Back to Journals » OncoTargets and Therapy » Volume 11
Application of weighted gene co-expression network analysis to identify key modules and hub genes in oral squamous cell carcinoma tumorigenesis
Authors Zhang X , Feng H, Li Z, Li D, Liu S , Huang H, Li M
Received 20 April 2018
Accepted for publication 11 July 2018
Published 19 September 2018 Volume 2018:11 Pages 6001—6021
DOI https://doi.org/10.2147/OTT.S171791
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Carlos E Vigil
Xiaoqi Zhang,* Hao Feng,* Ziyu Li, Dongfang Li, Shanshan Liu, Haiyun Huang, Minqi Li
Department of Bone Metabolism, School of Stomatology, Shandong University, Shandong Provincial Key Laboratory of Oral Tissue Regeneration, Jinan, China
*These authors contributed equally to this work
Purpose: Oral squamous cell carcinoma (OSCC) is one of the most common malignant diseases worldwide, yet its molecular mechanisms are largely unknown. We aimed to construct gene co-expression networks to identify key modules and hub genes involved in the pathogenesis of OSCC.
Patients and methods: We used dataset GSE30784 to construct co-expression networks by weighted gene co-expression network analysis (WGCNA). Gene Ontology (GO) and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analyses were performed by Database for Annotation, Visualization and Integrated Discovery (DAVID). Hub genes were screened and validated by other datasets.
Results: Turquoise and brown modules were found to be the most significantly related to tumorigenesis. Functional enrichment analysis showed that the turquoise module was associated with cell–cell adhesion, extracellular matrix and collagen catabolic process. A total of 10 hub genes (MMP1, TNFRSF12A, PLAU, FSCN1, PDPN, KRT78, EVPL, GGT6, SMIM5 and CYSRT1) were identified and validated at transcriptional and translational levels. Their genetic alteration and survival analysis were also revealed.
Conclusion: We identified two modules and 10 hub genes, which were associated with the tumorigenesis of OSCC. The two modules provided references that will advance the understanding of mechanisms of tumorigenesis in OSCC. Moreover, the hub genes may serve as biomarkers and therapeutic targets for precise diagnosis and treatment of OSCC in the future.
Keywords: oral squamous cell carcinoma, co-expression, WGCNA, hub gene
Introduction
Head and neck cancer is a type of upper gastrointestinal cancer based on anatomical structure. Head and neck squamous cell carcinoma (HNSC) is a common malignancy in the world, which accounts for 90% of head and neck cancers, and oral squamous cell carcinoma (OSCC) is the main subtype of HNSC. OSCC is characterized by high incidence of masses in the oral cavity, which leads to high mortality.1 The incidence of OSCC is associated with smoking, drinking and diet. In addition, viral infections, especially human papillomavirus (HPV), and the lack of vitamins and trace elements such as folic acid; vitamins A, C and E; zinc and selenium are also important etiological factors that can contribute to the incidence of OSCC.2–4 Accumulation of genetic alterations in oral epithelial cells is also considered to trigger the initiation and progression of OSCC.5 Considering recent developments in molecular biological research, it has been discovered that the occurrence and development of OSCC are related to the activation of oncogenes such as Bcl-2, c-Myc and EGFR6,7 and the inactivation of p53, Rb, p16 and p21 tumor suppressor genes.8,9 However, the occurrence of tumors is a complex and progressive process that is multifactorial, multistage and multistep. The molecular pathogenesis of OSCC remains unclear, and therefore, it is difficult to prevent the pathogenesis of OSCC and improve the survival rate of OSCC patients. In order to diagnose OSCC at its early stage and improve its treatment effect, better understanding of the pathomechanism of OSCC development is needed.
With the discovery and development of high-throughput research methods, systematic description, high-throughput data analysis and screening of important information are the basis for subsequent research. Previous research has been largely focused on studying individual molecules with little focus on functional networks. However, the development of cancer is a systematic biological process (BP) that traverses different functional networks. Weighted gene co-expression network analysis (WGCNA)10 is a new tool that is used to analyze the potential gene modules that function in the gene expression data. It has been reported that WGCNA can be used to analyze many BPs, such as genetics, multiple cancers and brain imaging data analysis,11 and hence can be useful to identify candidate biomarkers or therapeutic targets. Besides, it can be applied in comparing differentially expressed genes and help investigating the interactions among genes in different modules.12 WGCNA has been successfully applied in various types of cancer, such as breast cancer,13 glioblastoma14 and prostate cancer,15 in which WGCNA is used to investigate the relationship between tissue microarray data and clinical traits to identify rules for predicting survival outcome of patients.
