Back to Journals » Cancer Management and Research » Volume 11
Arm port vs chest port: a systematic review and meta-analysis
Authors Li G , Zhang Y, Ma H, Zheng J
Received 19 February 2019
Accepted for publication 23 May 2019
Published 3 July 2019 Volume 2019:11 Pages 6099—6112
DOI https://doi.org/10.2147/CMAR.S205988
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Kenan Onel
Guanhua Li,1,* Yu Zhang,2,* Hongmin Ma,3 Junmeng Zheng1
1Department of Cardiovascular Surgery, Sun Yat-sen Memorial Hospital, Sun Yat-sen University, Guangzhou, Guangdong 510120, People’s Republic of China; 2Department of Pathology, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangdong Provincial Hospital of Chinese Medicine, Guangzhou, Guangdong 510120, People’s Republic of China; 3Department of Surgery, Guangzhou Women and Children’s Medical Center, Guangzhou, Guangdong 510623, People’s Republic of China
*These authors contributed equally to this work
Background: Two prevailing, totally implantable venous access ports are routinely utilized in oncology: chest port or arm port. This systematic review with meta-analysis was conducted to compare safety and efficiency of the two techniques.
Methods: We performed evidence acquisition intensively from PubMed, Embase, and Cochrane Library. Available comparative studies that evaluated both techniques were identified. The outcomes of interest included total complication events, procedure-related infections, thrombosis, intra-operative complications, mechanical complications, conversion rate, early port removal, and operating time.
Results: Thirteen comparative studies including 3,896 patients (2,176 for chest ports, and 1,720 for arm ports) were identified. The present study showed that arm port was associated with higher procedure conversion rate (2.51% in chest port group and 8.32% in arm port group; odd ratios [OR] 0.27, 95% confidence interval [CI] 0.15-0.46; p<0.001), but lower incidence of intra-operative complications (1.38% in chest port group and 0.41% in arm port group; OR 2.38, 95% CI 1.07–5.29; p=0.03). There were no between-group differences with respect to total complication events, procedure-related infections, thrombosis, mechanical complications, early port removal, and operating time. Subgroup analysis of patients under 60 years revealed that no significant difference was detected in intra-operative events (1.19% in chest port group and 0.02% in arm port group, OR 2.59, 95% CI 0.74–9.08; p<0.14), indicating that age may be a risk factor for intra-operative events. Sensitivity analysis did not change conclusions of all endpoints of interest.
Conclusion: Arm port is associated with higher procedure conversion rate, but lower incidence of intra-operative complications, and age may be a risk factor for intra-operative events.
Keywords: chest port, arm port, total implantable venous access port, systematic review, meta-analysis
Introduction
Totally implantable venous access ports (TIVAPs), a long-term indwelling infusion system, was first introduced by Niederhuber in 1982.1 With the increasing use of chemotherapeutic drugs in oncologic patients, TIVAP gains its popularity due to the fact that this system enables long-term administration of chemotherapeutic agents, attenuates the burden of chemotherapy, and thus greatly improves quality of life in oncologic patients, meanwhile, this system preserves peripheral vessels and prevents venous-related infections.2–4 Two prevailing approaches are routinely utilized in oncology: chest ports or arm ports. Chest ports are most frequently implanted under the guidence of ultrasonography, via internal jugular vein, external jungular vein or subclavian vein puncture, while arm ports are placed through forearm veins such as basilic vein, cephalic vein or brachial vein, either through percutaneous puncture or surgical cut-down, both generally showing satisfactory results with respect to technical success and low incidence of postprocedural complications.5,6
For years, the chest has been the most popular and reliable access site of implantation for long-term indwelling ports, however, chest ports might not be eligible for patients undergoing radiotherapy with chest wall involvement, or those with chest wall skin lesions, meanwhile, many female patients experience perception of “foreign body”, “bra inconvenience”, and discomfort when wearing safety belt or bag strap. In 1993, an alternative arm port procedure was introduced by surgeons at the School of Medicine, Yale University.7 The arm ports have some unique advantages compared with chest ports, such as no risk for hemothorax or pneumothorax, lower incidence of arterial puncture,8 better cosmetic outcome,9 and more “bra convenience”.10 These advantages are especially beneficial to breast cancer patients requiring radiotherapy, flap transferring for reconstructive surgeries, as well as those patients with radiodermatitis or compromised respiratory function.11 Nevertheless, the most significant disadvantage of arm port implantation is high incidence of failure (technically unsuccessful port placement), which is around 6.4%.12,13
Despite the wide application of TIVAPs, there is no consensus whether one or the other access site of implantation for TIVAP is clinically superior. Although several studies that compared chest access and arm access had been reported by various institutions, some of the results are conflicting and within small population of patients.14–16 In the present study, we attempted to investigate and assemble the most comprehensive clinical data currently available in the literature to address a debatable issue: which approach, the arm port or the chest port, is more clinically beneficial to oncologic patients for the implantation of TIVAPs?
