Back to Journals » Journal of Pain Research » Volume 10
Changes in pain and concurrent pain medication use following compounded topical analgesic treatment for chronic pain: 3- and 6-month follow-up results from the prospective, observational Optimizing Patient Experience and Response to Topical Analgesics study
Authors Gudin JA, Brennan MJ, Harris ED , Hurwitz PL , Dietze DT , Strader JD
Received 8 June 2017
Accepted for publication 30 August 2017
Published 3 October 2017 Volume 2017:10 Pages 2341—2354
DOI https://doi.org/10.2147/JPR.S143513
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor E Alfonso Romero-Sandoval
Jeffrey A Gudin,1 Michael J Brennan,2 E Dennis Harris,3 Peter L Hurwitz,3 Derek T Dietze,4 James D Strader5
1Pain Medicine and Palliative Care, Englewood Hospital and Medical Center, Englewood, NJ, 2The Pain Center of Fairfield, Fairfield, CT, 3Clarity Science, Austin, TX, 4Metrics for Learning, LLC, Queen Creek, AZ, 5Safe Harbor Compliance and Clinical Services, LLC, Austin, TX, USA
Background: Opioids and other controlled substances prescribed for chronic pain are associated with abuse, addiction, and death, prompting national initiatives to identify safe and effective pain management strategies including topical analgesics.
Methods: This prospective, observational study evaluated changes from baseline in overall mean severity and interference scores on the Brief Pain Inventory scale and the use of concurrent pain medications at 3- and 6-month follow-up assessments in chronic pain patients treated with topical analgesics. Changes in pain severity and interference and medication usage were compared between treated patients and unmatched and matched controls.
Results: The unmatched intervention group (unmatched-IG) included 631 patients who completed baseline and 3-month follow-up surveys (3-month unmatched-IG) and 158 who completed baseline and 6-month follow-up assessments (6-month unmatched-IG). Baseline and 3-month follow-up data were provided by 76 unmatched controls and 76 matched controls (3-month unmatched-CG and matched-CG), and 51 unmatched and 36 matched patients completed baseline and 6-month follow-up surveys (6-month unmatched-CG and matched-CG). Baseline demographic characteristics and mean pain severity and interference scores were similar between groups. There were statistically significant decreases from baseline in mean pain severity and interference scores within the 3- and 6-month unmatched-IG (all P<0.001). Significantly greater decreases in the mean change from baseline in pain severity and interference scores were evident for the 3- and 6-month unmatched-IG versus unmatched-CG (all P<0.001), with similar results when the 3- and 6-month matched-IG and matched-CG were compared. A higher percentage of the 3- and 6-month unmatched-IG and matched-IG de-escalated use of concurrent pain medications (all P<0.001), while significantly higher percentages of the unmatched-CG and matched-CG escalated medication use. Side effects were reported by <1% of the unmatched-IG.
Conclusion: Topical analgesics appear to be effective and safe for the treatment of chronic pain, with randomized controlled trials needed to confirm these findings.
Keywords: chronic pain, opioids, pain interference, pain severity, topical analgesics, OPERA
Introduction
Chronic pain is a leading cause of disability in the US and worldwide1–8 and a frequent reason for consultations with primary care and specialty clinicians.9–14 Among a weighted total of 234.9 million who responded to the Functioning and Disability Supplement of the 2012 National Health Interview Survey, 126.1 million (weighted percentage, 55.7) reported some pain in the past 3 months.15 Of these, 25.3 million experienced daily chronic pain, 23.4 million reported that they experienced a lot of pain, and 10.5 million suffered a lot of pain every day.15
Chronic pain is associated with profound negative effects on personal, social, and psychological well-being,6,7,16–19 increased morbidity and mortality,16,18,19 absenteeism and job loss,6,16,20–22 and presenteeism,23 which is defined as reduced productivity attributable to physical, mental, or emotional illness or injury. Chronic pain is the number one cause of years lived with disability24 and increases the risk for other chronic illnesses,5,16,22,25,26 as well as all-cause mortality and death from cardiovascular disease.19
Treatment goals for chronic pain focus on effective pain relief, improved quality of life, and enhanced functional ability.14,27,28 Medications, including opioids and nonsteroidal anti-inflammatory drugs (NSAIDs), are commonly prescribed for chronic pain.29 The imperative to effectively treat patients with chronic pain, the established efficacy of opioid analgesics for pain management, and limited therapeutic alternatives to opioids are considered factors contributing to the overutilization of opioid medications in the US.30
The number of prescriptions for opioids and other controlled substances increased dramatically in recent years. During this time, rates of misuse, overuse, diversion, abuse, addiction, and death also escalated.30–35 Together, these events prompted a national initiative to develop more effective regulatory policies and implement clinical strategies to safely and effectively manage pain.36 These efforts included national guidelines for clinicians,37 policy reports and recommendations by the US Surgeon General,38 and the development of the National Pain Strategy.39
One component of these efforts focused on topical agents, which have the potential to provide analgesic effects without the risk of abuse, misuse, and addiction or systemic adverse events (AEs) associated with oral analgesics.40–44 Topical medications lower the risk of systemic AEs and drug–drug interactions, have limited systemic absorption, offer simple dose determination, provide direct access to the target site, and are easy for patients to apply.43,45–47 A systematic review concluded that topical analgesics have an important place in the management of acute and chronic pain conditions and warrant further study.29 Similar results are reported for the use of topical NSAIDs for the treatment of musculoskeletal conditions.48,49
The Optimizing Patient Experience and Response to Topical Analgesics (OPERA) study evaluated the effect and safety of topical analgesic formulations on changes from baseline in overall mean pain severity and interference pain scores and the use of concurrent pain medications at 3- and 6-month assessment intervals in patients with chronic pain conditions. Changes in severity and interference scores and pain medication usage were also compared between patients treated with topical agents and two separate groups of patients who did not receive topical therapy.
