment changes. Female patients were significantly more likely than their male counterparts to experience switches to another atypical (P<0.010), but less likely to switch to an LAI of the same molecule (P<0.010). Higher CCI scores were associated with significant increases in predicted augmentations with another atypical (P<0.050), but with fewer augmentations with another SMI drug (P<0.010). Relative to those with a diagnosis of major depressive disorder, having a diagnosis of schizophrenia or bipolar disorder was positively associated with experiencing changes in treatment patterns (with P<0.010 for all cases except in the case of LAI use among patients with bipolar disorder). Compared to patients covered by Medicare, those with commercial insurance were significantly less likely to switch to another atypical or to an LAI of the same molecule (P<0.010). Coverage with Medicaid was associated with augmentation with another atypical (P<0.050) or SMI (P<0.050).
Among non-adherent patients who initiated an antipsychotic, those with documented non-compliance were more likely to experience a dose increase of their first atypical drug, switch to another atypical drug, or switch to an LAI of the same molecule; however, they were less likely to augment with another SMI drug or atypical. The predicted probability of experiencing a dose increase was 10.4% higher (24.4% vs 22.1%, P=0.004) among patients with documented non-compliance compared to those without. Moreover, the predicted probability of switching to another atypical drug was 32.8% higher (32.8% vs 24.7%, P<0.001), the predicted probability of switching to an LAI of the same molecule was 125.0% higher (0.09% vs 0.04%, P=0.008), and the predicted probability of augmenting with another atypical drug was 15.4% lower (1.1% vs 1.3%, P=0.035) among patients with documented non-compliance compared to those without. Finally, the difference in the probability of augmenting with another SMI drug was not statistically significant (32.5% vs 31.9%, P=0.526) between the two groups (Figure 1).
  | Figure 1 Predicted treatment patterns for non-adherent new user patients (PDC <0.8) with SMI. Abbreviations: LAI, long-acting injectable; SMI, serious mental illness; PDC, proportion of days covered. |
Sensitivity analyses
The results of our study were qualitatively similar across all sensitivity analyses considered. Our first sensitivity analysis found that baseline results were largely driven by changes in treatment patterns among patients with schizophrenia. Among patients with schizophrenia, predicted probability among those with documented non-compliance compared to those without was 23.5% higher for switching to another atypical drug (55.1% vs 44.6%, P<0.001) and 84.6% higher for switching to an LAI of the same molecule (2.4% vs 1.3%, P<0.001). In contrast, the predicted probability of augmenting with another atypical drug was 33.9% lower (3.7% vs 5.6%, P<0.001) among patients with documented non-compliance. At the same time, the predicted probabilities of experiencing a dose increase and augmenting with another SMI drug were not significantly different between the two groups (26.0% vs 25.0%, P=0.332% and 33.0% vs 34.5%, P=0.175, respectively).
In our second sensitivity analysis, we found that the rate of changes in treatment patterns among patients with and without documented non-compliance were similar when non-compliance was defined based on having a V15.81 diagnosis code within 12 or within 6 months from first atypical fill, and qualitatively similar but smaller in magnitude when defined based on the V15.81 diagnosis code appearing at any time in the patient’s history (Table 4).
In our third set of sensitivity analyses, we found that the unweighted results were similar to results weighted by payer type. For example, the predicted probabilities of switching to another atypical antipsychotic among patients with and without documented history of non-compliance were 32.8% and 24.7% (a change of 32.8%; P<0.001) in our baseline analysis, and 32.7% and 24.8% (a change of 31.9%; P<0.001) in the unweighted analysis. Similarly, augmenting with another atypical was more likely among patients without documented non-compliance than among patients with it in both the baseline analysis (1.3% vs 1.1%; P=0.035) and the unweighted analysis (1.4% vs 1.0%; P<0.001). Also, analyses by payer type produced results that were consistent with those in our baseline analysis. As demonstrated in Table 4, changes in treatment patterns are similar for patients covered by Medicare and Medicaid.