Evidence acquisition
Clinical data search strategy
The present study was carried out according to the Meta-analysis Of Observational Studies in Epidemiology (MOOSE) recommendations and the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) protocol.17,18 Systematic review registration was not available. Literature search was executed in August 2018, regardless of article types, regions or language of publication. Eligible studies were primarily from on-line databases of PubMed, Embase, and the Cochrane Library. Medical Subject Headings (MeSH) terms, as well as their combinations were manually searched via (Title/Abstract) as follows: basilic/cephalic/brachial/subclavian/jugular AND totally implantable venous access port/totally implantable venous access device/venous port/indwelling port, with “Related Articles” function applied to expand the search results. Corresponding author was contacted if additional information was required. When the same group of authors published multiple but similar reports of studies, the most comprehensive or the most recent result was taken into consideration.
Inclusion and exclusion criteria
Inclusion criteria: 1) patients: patients who were diagnosed with malignancies requiring long-term indwelling ports, or those with gastrointestinal disorders requiring long-term venous port for parenteral nutrition support; 2) intervention: arm ports (basilic/cephalic/brachial vein insertion) vs chest ports (subclavian or jugular vein insertion), regardless of implantation methods, including surgical cut-down (peripheral vein cut-down method through surgical approach) and percutaneous puncture (radiology-guided placement, ultrasound-guided implantation, fluoroscopy-guided insertion, direct puncture, and so forth); 3) study types: all randomized controlled trials (RCTs), comparative studies available in the literature including cohort and case control studies which compared arm ports and chest ports were included; 4) outcomes: studies quantitatively reporting at least one of the outcomes described in the next section of this paper were included.
Exclusion criteria: 1) letters to the editor, editorials, reviews, case reports, laboratory-based animal studies, non-clinical studies, conference abstracts, and meta-analysis papers; 2) studies that failed to clearly clarify the outcomes of interest were excluded. If multiple access sites of implantation were compared in a single study (cephalic, jugular and subclavian vein), results of chest port/arm port category were combined.
Data extraction
Two independent authors (YZ, GL) conducted data extraction. Any discrepancy was resolved by discussion and consultation with senior authors in this paper (HM and JZ). Data on clinical characteristics (age, diagnosis), type of port, and technique used (approach of insertion) were collected. Port-related complications were classified into four categories: intra-operative complications, infectious complications, thrombotic complications, and mechanical complications.19,20
The primary outcomes of interest were the incidences of port-related complications from implantation to removal of TIVAP. Intra-operative complications included arterial puncture, hemothorax, and pneumothorax. Procedure-related infections, composed of port-site infection and catheter-related bloodstream infection, were diagnosed in accordance with the latest guideline of the Infectious Diseases Society of America.21 Procedure-related thrombotic complications were identified as any mural thrombi formed from the port catheter to the vessel lumen, giving rise to catheter occlusion, regardless of the presence of symptoms.22 On the basis of Clavien-Dindo classification of surgical complications,23 mechanical complications included port misfunctioning (such as deficiency of infusion or catheter occlusion), catheter fracture (defined as physical damage to the catheter of the port system), port dislocation (also named catheter migration or malposition), pinch-off syndrome, and hemorrhage. The second outcomes of interest were conversion to other access site of placement, early port removal (port removal before completion of treatment), and operating duration.
Quality assessment
Evaluation of RCT quality was subject to the Cochrane Collaboration’s tool, through which risk of bias was assessed based on Cochrane Handbook for Systematic Reviews of Interventions.24 Judgment of overall risk of bias for each included RCT was assessed as: low, moderate, or high.24 RCTs with low risk of bias were regarded as high quality. Quality of observational study was assessed according to the Newcastle-Ottawa scale (NOS), consisting of three aspects: patient selection, comparability of the study groups, and assessment of outcome.25 A quality score ≥8 points was regarded as high quality study.