Methods
Study design
This was an exploratory, prospective, observational study of patients who were prescribed one of four formulations of a topical pain medication. This nonprobability sample formed the overall intervention group (IG). In order to compare changes in pain severity and interference and the use of concurrent pain medications between patients treated with a topical agent and those not administered a topical medication, a convenience sample of patients was included. These patients met the study eligibility criteria, consented to participate in this study, and completed follow-up assessments but did not receive a topical agent. This group formed the unmatched control group (unmatched-CG) for comparison with the overall unmatched intervention group (unmatched-IG). A subset of patients in the unmatched-CG was matched retrospectively with patients in the unmatched-IG on sex, primary pain complaint, and age (±5 years), forming a matched control group (matched-CG) and a matched intervention group (matched-IG).
The study was performed in full accordance with the rules of the US Health Insurance Portability and Accountability Act of 1996 and the principles of the Declaration of Helsinki and the International Council for Harmonisation/Good Clinical Practice. The study protocol was approved by the IntegReview Institutional Review Board.
Patients
Physicians at the 85 participating clinical sites in the US invited eligible patients to enroll in this study. Inclusion criteria were the following: 1) age 18–64 years; 2) currently experiencing chronic pain attributed to any cause including neurological disorders, musculoskeletal disease, or other medical conditions; 3) a current prescription for topical (cream or patch) pain medication(s); and 4) insurance benefits to cover the costs of prescribed medication(s). Patients were ineligible if they had a current or past history of misuse of illicit or prescription drugs or were a current beneficiary of a government-funded health care program.
Study participants provided written informed consent at enrollment. Physicians and patients were assigned unique identification numbers to allow a linked analysis of patient- and physician-completed surveys. Aggregated patient and physician survey data were submitted to Clarity Science with no identifying information about patients or clinicians recorded on submitted surveys.
Patients could withdraw from this study at any time during the 6-month follow-up period, with the assurance of no harmful or unfavorable impact on their medical care. All diagnostic tests and treatment decisions were made at the discretion of physicians, with no tests, treatments, or investigations performed as part of this study.
Topical intervention
Four classes of prescription compounds were prescribed based on the major constituent in each formulation. These included compounds consisting primarily of 1) diclofenac, 2) ketoprofen, 3) flurbiprofen, or 4) other formulations not containing an NSAID. At the discretion of prescribing clinicians, the compounds could be combined with nonprescription neuropathic supplements including L-carnitine, lipoic acid, methyl B-12, pyridoxal-5-phosphate, folinic acid, vitamin D3, or magnesium chloride, which may or may not have analgesic properties themselves. Physicians recorded the compounded topical agent on prescription order forms, and a unique patient identification number was assigned to match the prescription to each patient.
Study procedures and assessments
Patients completed baseline surveys at the time of study enrollment and 3- and 6-month follow-up surveys. Patients responding to the baseline and 3- and 6-month follow-up surveys in the unmatched and matched IGs and CGs were not always the same, with some patients completing only one of the two follow-up assessments and others completing all three assessments. Those who responded only to the 3-month assessment comprised the 3-month follow-up group, while patients who responded to both the 3- and 6-month surveys or just the 6-month assessment formed the 6-month follow-up group.
The baseline and follow-up surveys of patients included questions to assess the primary pain complaint categorized as 1) arthritis, 2) neuropathy or radiculopathy, 3) myofascial or musculoskeletal pain, 4) tendonitis, or 5) other type of pain complaint. Patients indicated the location of each pain complaint, such as hands, elbow, wrist, feet, hips, knees, neck, shoulders, back, and other. They could indicate more than one type of pain complaint affecting multiple locations.
Patients completed the Brief Pain Inventory short form (BPI-sf)50 at each assessment, with all surveys completed during the usual 8-hour business interval for a medical clinic. The BPI-sf is a commonly used measure of pain for diverse conditions, including cancer, musculoskeletal disorders, depressive conditions, and surgical pain. The BPI-sf is recommended for use in clinical trials of patients with chronic pain27 and has adequate internal consistency, acceptable-to-excellent test–retest reliability, satisfactory-to-good construct validity, criterion validity, and is sensitive to change.51–54 Pain ratings are on a 0–10 numerical scale, with 0 equal to no pain and 10 equal to pain as bad as one can imagine. The BPI-sf yields an overall score for pain severity and pain interference as well as a score for each of the questions comprising the overall severity and interference measures. Patients receiving the topical therapy also indicated any side effects they experienced while using the formulation.