Finally, the results of the propensity score-matched analyses were largely consistent with our baseline model. As shown in Table 4, measured differences in predicted probability of various treatment changes between patients with and without documented non-compliance followed the same pattern as in the baseline analysis. Patients with documented non-compliance had higher predicted probabilities of dose increases and of switches to another atypical or LAI, but statistically similar probabilities of augmenting with another SMI; these results held both in the baseline and in the propensity score-matched analyses.
Discussion
Among non-adherent SMI patients initiating an antipsychotic, those with documented non-compliance were more likely than those without to experience a dose increase of their first atypical drug, switch to another atypical drug, or switch to an LAI of the same molecule; however, they were less likely to augment with another atypical.
Clinical management of SMI is complex, but our findings suggest that physicians respond as expected to reliable information of patient medication non-adherence. After obtaining such information, it is reasonable to expect that physicians may decide to switch the patient to a different antipsychotic or LAI in an attempt to improve adherence by changing the side-effect profile or addressing social factors that hinder frequent oral administration. It is similarly reasonable to expect that prescribers will be reluctant to prescribe additional drugs to patients that are non-adherent to the first prescribed medication. Our results are consistent with these expectations.
The only unexpected result, however, is a modestly higher rate of dose increases for patients with known non-adherence to medication. This finding is surprising for the reasons just described. Because physicians are not randomized according to whether they document non-adherence, it is plausible that physicians who are more likely to proactively titrate the patient’s dose are also the same physicians that are paying more attention to a patient’s underlying adherence. If so, the small difference in estimates across patient types may not reflect causal effect.
Results of our analyses align with prior studies of non-adherence among patients with SMIs, and fall on the high end of the reported range of proportion of patients who are non-adherent. In our analysis, we identified 68.2% of SMI patients initiating an antipsychotic that were non-adherent according to the claims-based PDC measure. Among other sources estimating the proportion of non-adherent patients, Shafrin et al found that 48% of SMI patients were non-adherent to their oral atypical antipsychotics,38 whereas Jiang et al39 determined that 64% of a sample of schizophrenia and bipolar patients with a prescription for non-injectable atypical antipsychotics were non-adherent (PDC <0.8). The cohort examined in our study consisted of new antipsychotic users only, which may explain the somewhat higher proportion of non-adherent patients as new users may be more likely to experience an initial adjustment period until their treatment regimen is stabilized.
Providing accurate adherence information to prescribers has been shown to lead to large cost savings as prescribers can optimize decision making with regard to treatment, especially among patients who are not responding to treatment due to non-adherence rather than physiological reasons. In a decision tree model building on results of previous studies, Shafrin et al3 showed that providing more accurate adherence information to providers treating patients with schizophrenia resulted in a cost savings of $1,977 (2018 USD) per patient. The current study serves to validate these findings in the real world, suggesting that cost savings are achievable by payers. Indeed, other studies found that avoiding unnecessary treatment changes saves an estimated $4,358 (2018 USD) per patient,40 while switching adherent patients to LAI treatments costs an estimated $1,841 (2018 USD) per patient.41
To our knowledge, no prior studies have examined how real-world prescribing behaviors differ for SMI patients with or without a history of claims-based documented non-compliance. However, this study does contribute to growing evidence that information on treatment adherence can improve provider decision making.28 New technologies, such as the Medication Event Monitoring System cap,42 a sensor on pill bottles used to monitor adherence, or the digital medicine system,43 an ingestible sensor that tracks adherence, may improve the quality of information available for providers and hence may lead to higher quality treatment decisions. In addition, prescribers may have varying experience (eg, training, access to guidelines, etc) in treating non-adherent SMI patients. It is likely that improving the quality of information and tools available to prescribers to assess and treat non-adherent SMI patients may lead to cost savings.38 Further research is needed to demonstrate the extent of these savings.