Statistical analysis
Review Manager 5.0 (Cochrane Collaboration, Oxford, UK) was used for this systematic review with meta-analysis. Weighted mean difference (WMD) was used to compare continuous variables, while odd ratio (OR) was employed to compare dichotomous variables. Ninety-five percent confidence interval (CI) was applied to all data throughout this study. When confronting with continuous variable expressed as mean and range value, standard deviation (SD) was converted as suggested by Hozo et al.26
Chi-squared test was used for the evaluation of statistical heterogeneity between studies, and significance level was set at p=0.10. For quantitative assessment of heterogeneity, I2 statistic was used, and high heterogeneity level was set at 75%.27 The random-effects model was applied if between-study heterogeneity was high, in order to make more conservative estimates,28 otherwise, the fixed-effects model was employed.24
Subgroup analyses were carried out for the outcomes of intra-operative complications, thrombotic complications, infectious complications, and mechanical complications. Available data were stratified based on patient’s age, method of placement (puncture or surgical cut-down), whether antibiotic or thromboprophylaxis was applied, so as to investigate whether these factors influenced the outcomes. Sensitivity analysis was performed and only outcomes with at least two studies were included. We performed sensitivity analysis for high quality studies (RCT with low risk of bias and non-randomized studies with quality score ≥8 points). We also used funnel plots to screen potential publication bias. A two-tailed p-value less than 0.05 was considered as statistical significance, except otherwise specified.
Results
A total of 914 possibly eligible studies were identified from databases (Figure 1). Thirteen studies including 3,896 cases (2,176 for chest ports, and 1,720 for arm ports) fulfilled the inclusion standard and were selected for final evidence synthesis. Agreement with respect to study selection between the two reviewers was 92%.
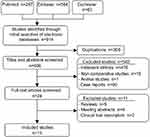 |
Figure 1 Flow diagram of study selection process. |
Characteristics of included studies
Table 1 depicts the characteristics of included studies in this study. Of these included studies, two29,30 were RCTs, and two10,31 were prospective non-randomized cohort studies. The remaining studies32-40 were retrospective. As for indications, the majority of included studies were about cancer patients with upcoming chemotherapies, and most of the reported studies used commercial venous port devices (BardPort, PowerPort, P-U Celsite, BraunMedical, and Meditec-R-Port). For port implantation, the majority of studies used percutaneous puncture approach, while there were three studies31,37,38 and four studies29,31,39 which used direct surgical cut-down methods to implant chest port devices and arm port devices, respectively.
 |
Table 1 Characteristics of included studies |
Based on Cochrane Handbook for Systematic Reviews of Interventions,24 Table 2 summarizes the risk of bias of two included RCTs, one RCT was considered as moderate risk, while the other p< was regarded as low risk. In Table 3, risk of bias analysis of the remaining included studies was summarized in line with the NOS.25 Among these non-randomized studies, two32,39 had a quality score below seven, and the remaining studies were considered as high-quality.
 |
Table 2 Risk of bias of included randomized controlled trials based on Cochrane Handbook for Systematic Reviews of Interventions |
 |
Table 3 Risk of bias for included non-randomized studies based on Newcastle-Ottawa scale |
Primary outcomes
The pooled data from 13 studies10,29–40 that evaluated primary outcomes in 3,896 patients (Figure 2) revealed no significant differences for total complication events between the chest port and arm port groups (11.8% and 12.8%; OR 0.94, 95% CI 0.77–1.15; p=0.53). Eleven studies in which 3,266 patients were included thoroughly reported the incidences of intra-operative complications, thrombotic complications, infectious complications, and mechanical complications. For procedure-related infections, no statistically significant differences were observed between the chest port and arm port groups (3.15% and 3.37%; OR 1.11, 95% CI 0.74–1.66; p=0.63). Differences for procedure-related thrombotic complications and mechanical complications were also absent between the two groups (2.87% and 3.91%; OR 0.75, 95% CI 0.49–1.13; p=0.17; and 4.86% and 2.96%; OR 1.34, 95% CI 0.92–1.95; p=0.13, respectively). However, when comparing the difference for intra-operative complications, the incidence in chest port group was significantly higher (1.38% and 0.41%; OR 2.38, 95% CI 1.07–5.29; p=0.03) (Figure 3).