Patients also reported if they took any additional medication(s) for pain relief at the time of the baseline survey and during the 3 days prior to completion of the follow-up surveys. These concurrent medications included over-the-counter (OTC) agents (eg, ibuprofen, naproxen, or acetaminophen), prescription NSAIDs (eg, naproxen sodium, celecoxib, meloxicam), prescription opioids (eg, fentanyl, hydrocodone, hydromorphone, morphine, or oxycodone), or prescription anticonvulsants (gabapentin or pregabalin). Patients could indicate more than one type of medication in these four classes.
All patient surveys were voluntarily and independently completed with no input or direction from treating physicians or other clinic staff. Completion of each of the surveys took approximately 10 minutes. Patients may have been compensated up to $25 for time or travel expenses associated with study participation at the discretion of the investigator.
Study end points
There were two primary end points and five secondary end points. The first primary end point evaluated changes from baseline within the unmatched-IG in the mean BPI-sf severity and interference scores for the 3- and 6-month follow-up groups. The second primary end point examined changes from baseline within the unmatched-IG in patient-reported use of concurrent pain medication(s) for the 3- and 6-month follow-up groups.
Secondary end points included the following:
- Differences from baseline between the unmatched-IG and unmatched-CG in the mean change in BPI-sf severity and interference scores for the 3- and 6-month follow-up groups
- Differences from baseline between the matched-IG and matched-CG in the mean change in BPI-sf severity and interference scores for the 3- and 6-month follow-up groups
- Changes from baseline between the unmatched-IG and unmatched-CG in the type of concurrent pain medication for the 3- and 6-month follow-up groups
- Changes from baseline between the matched-IG and matched-CG in the type of concurrent pain medication for the 3- and 6-month follow-up groups
- Patient- and investigator-reported AEs or serious adverse events (SAEs) in the 3- and 6-month follow-up groups
Statistical analysis
Descriptive statistics were calculated for all variables, including frequencies and percent responses for categorical variables and means and standard deviation (SD) for continuous variables. The maximum sample available for each variable was used when calculating descriptive statistics. The Shapiro–Wilk test was performed to determine normality of all continuous variables. Continuous variables that were not normally distributed were analyzed with nonparametric statistical tests.
Patient-reported changes from baseline in the use of concurrent medications were categorized as de-escalation, escalation, or no change, with the data reported as frequencies and percents. The number of patients in the study groups who used anticonvulsants at baseline was not sufficient to evaluate changes at the 3- and 6-month follow-up (Table 1). The assessment of de-escalation, escalation, and no change included only patients who reported changes in the use of opioids, prescription NSAIDs, and OTC pain medications. De-escalation was defined as a reduction in the use of one or more classes of medications at the follow-up surveys compared to the class or classes of medications indicated at baseline. For example, patients who reported the use of one, two, or three of the classes of medications at baseline and then indicated a decrease to none, one, or two concurrent medications, respectively, in the 3- and 6-month follow-up groups were categorized under de-escalations. An escalation was defined as the addition of a medication class at a follow-up assessment compared to the class or classes of medications indicated at baseline. For example, patients who reported none, one, or two concurrent medications at baseline and subsequently reported use of one, two, or three classes of medications, respectively, in the 3- or 6-month assessment groups were categorized under escalations. Under no change in concurrent medication use were included all patients who reported no changes in the class of pain medication used at baseline compared to the class or classes of medications indicated in the 3- or 6-month follow-up groups.
The Mann–Whitney test compared changes in BPI-sf severity and interference scores from baseline between the unmatched-IG and unmatched-CG for the 3- and 6-month groups. The Wilcoxon signed-rank test for continuous data evaluated changes in the BPI-sf severity and interference scores from baseline within the unmatched-IG and between the matched-IG and matched-CG for the 3- and 6-month follow-up groups. Results for each analysis were reported as mean, SD, statistic, and 95% confidence interval for the difference from baseline to the 3-month follow-up and baseline to the 6-month follow-up. Fisher’s exact test for categorical data evaluated changes from baseline between the unmatched-IG and the unmatched-CG in the type of concurrent pain medication reported by the 3- and 6-month follow-up groups. The McNemar test for categorical, matched data evaluated the changes from baseline between the matched-IG and matched-CG in the type of concurrent pain medication reported by the 3- and 6-month assessment groups.
A two-tailed alpha was set to 0.05 for all statistical comparisons. All analyses were performed with SPSS v. 23.55
Results
Baseline demographic and clinical characteristics of patients
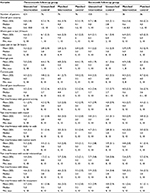
A total of 631 patients enrolled in the unmatched-IG at baseline and completed a 3-month follow-up assessment, which formed the 3-month follow-up group. There were 158 patients in the unmatched-IG who completed the 6-month assessment, comprising the 6-month follow-up group. The unmatched-CG included 76 patients who provided baseline and follow-up data at the 3-month assessment (3-month group) and 51 patients who responded to the baseline and 6-month follow-up surveys (6-month group). A total of 76 matched patients comprised the matched-IG and the matched-CG for the 3-month follow-up group, and 36 matched pairs were included in the 6-month group.