In this study, WGCNA was constructed on the basis of data from GSE30784, which included 167 OSCC samples, 17 dysplasia samples and 45 normal samples. Key gene modules associated with tumorigenesis of OSCC were identified, and the biological functions and pathways of genes in the two modules were detected and analyzed. Hub genes in turquoise and brown modules were also revealed and were validated by other datasets of GSE74530 and TCGA. Information on their genetic alterations and clinical features were also revealed. We postulated that these genes and modules might be potential biomarkers for the diagnosis and treatment of OSCC and might contribute to our understanding of the molecular mechanisms of its tumorigenesis.
Patients and methods
Data information
The OSCC datasets of GSE30784 and GSE74530 were obtained from NCBI Gene Expression Omnibus (GEO) (https://www.ncbi.nlm.nih.gov/geo/). GSE30784 consists of 167 OSCC samples, 17 dysplasia samples and 45 normal samples, and the platform is [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array. As for the GSE74530, it contains six OSCC samples and six normal samples, and its platform is also [HG-U133_Plus_2] Affymetrix Human Genome U133 Plus 2.0 Array. The R packages affy and annotate were used to process the raw data, make expression matrix and match the probe to their gene symbol.
Construction of WGCNA
The raw data of GSE30784 were downloaded from the GEO database. We used the affy package (under the R environment, version 3.4.3) to preprocess and normalize (RMA normalization) the raw data (.CEL files). The parameters were set as RMA (for background correction) and impute (for supplemental missing value). Ranked by SD from large to small (including normal, dysplasia and OSCC samples), we chose the top 5,000 genes for WGCNA. R package WGCNA was conducted, and the power parameter was pre-calculated by the pickSoftThreshold function. It can provide the appropriate soft-thresholding power for network construction by calculating the scale-free topology fit index for several powers. Turning adjacency into topological overlap was carried out, which could measure the network connectivity of a gene defined as the sum of its adjacency with all other genes for the network generation. Hierarchical clustering function was used to classify genes with similar expression profile into modules based on the TOM dissimilarity with a minimum size of 50 for the gene dendrogram. The dissimilarity of module eigengenes was calculated to choose a cutline to merge some modules. The gene network was visualized with randomly selected 1,000 genes. Last visualization in gene network of eigengenes was also carried out.
Identification of clinical significant modules
We calculated the correlation between MEs and clinical trait to identify the relevant module. Afterward, gene significance (GS) was defined as the log10 transformation of the P-value (GS=lgP) in the linear regression between gene expression and clinical information. In addition, module significance (MS) was defined as the average GS for all the genes in a module. In general, the module with the absolute MS ranked first among all the selected modules was considered as the one related with clinical trait.
Function enrichment analysis
To get further insight into the function of genes in key modules, we performed the Gene Ontology (GO) enrichment analysis for modules by using the online Database for Annotation, Visualization and Integrated Discovery (DAVID; https://david.ncifcrf.gov/summary.jsp).16,17 The gene lists of modules were uploaded, and we got the results of BP, cellular component (CC), molecular function (MF) and Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway. P-value <0.05 was regarded as significant.
Identification of hub genes in key modules
Hub genes that are highly interconnected with nodes in a module have been considered as functionally significant. In this study, key modules were selected and were visualized by Cytoscape and hub genes in networks were screened out by degree. Hub genes ranked top 30 in module networks were chosen as the candidates for further analysis and validation. GEPIA (http://gepia.cancer-pku.cn/)18 is a Web server for analyzing the RNA sequencing expression data of 9,736 tumors and 8,587 normal samples from the TCGA and the GTEx projects, using a standard processing pipeline. Survival analysis of candidate genes was carried out in GEPIA, and the significant results were picked out (logrank P<0.05). We chose the top five genes in each module with significant results of survival analysis and ranked them by node degree as hub genes in OSCC tumorigenesis.