 |
Figure 2 Forest plot and meta-analysis of total complication events. Abbreviation: M-H, Mantel-Haenszel method. |
 |
Figure 3 Forest plot and meta-analysis of primary outcomes. Abbreviation: M-H, Mantel-Haenszel method. |
Secondary outcomes
Data on procedure conversion rate were obtained in six studies,26,29,31,38–40 which assessed 1,314 and 914 patients in chest port group and arm port group, respectively. The procedure conversion rate was significantly higher in arm port group than that in chest port group (2.51% in chest port group and 8.32% in arm port group; OR 0.27, 95% CI 0.15–0.46; p<0.001) (Figure 4A). When incidences of early port removal were analyzed, there were three studies29,36,39 including 1,189 patients evaluated, and no significant difference was present between groups (7.29% and 4.37%; OR 1.27, 95% CI 0.26–6.19; p=0.77) (Figure 4A). As for operating time, three studies including 707 patients were reported, however, one study30 presented operating time as “mean ± standard deviation”, one study39 presented as “mean and range”, and the last one31 displayed as “mean and interquartile range”. Using Hozo’s26 method, ranges were converted to estimated SD for further comparison.41 Result of the analysis of operating time revealed that there were no statistically significant differences between chest port group and arm port group (WMD −4.31; 95% CI −17.81–9.19; p=0.53), with high between-study heterogeneity (I2=0.98, p<0.01) (Figure 4B).
Subgroup analysis
In subgroup analysis of antibiotic prophylaxis, there were no statistically significant differences with respect to infection rate in comparison of the previous results, suggesting that prophylactic administration of antibiotics did not interfere with procedure-related infection rate (Table 4). Three studies29,35,38 reported the anticoagulant profiles, and results showed that thromboprophylaxis did not influence the risk for thrombosis either. In the subgroup analysis of insertion methods, only one study31 used surgical cut-down approach in all patients, and six studies10,33–36,40 employed puncture approach for all patients. The results did not change the conclusions for the primary outcomes from original analyses, and pooled data from six studies including 1,781 patients who underwent percutaneous puncture approach indicated consistent results showing that the incidences of intra-operative events in chest port group were significantly higher (2.05% and 0.12%, OR 7.87, 95% CI 1.82–34.13; p<0.01) (Table 4). Finally, two subgroups were stratified according to patients’ age group (<60 years old and ≥60 years old). After pooling data from eight studies including 2,063 patients less than 60 years old, the result showed that no significant difference was detected with regard to intra-operative events between chest ports and arm ports (1.19% and 0.02%, OR 2.59, 95% CI 0.74–9.08; p<0.14), suggesting that age may be a risk factor for intra-operative events (Table 4).
 |
Table 4 Subgroup analysis comparing chest ports and arm ports |
Sensitivity analysis and publication bias
One low risk RCT29 and six non-randomized comparative studies10,33,35–38 that scored over eight points according to NOS were included in the sensitivity analysis. The results of sensitivity analysis did not change the conclusions for total complication events, all of the primary outcomes, and incidence of procedure conversion from original analyses. However, between-study heterogeneity decreased slightly in all of these parameters (Table 5). Due to insufficient data, sensitivity analyses for early port removal and operating time were not performed.
 |
Table 5 Sensitivity analysis comparing chest ports and arm ports |
Figure 5 depicts a funnel plot of included studies that assessed primary outcomes of interest. The distribution was even and around the vertical. Most of the studies were within the 95% CIs, suggesting no apparent publication bias.
 |
Figure 5 Funnel plot demonstrating meta-analysis of primary outcomes of interest. Abbreviation: SE, standard error. |
Discussion
This systematic review with meta-analysis compared the clinical efficacy of two types of TIVAPs: chest port and arm port. Our results showed that arm port is a safe site for implantation, with lower incidence of intra-operative complications, however, arm port is associated with higher procedure conversion rate. No statistically significant differences between chest port and arm port were found in total complication events, procedure-related infections, procedure-related thrombotic complications, incidence of early port removal, and operating duration. On subgroup analysis, we found that antibiotic prophylaxis and method of insertion did not interfere with the conclusions for the primary outcomes from original analyses, but results revealed that there were no statistically significant differences in intra-operative events in those patients under 60, suggesting that age may affect the risk for intra-operative complications. Sensitivity analysis did not change the conclusions of all endpoints of interest.
For the utilization of a new technique, patients’ safety is always the first priority. Chest ports are still popular and their application is continuously expanding among non-surgical physicians.15 Under the guidance of ultrasound or fluoroscopy, the risks for intra-operative events, such as pneumothorax, hemothorax, and arterial injury, are therefore minimized during percutaneous cannulations of subclavian vein or jugular vein.42 Implantation of arm ports through peripheral forearm vessels is more surgically challenging, but arm port users experience less perception of “foreign body”, with satisfactory cosmetic results. Pooled data from 2,176 chest port and 1,720 arm port users from included studies of this study, showed that incidences for total complication events were 11.8% and 12.8%, respectively. Our result of total complication events is in agreement with previous studies that assessed overall complication rate,4,19,43 with no significant difference between chest ports and arm ports. Although the technique of implanting arm ports is newly developed, and more challenging for clinicians, our findings indicate that arm ports are at least as safe as conventional chest ports with proper patient indications.