Limitations
There are several limitations to our study. First, identification of prescriber knowledge of non-adherence is based on observing the V15.81 diagnosis code, a general ICD-9 code for non-compliance to medical treatment, in the patient’s medical claims, and thus this study may overestimate physician-documented non-compliance “with antipsychotic” medications specifically. On the other hand, our study likely underestimates the effect of provider knowledge of patient non-compliance as the V15.81 diagnosis code is almost certainly under-coded. To examine the validity of our measure, we analyzed the association between appearance of the V15.81 diagnosis code and claims-based adherence measures and found that the presence of V15.81 diagnosis code was negatively correlated with measured adherence. Future studies examining this relationship may use ICD-10 codes, which are able to differentiate between clinical and pharmacologic non-adherence. Previous research indicates that errors in measurement of non-adherence is only likely to bias our results toward finding no effect.38
Second, our current study design cannot definitively establish causality. Our results suggest that prescribers react to information on non-adherence by adjusting treatment regimen, but it is also possible that providers who use the V15.81 diagnosis code engage in different treatment strategies for other reasons that are correlated with documenting patient non-compliance in medical claims. Nevertheless, in several sensitivity analyses we have confirmed our primary results.
Third, Truven Health MarketScan data is a convenience sample and therefore may not be nationally representative. To address this issue, we reweighted our sample to match the nationally representative distribution of SMI patients by payer and found similar results. Nevertheless, our sample does not include uninsured individuals, those covered by the Veterans Affairs, Indian Health Services, and a variety of other populations.
Fourth, we used PDC as a measure of adherence. Although there are numerous alternative measures of adherence,44 PDC is often used for patients with SMIs and other conditions.45–48 Particularly, we believe that PDC is more appropriate in the SMI context than alternative measures such as medical possession ratio as it captures discontinuation as non-adherence. While typically used in the literature for serious mental illnesses and other conditions,46–51 to our best knowledge, this threshold is arbitrary and lacks validation for patients with serious mental illness. Moreover, as any claims-based measure, PDC may overestimate adherence as patients may fill prescriptions they do not actually ingest. Our measures of prescribed treatment patterns may also be biased if patients do not fill prescriptions received from the provider. Thus, our cohort of non-adherent patients may be missing some patients who are in fact non-adherent but who still fill their prescription. Finally, our study includes a limited number of treatments and may unintentionally exclude interventions – such as counseling within a standard physician visit – that are not captured in health insurance claims data.
Conclusion
SMI patients with documented non-compliance were more likely to experience a dose increase of their first atypical drug, switch to another atypical drug, or switch to an LAI of the same molecule, but less likely to augment with another SMI medication. Despite high level of medication non-adherence among patients with SMI, non-compliance was rarely documented in claims data. Access to adherence information may help prescribers avoid sub-optimal treatment decisions such as unnecessary augmentation with another atypical drug among patients who do not respond to therapy because of non-compliance.
Acknowledgments
The contents of this manuscript were previously presented at the US 2017 Psych Congress and AMCP Managed Care and Specialty Pharmacy Nexus 2017 Annual Meeting. The work for this manuscript was conducted at Precision Health Economics offices in Los Angeles, Austin, and Boston.
Author contributions
All authors have contributed sufficiently to the conception and design, data acquisition, data analysis and interpretation, drafting and critical revision, and final approval of the manuscript to be published and have agreed to be accountable for all aspects of the work.
Disclosure
JS, KB, KE, and MB are employees of Precision Health Economics, a health care consulting firm that received funding for this study from Otsuka Pharmaceutical Development & Commercialization, Inc. DNL is a consulting Scientific Advisor at Precision Health Economics. DNL and JS are investors in Precision Health Economics’ parent company, Precision Medicine Group. FMF is an employee of Otsuka Pharmaceutical Development & Commercialization, Inc. The authors report no other conflicts of interest in this work.