Validation of hub genes
The “limma” R package was used to screen the differently expressed genes (DEGs) between OSCC samples and normal samples in dataset GSE74530 for validation. The cutoff value was log2FC>|2|, adjusted P-value <0.01. Volcano plot and hierarchical clustering analysis were carried out by R packages ggplot2 and pheatmap, respectively. Venn diagram was performed by online tool jvenn (http://jvenn.toulouse.inra.fr/app/example.html)19 to overlap the gene in key modules and DEGs. The expression statuses of hub genes in GSE30784, GSE74530 and TCGA HNSC were revealed for validation. P-value <0.05 was regarded as significant. The translational-level validation of hub genes was carried out using The Human Protein Atlas database (https://www.proteinatlas.org/).20
Genetical alteration of hub genes
CBio Cancer Genomics Portal (http://www.cbioportal.org/)21,22 is an open platform that provides visualization, analysis and downloads of large-scale cancer genomic datasets of various cancers. Complex cancer genomic profiles can be easily obtained by using the query interface of the portal, enabling researchers to explore and compare genetic alterations across samples. We used CBioPortal to explore genetic alterations connected with the 10 hub genes and their correlation with other famous genes and drugs.
Results
Expression value analysis of microarray data
To construct the gene co-expression networks, the raw data of GSE30784 were downloaded from GEO database. Raw data were preprocessed identically by using R for background correction and normalization. R package annotation were conducted to match probes and gene symbols, and the probes matching several genes were removed, and for the gene matched by multiple probes, the median was regarded as the final expression value. At last, we obtained a total 20,027 genes. We calculated SD for each gene and ranked them from large to small, and then, the top 5,000 genes were chosen for WGCNA. The 5,000 genes were used for cluster analysis by fashClust function of the WGCNA package (Figure 1A). As we can see, 229 samples were divided into two clusters on the whole.
Construction of weighted co-expression network and identification of key modules
Selection of the soft-thresholding power is an important step when constructing a WGCNA. We performed the analysis of network topology for thresholding powers from 1 to 20 and identified the relatively balanced scale independence and mean connectivity of the WGCNA. As shown in Figure 1B and C, power value 9, which was the lowest power for the scale-free topology fit index on 0.9, was selected to produce a hierarchical clustering tree (dendrogram) of 5,000 genes. We set the MEDissThres as 0.25 to merge similar modules (Figure 2A), and 11 modules were generated (Figure 2B). There were 223 genes in the black module, 518 genes in the blue module, 954 genes in the brown module, 139 in genes in the greenyellow module, 172 genes in the magenta module, 302 genes in the pink module, 143 genes in the purple module, 379 genes in the red module, 112 genes in the salmon module, 530 genes in the turquoise module and 471 genes in the yellow module. The genes that could not be included in any modules were put into the gray module, which was removed in the subsequent analysis. Module preservation analyses for the process of normal to dysplasia and dysplasia to neoplasm are shown in Figure S1A and B.
Correlation between modules and identification of key modules
Interaction relationships of the 11 modules were analyzed, and the network heatmap was plotted (Figure 3A). The results revealed that each module was an independent validation to each other, which demonstrated a high level of independence among the modules and relative independence of genes expression in each module. Besides, we calculated eigengenes and clustered them according to their correlation in order to explore co-expression similarity of all modules (Figure 3C). We found that the 11 modules were mainly divided into two clusters. Similar results were demonstrated by the heatmap plotted according to adjacencies (Figure 3D). Furthermore, the ME of the turquoise module and brown module revealed a high correlation with disease status (normal, dysplasia and OSCC) compared with other modules (Figure 3B). The turquoise module was positively correlated with the disease status, while the brown module was negatively correlated with the disease status, indicating that the turquoise module may play an important role in tumorigenesis of OSCC and the brown module may function as an antitumor. We identified the turquoise module and brown module as the modules most relevant to the disease status of OSCC. Figure 4A and B illustrates the correlation between module membership and GS in turquoise and brown modules, respectively.
Identification of hub genes in the turquoise module and brown module
We visualized the turquoise module and the brown module as networks in Cytoscape and screened out 30 genes by sorting node degree candidate gene for further analysis. Figure 4C and D shows the top 30 hub genes in the turquoise module and brown module. We used GEPIA to perform survival analysis; P<0.05 was regarded as statistically significant. The genes with a significant result of survival analysis were selected and ranked by node degree. The top five genes in each module were considered as hub genes. Figure 5 shows the survival analysis of hub genes (A–E were hub genes in the turquoise module and F–J were hub genes in the brown module). They were MMP1, TNFRSF12A, PLAU, FSCN1 and PDPN in the turquoise module and KRT78, EVPL, GGT6, SMIM5 and CYSRT1 in the brown module. The survival analysis of the 10 hub genes were validated in another GEO dataset, GSE41613 (Figure S3).