The procedure conversion rate is significantly higher for arm ports as compared to chest ports. Additional challenges for arm port implantation include anatomic variations, longer distance for implantation, and frequent use of peripheral intravenous access, which make cannulation of peripheral forearm vessels not always achievable. Anatomic variations of basilic, cephalic or brachial vein frequently occur, and these peripheral vessels are much smaller in diameter as compared with central veins. These peripheral forearm vessels may be difficult to recognize or too limited in size to use.44 Therapeutic usage of peripheral venous access is frequent for oncologic and critically-ill patients, however, frequent puncture of these vessels leads to adhesion, spasm, or shrinkage, and thus complicates anatomy of surrounding structures. Moreover, patients with conversion might reveal higher risks for other complications. As implantation of indwelling TIVAPs is performed not only by surgeons, but also widely performed by non-surgical practitioners (such as trained physicians, radiologists, anesthesiologists, oncologists, and so forth), arm port implantation may need to be placed in selected patients to avoid procedure conversion. In our experience, no matter how experienced the clinician is in arm port implantation, preoperative ultrasound evaluation of forearm vessels is indicated in every case to minimize this unfavorable event.
Our findings showed that chest port was associated with higher incidence of intra-operative complications. Due to specific anatomy, accidental pneumothorax or arterial puncture could not be eliminated completely, even if ultrasound or fluoroscopy guidance is routinely available. But interestingly, in subgroup analysis, no between-group differences in intra-operative events were seen in those patients under 60. Patients’ age appeared to be relevant to the risk for intra-operative events, although it is still poorly illustrated. Similar results were previously reported: high age is an independent risk factor for procedural complications in subclavian vein puncture, and one implication is closely related to variations in body habitus.45,46 Based on our experiences, our explanation is that vessel fragility and decreased vessel elasticity may be responsible for this issue, as the elderly are prone to compromised vascular compliance, which makes the aged population more susceptible to vascular interference and trauma. Another potential reason is higher morbidities of chronic obstructive pulmonary diseases and obesity that attribute to structural elevation of apex pulmonis, thereby facilitating the occurrence of pneumothorax.46 Certainly, special caution in terms of intra-operative complications in aged patients is alerted when performing implantation of chest ports.
The pooled analyses of procedure-related infections, thrombolytic complications, and mechanical complications showed no between-group differences. Subgroup analysis of antibiotic prophylaxis demonstrated that administration of antibiotics did not affect the outcome of overall infection rate. But we still recommend that anti-septic protocols should be followed for the sake of infection prevention and management in oncologic patients who are vulnerable to infection owing to leukocytopenia induced by immune suppression.47 Subgroup analysis of thromboprophylaxis did not alter any original conclusion, which was consistent with Chaukiyal’s result48 showing that thromboprophylaxis had no beneficial effects on the risks of catheter-related thrombolytic complications in oncologic patients. Nonetheless, as regular heparin flushing has become routine practice for TIVAPs in almost every institution,49 it seems to be enough for the prevention of port-related thrombosis.50
Two methods of implanting TIVAPs are used in current clinical practice: surgical cut-down or percutaneous puncture. No consensus has ever been reached regarding implantation methods because percutaneous puncture is less time-consuming, but surgical cut-down method lowers the risk for complications such as pneumothorax.31 With the worldwide spread of ultrasound monitoring for venous access, venous surgical cut-down method is on the decrease.31 No significant difference in terms of insertion approach could be addressed in this study. Of the three included studies in which operating time was reported, detailed procedure description was not available in one study,30 one study31 inserted TIVAPs through surgical cut-down, and the last39 separately used surgical approach and puncture to cannulate arm ports and chest ports, respectively. Because of the heterogeneous characteristic and data insufficiency, it is difficult to reach a definite conclusion for the comparison of inserting methods in this instance.
Arm port implantation is especially attractive to young females for cosmetic reasons, as it leaves no scars on the chest. Arm port users experience less “bra inconvenience”, and psychologically, patients’ anxiety of indwelling arm ports might be less as compared to chest ports.40 Moreover, arm port is especially appropriate for patients who require neck or chest radiation therapies.40 Comparison of these quality-of-life related outcomes is of importance, however, only a limited number of studies reported these parameters, which were measured based on various evaluating methods, such as self-administered questionnaire10,36 and quality of life score,37,46 and non-uniformity of these measurements made these data incomparable in this study.