References
Kessler RC, Berglund PA, Zhao S, et al. The 12-month prevalence and correlates of serious mental illness (SMI). In: Manderscheid RW, Sonnenschein MA, editors. Mental Health: United States; Washington, DC: US Government Printing Office; 1996:59–70. | ||
Insel TR. Assessing the economic costs of serious mental illness. Am J Psychiatry. 2008;165(6):663–665. | ||
Shafrin J, Schwartz TT, Lakdawalla DN, Forma FM. Estimating the value of new technologies that provide more accurate drug adherence information to providers for their patients with schizophrenia. J Manag Care Spec Pharm. 2016;22(11):1285–1291. | ||
Subotnik KL, Nuechterlein KH, Ventura J, et al. Risperidone nonadherence and return of positive symptoms in the early course of schizophrenia. Am J Psychiatry. 2011;168(3):286–292. | ||
Lindenmayer JP, Liu-Seifert H, Kulkarni PM, et al. Medication nonadherence and treatment outcome in patients with schizophrenia or schizoaffective disorder with suboptimal prior response. J Clin Psychiatry. 2009;70(7):990–996. | ||
Offord S, Lin J, Wong B, Mirski D, Baker RA. Impact of oral antipsychotic medication adherence on healthcare resource utilization among schizophrenia patients with Medicare coverage. Community Ment Health J. 2013;49(6):625–629. | ||
Weiden PJ, Kozma C, Grogg A, Locklear J. Partial compliance and risk of rehospitalization among California Medicaid patients with schizophrenia. Psychiatr Serv. 2004;55(8):886–891. | ||
Offord S, Lin J, Mirski D, Wong B. Impact of early nonadherence to oral antipsychotics on clinical and economic outcomes among patients with schizophrenia. Adv Ther. 2013;30(3):286–297. | ||
Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161(4):692–699. | ||
Eaddy M, Grogg A, Locklear J. Assessment of compliance with antipsychotic treatment and resource utilization in a Medicaid population. Clin Ther. 2005;27(2):263–272. | ||
Law MR, Soumerai SB, Ross-Degnan D, Adams AS. A longitudinal study of medication nonadherence and hospitalization risk in schizophrenia. J Clin Psychiatry. 2008;69(1):47–53. | ||
Velligan DI, Weiden PJ, Sajatovic M, et al. Strategies for addressing adherence problems in patients with serious and persistent mental illness: recommendations from the expert consensus guidelines. J Psychiatr Pract. 2010;16(5):306–324. | ||
Svarstad BL, Shireman TI, Sweeney JK. Using drug claims data to assess the relationship of medication adherence with hospitalization and costs. Psychiatr Serv. 2001;52(6):805–811. | ||
Marcus SC, Olfson M. Outpatient antipsychotic treatment and inpatient costs of schizophrenia. Schizophr Bull. 2008;34(1):173–180. | ||
Predmore ZS, Mattke S, Horvitz-Lennon M. Improving antipsychotic adherence among patients with schizophrenia: savings for states. Psychiatr Serv. 2015;66(4):343–345. | ||
Hansen RA, Maciejewski M, Yu-Isenberg K, Farley JF. Adherence to antipsychotics and cardiometabolic medication: association with health care utilization and costs. Psychiatr Serv. 2012;63(9):920–928. | ||
Thieda P, Beard S, Richter A, Kane J. An economic review of compliance with medication therapy in the treatment of schizophrenia. Psychiatr Serv. 2003;54(4):508–516. | ||
Higashi K, Medic G, Littlewood KJ, Diez T, Granström O, de Hert M. Medication adherence in schizophrenia: factors influencing adherence and consequences of nonadherence, a systematic literature review. Ther Adv Psychopharmacol. 2013;3(4):200–218. | ||
Chapman SC, Horne R. Medication nonadherence and psychiatry. Curr Opin Psychiatry. 2013;26(5):446–452. | ||