Demographic characteristics were similar between the unmatched-IG and unmatched-CG in both follow-up groups, with a mean age of 46.3 years for the unmatched-IG at baseline among those in the 3-month follow-up group and 46.8 for those in the 6-month assessment group. Patients in the unmatched-CG at baseline were 45.4 and 43.2 years, respectively, in the 3- and 6-month follow-up groups. The matched-IG and matched-CG were similar to the unmatched-IG and unmatched-CG with respect to age, sex, and geographic location in both follow-up groups (Table 1).
The most frequently prescribed topical formulation at baseline for patients in the unmatched-IG was diclofenac, at 44.4% of those in the 3-month group and 48.1% of the 6-month group. Approximately one-half of patients in the matched-IG were prescribed a diclofenac-based formulation at baseline in both the 3- and 6-month follow-up groups.
An OTC agent was the class of concurrent pain medication that was the most frequently reported at baseline by patients in the 3- and 6-month follow-up groups for unmatched-IG, unmatched-CG, matched-IG, and matched-CG. Use of an opioid alone or in combination with an OTC, a prescription NSAID, or an anticonvulsant at baseline was reported by 19.2% of the unmatched-IG in the 3-month assessment group and 19.6% of those in the 6-month group. Use of an opioid alone or in combination with other medications at baseline was reported by 3.9% and 9.9% of patients in the unmatched-CG in the 3- and 6-month assessment groups, respectively. Use of opioid monotherapy or in combination with either an OTC, prescription NSAID, or anticonvulsant at baseline was reported by 14.5% of the matched-IG and 3.9% of the matched-CG in the 3-month group and 8.4% of the matched-IG and 11.2% of the matched-CG in the 6-month group (Table 1). Patient responses regarding their primary pain complaint at baseline were heterogeneous for the unmatched-IG and unmatched-CG and the matched-IG and matched-CG in the 3- and 6-month assessment groups (Table 2).
Baseline Brief Pain Inventory scores
The overall mean pain severity scores at baseline were similar between the unmatched-IG and unmatched-CG, with a mean score of 4.8 for the unmatched-IG and 4.3 for the unmatched-CG for the 3-month group and similar results for the unmatched-IG (4.7) and unmatched-CG (4.4) for the 6-month assessment group. The overall mean pain interference scores at baseline were 4.5 and 4.2 for the unmatched-IG for the 3- and 6-month groups, respectively, compared to 3.2 and 3.3, respectively, for the unmatched-CG in the 3- and 6-month groups.
Evaluation of the overall pain severity score at baseline revealed a mean score of 4.6 and 4.3, respectively, for the matched-IG and matched-CG in the 3-month assessment group and 4.6 for both the matched-IG and matched-CG in the 6-month follow-up group. Baseline overall mean pain interference scores were 4.3 for the matched-IG and 3.2 for the matched-CG respondents in the 3-month group, changing slightly to 4.0 in the matched-IG and 3.6 in the matched-CG for those in the 6-month assessment group (Table 3).
Change from baseline in the mean pain severity and interference scores within the unmatched-IG
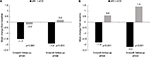
Evaluation of changes in overall mean pain severity and pain interference scores within the unmatched-IG revealed statistically significant decreases from baseline for both the 3- and 6-month follow-up groups (P<0.001 for both groups). The overall mean pain severity score decreased from 4.8 at baseline to 3.3 with a similar decline in the overall mean pain interference score for the 3-month follow-up group (Figure 1A). Statistically significant reductions in pain severity and interference (P<0.001 for both end points) were also evident for the 6-month follow-up group of patients in the unmatched-IG (Figure 1B).
  | Figure 1 Change from baseline in overall mean pain severity and interference scores within the (A) 3- and (B) 6-month unmatched intervention follow-up group. |
Change from baseline in the overall mean pain severity and interference scores between the unmatched and matched 3- and 6-month follow-up groups
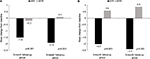
Evaluation of the mean change from baseline in the overall pain severity score in the 3-month follow-up group revealed a decrease of 1.5 in the unmatched-IG compared to a decrease of 0.2 in the unmatched-CG (P<0.001). There was a mean decrease in the overall pain severity score of 1.9 in the unmatched-IG and an increase of 0.2 in the unmatched-CG (P<0.001) for the 6-month group (Figure 2A). The change from baseline in the overall interference score for the 3-month follow-up group was a decrease of 1.8 for the unmatched-IG and an increase of 0.6 (P<0.001) in the unmatched-CG (Figure 2B). Among the 6-month follow-up group, the mean change from baseline in the overall interference score for the unmatched-IG was a decrease of 2.2, which was significantly different (P<0.001) from the mean increase of 1.4 in the unmatched-CG (Figure 2B).
Restricting the analysis to the matched-IG and matched-CG revealed a similar pattern in the mean change from baseline for the 3- and 6-month follow-up groups for overall pain severity scores, with statistically significant differences evident in both groups (P=0.001 for both comparisons; Figure 3A). Similarly, the mean change from baseline in overall pain interference score was a 2-point decrease in the matched-IG and a 0.6 increase in the matched-CG for the 3-month assessment group (P<0.001) and a mean decrease of 2.4 in the matched-IG compared to a mean increase of 0.9 in the matched-CG (P<0.001) for patients in the 6-month group (Figure 3B).