Our study is distinctive and provides valuable information to oncology-related clinicians in that, to the best of our knowledge, this systematic review with meta-analysis is the first one to comprehensively investigate the clinical efficacies and represents the most up-to-date information with respect to chest ports and arm ports. We used multiple strategies to launch evidence acquisition, strict standards to assess quality of included studies, scientific statistics for data analysis, subgroup and sensitivity analysis to control study heterogeneity. Our study has several limitations which should be taken into consideration. First of all, the most apparent limitation of the present study is that most of the included data were from retrospective cohort studies. Inadequacy of randomization and lack of blinding will increase the risk of bias. Secondly, this study was based on the premised assumption that demographic subgroups (age, sex, race, nationality, and disease distribution) were similar enough for comparison. However, subgroup analysis with regard to age generated some different outcomes, hence, further systematic reviews should be conducted when sufficient future results are released. And then, the included studies were from different institutions with various levels of clinician expertise. Not only the between-study time-span, which is more than 10 years, but also the different backgrounds of the TIVAP operators with different insertion approaches will affect the outcomes of interest. Next, some outcomes of interest, especially the mechanical complications, might not be clearly defined in some studies, yielding misinterpretation of results or data omission. So, the incidence of mechanical complications might be underrated, and caution should be taken when interpreting results of this section. Finally, follow-up period of included studies was generally short. But there are increasing cumulative risks for TIVAP-related infection, thrombosis, and mechanical mis-functioning along with the duration of port indwelling, some incidences of outcomes might be underestimated because of insufficient follow-up period. Surely, long-term outcomes of arm ports, especially for oncologic efficacy and safety, require further investigation.
Conclusion
In this systematic review with meta-analysis that compared clinical efficacy of two types of TIVAPs: chest port and arm port, we found that arm port is associated with higher procedure conversion rate, but lower incidence of intra-operative complications. There are no statistically significant differences between chest port and arm port with regard to in total complication events, procedure-related infections, procedure-related thrombotic complications, incidence of early port removal, and operating duration. Age may be a risk factor for intra-operative complications. Further confirmation of these results is warranted and our findings should be updated with larger scale and well-designed studies with longer follow-up.
Acknowledgment
This study received no specific grant from any funding agency.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Niederhuber JE, Ensminger W, Gyves JW, Liepman M, Doan K, Cozzi E. Totally implanted venous and arterial access system to replace external catheters in cancer treatment. Surgery. 1982;92:706–712.
2. Bow EJ, Kilpatrick MG, Clinch JJ. Totally implantable venous access ports systems for patients receiving chemotherapy for solid tissue malignancies: a randomized controlled trial examining the safety, efficacy, costs, and impact on quality of life. J Clin Oncol. 1999;17:1267. doi:10.1200/JCO.1999.17.4.1267
3. Otsubo R, Hatachi T, Shibata K, et al. Evaluation of totally implantable venous access devices with the cephalic vein cut-down approach: usefulness of preoperative ultrasonography. J Surg Oncol. 2016;113:114–119. doi:10.1002/jso.24100
4. Cavallaro G, Iorio O, Iossa A, et al. Surgical approach for totally implantable venous access devices (TIVADs). Consideration after 753 consecutive procedures. Am Surg. 2014;80:513–515.
5. Kawamura J, Nagayama S, Nomura A, et al. Long-term outcomes of peripheral arm ports implanted in patients with colorectal cancer. Int J Clin Oncol. 2008;13:349–354. doi:10.1007/s10147-008-0766-2
6. Goltz JP, Scholl A, Ritter CO, Wittenberg G, Hahn D, Kickuth R. Peripherally placed totally implantable venous-access port systems of the forearm: clinical experience in 763 consecutive patients. Cardiovasc Intervent Radiol. 2010;33:1159–1167. doi:10.1007/s00270-010-9854-6
7. Salem RR, Ward BA, Ravikumar TS. A new peripherally implanted subcutaneous permanent central venous access device for patients requiring chemotherapy. J Clin Oncol. 1993;11:2181–2185. doi:10.1200/JCO.1993.11.11.2181
8. Shiono M, Takahashi S, Takahashi M, Yamaguchi T, Ishioka C. Current situation regarding central venous port implantation procedures and complications a questionnaire based survey of 11693 implantations in Japan. Int J Clin Oncol. 2016;216:1172–1182. doi:10.1007/s10147-016-1003-z
9. Angelo FAD, Ramacciato G, Aurello P, et al. Alternative insertion sites for permanent central venous access devices. Eur J Surg Oncol. 1997;23(6):547–549.