Kulkarni J, Reeve-Parker K. Psychiatrists’ awareness of partial- and non-adherence to antipsychotic medication in schizophrenia: results from the Australian ADHES survey. Australas Psychiatry. 2015;23(3):258–264. | ||
Loayza N, Crettol S, Riquier F, Eap CB. Adherence to antidepressant treatment: what the doctor thinks and what the patient says. Pharmacopsychiatry. 2012;45(5):204–207. | ||
Olivares JM, Alptekin K, Azorin JM, et al. Psychiatrists’ awareness of adherence to antipsychotic medication in patients with schizophrenia: results from a survey conducted across Europe, the Middle East, and Africa. Patient Prefer Adherence. 2013;7:121–132. | ||
Stephenson JJ, Tunceli O, Tuncelli O, et al. Adherence to oral second-generation antipsychotic medications in patients with schizophrenia and bipolar disorder: physicians’ perceptions of adherence vs. pharmacy claims. Int J Clin Pract. 2012;66(6):565–573. | ||
Velligan DI, Weiden PJ, Sajatovic M, et al. Assessment of adherence problems in patients with serious and persistent mental illness: recommendations from the Expert Consensus Guidelines. J Psychiatr Pract. 2010;16(1):34–45. | ||
Sawada N, Uchida H, Watanabe K, et al. How successful are physicians in eliciting the truth from their patients? A large-scale Internet survey from patients’ perspectives. J Clin Psychiatry. 2012;73(3):311–317. | ||
Kvedar JC, Fogel AL, Elenko E, Zohar D. Digital medicine’s march on chronic disease. Nat Biotechnol. 2016;34(3):239–246. | ||
Comstock J. Proteus, Otsuka submit first commercial drug with built-in sensor to FDA; 2015. Available from: http://www.mobihealthnews.com/46680/proteus-otsuka-submit-first-commercial-drug-with-built-in-sensor-to-fda. Accessed August 2, 2017. | ||
Shafrin J, May SG, Shrestha A, et al. Access to credible information on schizophrenia patients’ medication adherence by prescribers can change their treatment strategies: evidence from an online survey of providers. Patient Prefer Adherence. 2017;11:1071–1081. | ||
Taylor TE, Zigel Y, de Looze C, Sulaiman I, Costello RW, Reilly RB. Advances in audio-based systems to monitor patient adherence and inhaler drug delivery. Chest. 2018;153(3):710–722. | ||
Kane JM, Kishimoto T, Correll CU. Non-adherence to medication in patients with psychotic disorders: epidemiology, contributing factors and management strategies. World Psychiatry. 2013;12(3):216–226. | ||
National Committee for Quality Assurance. Adherence to Antipsychotic Medications for People with Schizophrenia –This HEDIS Measure; 2017. Available from: http://www.ncqa.org/report-cards/health-plans/state-of-health-care-quality/2017-table-of-contents/antipsychotic-medications. Accessed July 18, 2018. | ||
Healthmonix. 2018 MIPS Measure #383: Adherence to Antipsychotic Medications For Individuals with Schizophrenia; 2018. Available from: http://healthmonix.com/mips_quality_measure/2018-mips-quality- measure-383/. Accessed May 31, 2018. | ||
Gosmanova EO, Lu JL, Streja E, Cushman WC, Kalantar-Zadeh K, Kovesdy CP. Association of medical treatment nonadherence with all-cause mortality in newly treated hypertensive US veterans. Hypertension. 2014;64(5):951–957. | ||
2012 ICD-9-CM Diagnosis Code V15.8. 2012. Alkaline Software. Available from: http://www.icd9data.com/2012/Volume1/V01-V91/V10-V19/V15/V15.81.htm. Accessed May 31, 2018. | ||
Quan H, Li B, Couris CM, et al. Updating and validating the Charlson Comorbidity Index and score for risk adjustment in hospital discharge abstracts using data from 6 countries. Am J Epidemiol. 2011;173(6):676–682. | ||