Change from baseline in the use of concurrent pain medications between the unmatched and matched 3- and 6-month follow-up groups
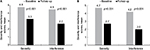
More than one-half (53.6%) of patients in the unmatched-IG reported a de-escalation in the use of concurrent pain medication compared to 4.0% of patients in the unmatched-CG for the 3-month group (P<0.001; Figure 4A). The percentage of de-escalations increased to 60.0% in the unmatched-IG and 5.9% in the unmatched-CG in the 6-month assessment group (P<0.001; Figure 4B). Medication escalation was reported by 10.0% of patients in the unmatched-IG and 52.0% of those in the unmatched-CG in the 3-month group (P<0.001; Figure 4A), which was similar to the changes in the use of concurrent medications in the unmatched-IG and unmatched-CG for the 6-month assessment group (P<0.001; Figure 4B). No change in patient-reported use of concurrent pain medications was reported by 36.4% of the unmatched-IG and 44.0% of those in the unmatched-CG in the 3-month follow-up group (P=0.206; Figure 4A), with similar percentages of patients reporting no change in the 6-month assessment group (P=0.234; Figure 4B).
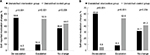  | Figure 4 Percent change from baseline in the use of concurrent pain medications between the (A) 3- and (B) 6-month unmatched intervention and control groups. |
The analysis of changes in the use of concurrent pain medications for the matched-IG and matched-CG revealed similar results, with significantly more patients in matched-IG reporting a de-escalation in the use of concurrent medications and a significantly higher percentage of patients in the matched-CG indicating an escalation in medication use in the 3-month follow-up group (P<0.001; Figure 5A), with similar results for the 6-month assessment (P=0.001; Figure 5B). There were no statistically significant differences in the percentage of patients in the matched-IG and matched-CG who reported no change in medication use at the 3- and 6-month follow-up assessments.
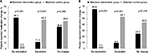  | Figure 5 Percent change from baseline in the use of concurrent pain medications between the (A) 3- and (B) 6-month matched intervention and control groups. |
Safety
Less than 1% (0.5%) of patients in the unmatched-IG reported a skin rash, with no AEs or SAEs reported by clinicians at the participating study sites.
Discussion
This is a report of the final results of the OPERA study, a prospective, non-randomized study of patients with chronic pain who were treated with compounded topical analgesics. Patients reported several types of chronic pain as their primary complaint, including neuropathic, arthritic, and musculoskeletal pain. They also indicated a variety of medical treatments for chronic pain at baseline, including OTC agents, prescription NSAIDs, opioids, anticonvulsants, or combinations of these four classes. Based on the anchors for the BPI-sf pain scores, patients experienced pain that was of moderate severity.
A recent systematic review noted that many trials of topical analgesics relied on a short duration of follow-up to evaluate efficacy and safety.29 In the present study, data were collected from patients at baseline and at 4-week intervals thereafter through 6 months. Changes in the BPI-sf and use of concurrent pain medications at the 3- and 6-month follow-up intervals were evaluated to assess the long-term safety and effectiveness of topical analgesics for the treatment of chronic pain. Sustained evidence of clinical benefit is essential if topical analgesics are used to offer patients and clinicians a safe, effective, feasible, and acceptable treatment for chronic pain.
The study showed that treatment with compounded topical analgesics led to a reduction in mean scores for overall pain severity and interference within the unmatched-IG in the 3- and 6-month assessment groups, with a trend for longer duration of topical therapy to improve overall mean pain severity and interference scores on the BPI-sf. Analysis of the secondary end points demonstrated statistically significant reductions in the mean change from baseline to the 3- and 6-month follow-up groups in overall severity and interference scores between the unmatched-IG and unmatched-CG. Samples of unmatched-IG and unmatched-CG patients were matched on sex, primary pain complaint, and age and were compared to validate study results. The comparison of mean changes from baseline in overall pain severity and interference scores between the matched-IG and matched-CG in the 3- and 6-month assessment groups was consistent with the comparison of the unmatched-IG and unmatched-CG, thereby offering additional support for the pain-relieving effects of compounded topical analgesics.
Importantly, a significantly higher percentage of patients in the unmatched-IG and matched-IG reported de-escalation of their use of concurrent pain medications in both the 3- and 6-month assessment groups, while patients in the unmatched-CG and matched-CG were significantly more likely to report escalation of their use of concurrent medications. The vast majority of patients reported no side effects associated with the topical therapy.
The dramatic increase in opioid use of 1,448% from 1996 to 2011, with increases of 690% from 1996 to 2004 and 100% from 2004 to 2011,32 is considered a major contributing factor in the development of a public health problem characterized by increased demand for opioids and rising rates of misuse, overuse, diversion, abuse, addiction, and death.30–35 Although effective for the relief of pain, opioids and other types of oral analgesics also frequently result in AEs, including potentially fatal respiratory depression, addiction, pruritus, nausea, and constipation.56–60 Oral NSAIDs can also cause serious AEs, including gastrointestinal bleeding, cardiovascular complications, and renal dysfunction or failure.61–66 While topical NSAIDs are associated with a higher incidence of local AEs, the occurrence of gastrointestinal events is significantly lower compared to oral NSAIDs.67
Results from this exploratory study suggest that topical analgesics may provide an effective and safe treatment alternative to opioids and prescription NSAIDs for the management of chronic pain. Topical compounded formulations warrant further evaluation in randomized, controlled, blinded clinical trials to more rigorously evaluate their efficacy and safety for this indication.