10. Goltz JP, Petritsch B, Kirchner J, Hahn D, Kickuth R. Percutaneous image guided implantation of totally implantable venous access ports in the forearm or the chest? A patients point of view. Support Care Cancer. 2013;21(2):505–510. doi:10.1007/s00520-012-1544-2
11. Klösges L, Meyer C, Boschewitz J, et al. Long term outcome of peripherally implanted venous access ports in the forearm in female cancer patients. Cardiovasc Intervent Radiol. 2015;38(3):657–664. doi:10.1007/s00270-014-0975-1
12. Goltz JP, Scholl A, Ritter CO, Wittenberg G, Hahn D, Kickuth R. Peripherally placed totally implantable venous-access port systems of the forearm: clinicalexperience in 763 consecutive patients. Cardiovasc Intervent Radiol. 2010;33(6):1159–1167. doi:10.1007/s00270-010-9854-6
13. Marcy PY, Magné N, Castadot P, et al. Is radiologic placement of an arm port mandatory in oncology patients? Analysis of a large bi-institutional experience. Cancer. 2007;110(10):2331–2338. doi:10.1002/cncr.23040
14. Lin CH, Yu JC, Lee YT, et al. Conversion from cephalic vein to external jugular vein: success rate increased on totally implantable venous access ports with cut-down technique. Surg Innov. 2013;20:566–569. doi:10.1177/1553350613479178
15. Di Carlo I, Pulvirenti E, Mannino M, Toro A. Increased use of percutaneous technique for totally implantable venous access devices. Is it real progress? A 27-year comprehensive review on early complications. Ann Surg Oncol. 2010;17:1649–1656. doi:10.1245/s10434-010-1005-4
16. Di Carlo I, Barbagallo F, Toro A, et al. External jugular vein cutdown approach, as a useful alternative, supports the choice of the cephalic vein for totally implantable access device placement. Ann Surg Oncol. 2005;12:1–4. doi:10.1245/ASO.2005.10.907
17. Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of Observational Studies in Epidemiology (MOOSE) group. Jama. 2000;283:2008–2012.
18. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65–W94.
19. McGee DC, Gould MK. Preventing complications of central venous catheterization. N Engl J Med. 2003;348:1123–1133. doi:10.1056/NEJMra011883
20. Jung P, Ryu H, Jung JH, et al. Complications of central venous totally implantable access port: internal jugular versus subclavian access. Korean J Crit Care Med. 2015;30:13–17.
21. Mermel LA, Allon M, Bouza E, et al. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;49:1–45. doi:10.1086/599376
22. Debourdeau P, Kassab Chahmi D, Le Gal G, et al. 2008 SOR guidelines for the prevention and treatment of thrombosis associated with central venous catheters in patients with cancer: report from the working group. Ann Oncol. 2009;20:1459–1471. doi:10.1093/annonc/mdp052
23. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi:10.1097/01.sla.0000133083.54934.ae
24. Higgins JPT, Green S(editors). Cochrane handbook for systematic reviews of interventions version 5.1.0. The Cochrane Collaboration 2011. Available from: http://www.cochrane-handbook.org/.
25. Wells G, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. Ottawa Hospital Research Institute; 2010. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp. Accessed October 15, 2018.
26. Hozo SP, Djulbegovic B, Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi:10.1186/1471-2288-5-27
27. Olivo SA, Macedo LG, Gadotti IC, Fuentes J, Stanton T, Magee DJ. Scales to assess the quality of randomized controlled trials: a systematic review. Phys Ther. 2008;88:156–175. doi:10.2522/ptj.20070147
28. Wu SY, Ling Q, Cao LH, et al. Real-time two-dimensional ultrasound guidance for central venous cannulation: a meta-analysis. Anesthesiology. 2013;118:361–375. doi:10.1097/ALN.0b013e31827bd172
29. Biffi R, Orsi F, Pozzi S, et al. Best choice of central venous insertion site for the prevention of catheter-related complications in adult patients who need cancer therapy a randomized trial. Ann Oncol. 2009;20(5):935–940. doi:10.1093/annonc/mdn701
30. D’Angelo FA, Ramacciato G, Aurello P, et al. Prospective randomised study of cephalic vein cut-down versus subclavian vein puncture technique in the implantation of subcutaneous venous access devices. Chir Ital. 2002;54(4):495–500.
31. Iorio O, Gazzanelli S, D’Ermo G, et al. A prospective comparative evaluation on totally implantable venous access devices by external jugular vein versus cephalic vein cutdown. Am Surg. 2018;84(6):841–843.