Center for Behavioral Health Statistics and Quality. National Survey on Drug Use and Health: Detailed Tables. Rockville, MD: Substance Abuse and Mental Health Services Administration. https://www.samhsa.gov/data/sites/default/files/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015/NSDUH-DetTabs-2015.pdf. Accessed May 31, 2018. | ||
Dehejia RH, Wahba S. Propensity score-matching methods for nonexperimental causal studies. Rev Econ Stat. 2002;84(1):151–161. | ||
Shafrin J, Forma F, Scherer E, Hatch A, Vytlacil E, Lakdawalla D. The cost of adherence mismeasurement in serious mental illness: a claims-based analysis. American Journal of Managed Care. 2017;23(5):e156–e163. | ||
Jiang Y, Ni W. Estimating the Impact of Adherence to and Persistence with Atypical Antipsychotic Therapy on Health Care Costs and Risk of Hospitalization. Pharmacotherapy. 2015;35(9):813–822. | ||
Faries DE, Ascher-Svanum H, Nyhuis AW, Kinon BJ. Clinical and economic ramifications of switching antipsychotics in the treatment of schizophrenia. BMC Psychiatry. 2009;9:54. | ||
Lin J, Wong B, Offord S, Mirski D. Healthcare cost reductions associated with the use of LAI formulations of antipsychotic medications versus oral among patients with schizophrenia. J Behav Health Serv Res. 2013;40(3):355–366. | ||
Diaz E, Levine HB, Sullivan MC, et al. Use of the Medication Event Monitoring System to estimate medication compliance in patients with schizophrenia. J Psychiatry Neurosci. 2001;26(4):325–329. | ||
US Food and Drug Administration. Highlights of prescribing information: Abilify MyCite. 2017; Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/207202lbl.pdf. Accessed May 3, 2018. | ||
Hess LM, Raebel MA, Conner DA, Malone DC. Measurement of adherence in pharmacy administrative databases: a proposal for standard definitions and preferred measures. Ann Pharmacother. 2006;40(7–8):1280–1288. | ||
Martin BC, Wiley-Exley EK, Richards S, Domino ME, Carey TS, Sleath BL. Contrasting measures of adherence with simple drug use, medication switching, and therapeutic duplication. Ann Pharmacother. 2009;43(1):36–44. | ||
Sacks NC, Burgess JF, Cabral HJ, Mcdonnell ME, Pizer SD. The effects of cost sharing on adherence to medications prescribed for concurrent use: do definitions matter? J Manag Care Spec Pharm. 2015;21(8):678–687. | ||
Krueger K, Griese-Mammen N, Schubert I, et al. In search of a standard when analyzing medication adherence in patients with heart failure using claims data: a systematic review. Heart Fail Rev. 2018;23(1):63–71. | ||
Karve S, Markowitz M, Fu DJ, et al. Assessing medication adherence and healthcare utilization and cost patterns among hospital-discharged patients with schizoaffective disorder. Appl Health Econ Health Policy. 2014;12(3):335–346. | ||
Ho PM, Rumsfeld JS, Masoudi FA, et al. Effect of medication nonadherence on hospitalization and mortality among patients with diabetes mellitus. Arch Intern Med. 2006;166(17):1836–1841. | ||
Gibson TB, Song X, Alemayehu B, et al. Cost sharing, adherence, and health outcomes in patients with diabetes. Am J Manag Care. 2010;16(8):589–600. | ||
Roebuck MC, Liberman JN, Gemmill-Toyama M, Brennan TA. Medication adherence leads to lower health care use and costs despite increased drug spending. Health Aff. 2011;30(1):91–99. |
Supplementary materials
  | Table S3 Descriptive statistics of study variables for all new starter patients Abbreviations: LAI, long-acting injectable; SMI, serious mental illness. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.