Limitations
This was an exploratory study based on a nonprobability sample of patients attending diverse clinical settings for the treatment of chronic pain who consented to participate in this study. The use of a convenience sample consisting of patients who volunteered for study participation is subject to concerns about bias. It was not the primary purpose of this study to identify the optimal agent or compare the efficacy of specific analgesics or combinations of topical agents. These findings are representative of patients who consulted a primary care physician or specialist for the treatment of chronic pain, which may limit the generalizability of the results to the general population of individuals with chronic pain who might not be seeking primary or specialty medical care. However, minimal eligibility criteria were imposed on subject participation to increase the likelihood that patients would be representative of the general population of patients with chronic pain.
There is also the possibility of differences between patients who agreed to undergo treatment with the topical analgesic and those who declined or were not prescribed this therapy. There were differences between the unmatched-IG and unmatched CG in baseline measures of type of primary pain complaint, overall mean pain severity and interference scores and subscales, and self-reported use of concurrent pain medications. Therefore, generalizability of the findings may be limited to those who accepted topical therapies. However, the statistical comparison of the matched-IG with the matched-CG on BPI-sf scores and use of concurrent pain medications yielded results that were similar to those for the unmatched-IG and unmatched-IG. This finding suggests that these results accurately represent changes from baseline in overall pain severity and interference scores and use of concurrent pain medications in both the 3- and 6-month follow-up groups.
Pain complaints and changes in use of concurrent medications were reported by patients rather than based on physician assessment, which may introduce bias in reporting. However, patient questions focused on current or recent circumstances, which was intended to reduce recall bias. Not all patients in the unmatched-IG and unmatched-CG completed the baseline and both follow-up assessments, with some patients completing the baseline and only one of the two follow-up surveys. This lack of consistency in the composition of the unmatched-IG and unmatched-CG could affect the accuracy of reporting these results.
Finally, less than 1% of patients reported a rash that was thought to be caused by the topical agent. This rate of AEs is lower than the frequency of AEs reported in earlier studies evaluating topical analgesics for the treatment of chronic pain.68,69 One possible explanation for our low rate of AEs is that compound manufacturers typically manufacture their products with a goal of being as reactively inert as possible. A randomized controlled trial will more accurately characterize the safety profile of topical analgesics.
Conclusion
These results suggest that the topical analgesics are effective and safe for the relief of moderately severe chronic pain attributed to arthritis, neuropathic conditions, and musculoskeletal disorders. Reductions in the interference of pain with activities of daily living were also noted. Importantly, the topical compounded formulations were associated with reductions in the use of concurrent pain medications, including oral opioid analgesics. Randomized, controlled trials will confirm the efficacy and safety results reported here.
Acknowledgment
Carole Alison Chrvala, PhD, of Health Matters, Inc., is acknowledged for her assistance with writing and editing this manuscript. This study by Clarity Science was funded by Safe Harbor Compliance and Clinical Services, Annie’s Apothecary, Boothwyn Pharmacy, and Cypress Compounding Pharmacy.
Disclosure
Jeffrey A Gudin and Michael J Brennan have received compensation from Clarity Science for their roles as principal investigators and for providing protocol-required services for the study. E Dennis Harris and Peter L Hurwitz are employees of Clarity Science. James D Strader is the CEO of Safe Harbor Compliance and Clinical Services. Derek T Dietze received compensation for study statistical analyses. The authors report no other conflicts of interest in this work.
References
Goldberg DS, McGee SJ. Pain as a global public health priority. BMC Public Health. 2011;11:770. | ||
Harker J, Reid KJ, Bekkering GE, et al. Epidemiology of chronic pain in Denmark and Sweden. Pain Res Treat. 2012;2012:371248. | ||
Langley PC. The prevalence, correlates and treatment of pain in the European Union. Curr Med Res Opin. 2011;27(2):463–480. | ||
Mansfield KE, Sim J, Jordan JL, Jordan KP. A systematic review and meta-analysis of the prevalence of chronic widespread pain in the general population. Pain. 2016;157(1):55–64. | ||