32. Alahyane A, Bounaim A, El Fahssi M. Complications of implanted venous sites for chemotherapy. J Afr Du Cancer. 2010;2(4):240–244. doi:10.1007/s12558-010-0115-z
33. Akahane A, Sone M, Ehara S, Kato K, Tanaka R, Nakasato T. Subclavian vein versus arm vein for totally implantable central venous port for patients with head and neck cancer a retrospective comparative analysis. Cardiovasc Intervent Radiol. 2011;34(6):1222–1229. doi:10.1007/s00270-010-0051-4
34. Goltz JP, Noack C, Petritsch B, Kirchner J, Hahn D, Kickuth R. Totally implantable venous power ports of the forearm and the chest: initial clinical experiencewith port devices approved for high-pressure injections. Br J Radiol. 2012;85(1019):e966–e972. doi:10.1259/bjr/33224341
35. Kuriakose P, Colon-Otero G, Paz-Fumagalli R. Risk of deep venous thrombosis associated with chest versus arm central venous subcutaneous port catheters a 5-year single-institution retrospective study. J Vasc Interv Radiol. 2002;13:179–184.
36. Li Y, Cai Y, Gan X, et al. Application and comparison of different implanted ports in malignant tumor patients. World J Surg Oncol. 2016;14(1):251. doi:10.1186/s12957-016-1002-6
37. Marcy PY, Magné N, Castadot P, et al. Radiological and surgical placement of port devices a 4-year institutional analysis of procedure performance, quality of life and cost in breast cancer patients. Breast Cancer Res Treat. 2005;92(1):61–67. doi:10.1007/s10549-005-1711-y
38. Marcy PY, Chamorey E, Amoretti N, et al. A comparison between distal and proximal port device insertion in head and neck cancer. Eur J Surg Oncol. 2008;34(11):1262–1269. doi:10.1016/j.ejso.2007.09.011
39. Matiotti-Neto M, Eskander MF, Tabatabaie O, et al. Percutaneous versus cut-down technique for indwelling port placement. Am Surg. 2017;83(12):1336–1342.
40. Shiono M, Takahashi S, Kakudo Y, et al. Upper arm central venous port implantation A 6-year single institutional retrospective analysis and pictorial essay of procedures for insertion. PLoS One. 2014;9(3):e91335. doi:10.1371/journal.pone.0091335
41. Wan X, Wang W, Liu J, et al. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res Methodol. 2014;14:135. doi:10.1186/1471-2288-14-135
42. Orci LA, Meier RP, Morel P, et al. Systematic review and meta-analysis of percutaneous subclavian vein puncture versus surgical venous cutdown for the insertion of a totally implantable venous access device. Br J Surg. 2014;101:8–16. doi:10.1002/bjs.9276
43. Knebel P, Fischer L, Huesing J, Hennes R, Büchler MW, Seiler CM. Randomized clinical trial of a modified Seldinger technique for open central venous cannulation for implantable access devices. Br J Surg. 2009;96:159–165. doi:10.1002/bjs.6457
44. Koketsu S, Samesima S, Yoneyama S, et al. Outcome of cephalic vein cut-down approach: a safe and feasible approach for totally implantable venous access device placement. Oncol Lett. 2010;1(6):1029–1031. doi:10.3892/ol.2010.189
45. Lefrant JY, Muller L, De La Coussaye JE, et al. Risk factors of failure and immediate complication of subclavian vein catheterization in critically ill patients. Intensive Care Med. 2002;28(8):1036–1041. doi:10.1007/s00134-002-1364-9
46. Marcy PY, Schiappa R, Ferrero JM, et al. Patient satisfaction and acceptance of their totally implanted central venous catheter: a French prospective multicenter study. J Vasc Access. 2017;18(5):390–395. doi:10.5301/jva.5000744
47. Pronovost P, Needham D, Berenholtz S, et al. An intervention to decrease catheter-related bloodstream infections in the ICU. N Engl J Med. 2006;355:2725–2732. doi:10.1056/NEJMoa061115
48. Chaukiyal P, Nautiyal A, Radhakrishnan S, et al. Thromboprophylaxis in cancer patients with central venous catheters. A systematic review and meta-analysis. Thromb Haemost. 2008;99:38–43. doi:10.1160/TH07-07-0446
49. Debourdeau P, Farge D, Beckers M, et al. International clinical practice guidelines for the treatment and prophylaxis of thrombosis associated with central venous catheters in patients with cancer. Thromb Haemost. 2013;11:71–80. doi:10.1111/jth.12071
50. Kefeli U, Dane F, Yumuk PF, et al. Prolonged interval in prophylactic heparin flushing for maintenance of subcutaneous implanted port care in patients with cancer. Eur J Cancer Care (Engl). 2009;18:191–194. doi:10.1111/j.1365-2354.2008.00973.x
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