Ohayon MM, Stingl JC. Prevalence and comorbidity of chronic pain in the German general population. J Psychiatr Res. 2012;46(4):444–450. | ||
Reid KJ, Harker J, Bala MM, et al. Epidemiology of chronic non-cancer pain in Europe: narrative review of prevalence, pain treatments and pain impact. Curr Med Res Opin. 2011;27(2):449–462. | ||
Reitsma ML, Tranmer JE, Buchanan DM, Vandenkerkhof EG. The prevalence of chronic pain and pain-related interference in the Canadian population from 1994 to 2008. Chronic Dis Inj Can. 2011;31(4):157–164. | ||
Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9(10):883–891. | ||
Friessem CH, Willweber-Strumpf A, Zenz MW. Chronic pain in primary care. German figures from 1991 and 2006. BMC Public Health. 2009;9:299. | ||
Hasselström J, Liu-Palmgren J, Rasjö-Wrååk G. Prevalence of pain in general practice. Eur J Pain. 2002;6(5):375–385. | ||
Hensler S, Heinemann D, Becker MT, et al. Chronic pain in German general practice. Pain Med. 2009;10(8):1408–1415. | ||
Jordan KP, Kadam UT, Hayward R, Porcheret M, Young C, Croft P. Annual consultation prevalence of regional musculoskeletal problems in primary care: an observational study. BMC Musculoskelet Disord. 2010;11:144. | ||
Lalonde L, Choinière M, Martin E, Berbiche D, Perreault S, Lussier D. Costs of moderate to severe chronic pain in primary care patients - a study of the ACCORD Program. J Pain Res. 2014;7:389–403. | ||
Mills S, Torrance N, Smith BH. Identification and management of chronic pain in primary care: a review. Curr Psychiatry Rep. 2016;18(2):22. | ||
Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16(8):769–780. | ||
Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain. 2006;10(4):287–333. | ||
Breivik H. A major challenge for a generous welfare system: a heavy socio-economic burden of chronic pain conditions in Sweden--and how to meet this challenge. Eur J Pain. 2012;16(2):167–169. | ||
Mäntyselkä PT, Turunen JH, Ahonen RS, Kumpusalo EA. Chronic pain and poor self-rated health. JAMA. 2003;290(18):2435–2442. | ||
Torrance N, Elliott AM, Lee AJ, Smith BH. Severe chronic pain is associated with increased 10-year mortality. a cohort record linkage study. Eur J Pain. 2010;14(4):380–386. | ||
de Sola H, Salazar A, Dueñas M, Ojeda B, Failde I. Nationwide cross-sectional study of the impact of chronic pain on an individual’s employment: relationship with the family and the social support. BMJ Open. 2016;6(12):e012246. | ||
Gerdle B, Björk J, Cöster L, Henriksson K, Henriksson C, Bengtsson A. Prevalence of widespread pain and associations with work status: a population study. BMC Musculoskelet Disord. 2008;9:102. | ||
Liedgens H, Obradovic M, De Courcy J, Holbrook T, Jakubanis R. A burden of illness study for neuropathic pain in Europe. Clinicoecon Outcomes Res. 2016;8:113–126. | ||
Langley P, Müller-Schwefe G, Nicolaou A, Liedgens H, Pergolizzi J, Varrassi G. The impact of pain on labor force participation, absenteeism and presenteeism in the European Union. J Med Econ. 2010;13(4):662–672. | ||
Murray CJ, Atkinson C, Bhalla K, et al; US Burden of Disease Collaborators. The state of US health, 1990-2010: burden of diseases, injuries, and risk factors. JAMA. 2013;310(6):591–608. | ||
Fine PG. Long-term consequences of chronic pain: mounting evidence for pain as a neurological disease and parallels with other chronic disease states. Pain Med. 2011;12(7):996–1004. | ||
Grimby-Ekman A, Gerdle B, Björk J, Larsson B. Comorbidities, intensity, frequency and duration of pain, daily functioning and health care seeking in local, regional, and widespread pain - a descriptive population-based survey (SwePain). BMC Musculoskelet Disord. 2015;16:165. | ||
Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain. 2008;9(2):105–121. | ||
Jackman RP, Purvis JM, Mallett BS. Chronic nonmalignant pain in primary care. Am Fam Physician. 2008;78(10):1155–1162. | ||
Argoff CE, Albrecht P, Irving G, Rice F. Multimodal analgesia for chronic pain: rationale and future directions. Pain Med. 2009;10 Suppl 2:S53–S66. | ||
Volkow ND, McLellan AT. Opioid abuse in chronic pain--misconceptions and mitigation strategies. N Engl J Med. 2016;374(13):1253–1263. | ||
Agarin T, Trescot AM, Agarin A, Lesanics D, Decastro C. Reducing opioid analgesic deaths in America: what health providers can do. Pain Physician. 2015;18(3):E307–E322. | ||
Atluri S, Sudarshan G, Manchikanti L. Assessment of the trends in medical use and misuse of opioid analgesics from 2004 to 2011. Pain Physician. 2014;17(2):E119–E128. | ||
Campbell G, Nielsen S, Larance B, et al. Pharmaceutical opioid use and dependence among people living with chronic pain: associations observed within the pain and opioids in treatment (POINT) cohort. Pain Med. 2015;16(9):1745–1758. | ||
Cheatle MD. Prescription opioid misuse, abuse, morbidity, and mortality: balancing effective pain management and safety. Pain Med. 2015;16 Suppl 1:S3–S8. | ||
Edlund MJ, Martin BC, Russo JE, DeVries A, Braden JB, Sullivan MD. The role of opioid prescription in incident opioid abuse and dependence among individuals with chronic noncancer pain: the role of opioid prescription. Clin J Pain. 2014;30(7):557–564. | ||
Brady KT, McCauley JL, Back SE. Prescription opioid misuse, abuse, and treatment in the United States: an update. Am J Psychiatry. 2016;173(1):18–26. | ||
Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain--United States, 2016. JAMA. 2016;315(15):1624–1645. | ||
U.S. Department of Health and Human Services (HHS), Office of the Surgeon General. Facing Addiction in America: The Surgeon General’s Report on Alcohol, Drugs, and Health. Washington, DC: HHS; 2016. | ||
Interagency Pain Research Coordinating Committee. National Pain Strategy. 2016. Available from: https://iprcc.nih.gov/National_Pain_Strategy/NPS_Main.htm. Accessed March 18, 2016. | ||
Casale R, Symeonidou Z, Bartolo M. Topical treatments for localized neuropathic pain. Curr Pain Headache Rep. 2017;21(3):15. | ||
Flores MP, Castro AP, Nascimento Jdos S. Topical analgesics. Rev Bras Anestesiol. 2012;62(2):244–252. | ||
Haroutiunian S, Drennan DA, Lipman AG. Topical NSAID therapy for musculoskeletal pain. Pain Med. 2010;11(4):535–549. | ||
McCarberg BH, D’Arcy Y. Target pain with topical peripheral analgesics. Nurse Pract. 2007;32(7):44–49. | ||
Sawynok J. Topical and peripherally acting analgesics. Pharmacol Rev. 2003;55(1):1–20. | ||
Cline AE, Turrentine JE. Compounded topical analgesics for chronic pain. Dermatitis. 2016;27(5):263–271. | ||
Peppin JF, Albrecht PJ, Argoff C, et al. Skin matters: a review of topical treatments for chronic pain. Part one: skin physiology and delivery systems. Pain Ther. 2015;4(1):17–32. | ||
Peppin JF, Albrecht PJ, Argoff C, et al. Skin matters: a review of topical treatments for chronic pain. Part two: treatments and applications. Pain Ther. 2015;4(1):33–50. | ||
Massey T, Derry S, Moore RA, McQuay HJ. Topical NSAIDs for acute pain in adults. Cochrane Database Syst Rev. 2010;(6):CD007402. | ||
Mason L, Moore RA, Edwards JE, Derry S, McQuay HJ. Topical NSAIDs for chronic musculoskeletal pain: systematic review and meta-analysis. BMC Musculoskelet Disord. 2004;5:28. | ||
Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. | ||
Erdemoglu AK, Koc R. Brief Pain Inventory score identifying and discriminating neuropathic and nociceptive pain. Acta Neurol Scand. 2013;128(5):351–358. | ||
Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, Cleeland CS. Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. Clin J Pain. 2004;20(5):309–318. | ||
Mendoza T, Mayne T, Rublee D, Cleeland C. Reliability and validity of a modified Brief Pain Inventory short form in patients with osteoarthritis. Eur J Pain. 2006;10(4):353–361. | ||
Tan G, Jensen MP, Thornby JI, Shanti BF. Validation of the Brief Pain Inventory for chronic nonmalignant pain. J Pain. 2004;5(2):133–137. | ||
IBM. SPSS Statistics: V. 23.0. Armonk, NY: IBM Corporation; 2016. | ||
Camilleri M. Opioid-induced constipation: challenges and therapeutic opportunities. Am J Gastroenterol. 2011;106(5):835–842. | ||
Dahan A, Aarts L, Smith TW. Incidence, reversal, and prevention of opioid-induced respiratory depression. Anesthesiology. 2010;112(1):226–238. | ||
Porreca F, Ossipov MH. Nausea and vomiting side effects with opioid analgesics during treatment of chronic pain: mechanisms, implications, and management options. Pain Med. 2009;10(4):654–662. | ||
Roy S, Wang J, Kelschenbach J, Koodie L, Martin J. Modulation of immune function by morphine: implications for susceptibility to infection. J Neuroimmune Pharmacol. 2006;1(1):77–89. | ||
Tey HL, Yosipovitch G. Targeted treatment of pruritus: a look into the future. Br J Dermatol. 2011;165(1):5–17. | ||
Farkouh ME, Greenberg BP. An evidence-based review of the cardiovascular risks of nonsteroidal anti-inflammatory drugs. Am J Cardiol. 2009;103(9):1227–1237. | ||
Harirforoosh S, Jamali F. Renal adverse effects of nonsteroidal anti-inflammatory drugs. Expert Opin Drug Saf. 2009;8(6):669–681. | ||
John R, Herzenberg AM. Renal toxicity of therapeutic drugs. J Clin Pathol. 2009;62(6):505–515. | ||
Lazzaroni M, Porro GB. Management of NSAID-induced gastrointestinal toxicity: focus on proton pump inhibitors. Drugs. 2009;69(1):51–69. | ||
Scarpignato C, Hunt RH. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin North Am. 2010;39(3):433–464. | ||
Trelle S, Reichenbach S, Wandel S, et al. Cardiovascular safety of non-steroidal anti-inflammatory drugs: network meta-analysis. BMJ. 2011;342:c7086. | ||
Derry S, Moore RA, Rabbie R. Topical NSAIDs for chronic musculoskeletal pain in adults. Cochrane Database Syst Rev. 2012;(9): CD007400. | ||
Baer PA, Thomas LM, Shainhouse Z. Treatment of osteoarthritis of the knee with a topical diclofenac solution: a randomised controlled, 6-week trial. BMC Musculoskelet Disord. 2005;6:44. | ||
Bookman AA, Williams KS, Shainhouse JZ. Effect of a topical diclofenac solution for relieving symptoms of primary osteoarthritis of the knee: a randomized controlled trial. CMAJ. 2004;171(4):333–338. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.