Function enrichment analysis in two key modules
We performed enrichment analysis to explore the BP and pathway in which the two key modules were involved. GO enrichment of BP was conducted by DAVID, and the detailed information is given in Table 1. As the results show, the brown module was mainly enriched in regulation of epidermis such as epidermis development, epithelial cell differentiation and keratinocyte differentiation, which were negatively correlated with tumorigenesis. In the turquoise module, the results of enrichment analysis were mainly concerned with tumorigenesis of malignancy such as positive regulation of cell migration, angiogenesis, cell adhesion, cell–matrix adhesion and extracellular matrix (ECM) organization, which played an important role in tumorigenesis, invasion, migration and metastasis of OSCC.
  | Table 1 GO enrichment analysis of turquoise module and brown module |
As for the pathways in which these two modules were involved, we performed KEGG pathway analysis, and the details of the results are shown in Table 2. As we can see, the brown module enriched in pathways of metabolism of biological compounds such as steroid biosynthesis, leucine and isoleucine degradation, biosynthesis of antibiotics and glycerolipid metabolism. In the turquoise module, pathways concerning with regulation of cell adhesion were significantly enriched such as focal adhesion, PI3K–Akt signaling pathway and ECM–receptor interaction, which were closely related with malignancy.
A direct comparison of the network constructed from tumor vs normal samples of GSE30784 for providing solid insights (Figure S2A and B). The GO enrichment analysis and the KEGG pathway analysis were carried out for the DEGs mentioned earlier (Tables S1 and S2). The enrichment analysis results between modules from WGCNA and DEGs were similar, which means that the results were reliable.
Validation of hub genes
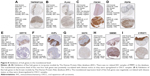
We used another dataset GSE74530 from GEO database to validate the expression status of the 10 hub genes. We set the cutoff as logFC>|1| and P<0.05 to screen DEGs. Figure 6A shows the volcano plot of DEGs, and Figure 6B shows hierarchical clustering heat map of DEGs. We overlapped the DEGs and genes in the turquoise module and brown module by venn diagram and found that the 10 hub genes were all present in DEGs and modules. The results are shown in Figure 6C and D. Figure 7A demonstrates the expression status of 10 hub genes in normal, dysplasia and OSCC samples of GSE30784. As shown in the figure, the expression status was in accordance with the disease status in which expression of five hub genes of the turquoise module was positively correlated with disease status and expression of five hub genes of the brown module was negatively correlated with disease status. Figure 7B illustrates the validation of 10 hub genes in GSE74530, which presents a similar conclusion with GSE30784, that the hub genes in the turquoise module were highly expressed in tumor samples, while hub genes in the brown module were highly expressed in normal samples. We further analyzed TCGA data of HNSCs to validate the results and found similar results as described earlier (Figure 7C). Moreover, immunohistochemistry (IHC) staining obtained from The Human Protein Atlas database also demonstrated the expression status of hub genes and the patient data as shown in Figure 8. Figure 8A–D shows hub genes in the turquoise module, whose results were in accordance with the transcriptional level, but there were no related IHC samples of MMP1 in the database. Figure 8E–I shows hub genes in the brown module, and results were in accordance with transcriptional level before. These results confirmed the validity of the hub genes that we found as reliable.
Genetical alteration of hub genes
OncoPrint of CBioPortal was used to show the 10 hub genes’ alteration statuses in TCGA HNSC patients. The 10 hub genes altered in 222 (44%) of 510 patients (Figure 9B), and the frequency of alteration of each hub gene is shown in Figure 9A. FSCN1 and MMP1 altered most (15% and 9%, respectively), amplification, and mRNA upregulation was the main type. Figure 9C demonstrates the relationship of the 10 genes and the other 50 most frequently altered neighbor genes (only MMP1, PLAU, FSCN1 and EVPL had connection with these 50 genes). PIK3CA and TP63 were significantly important in the network. The correlation of antitumor drugs and hub genes was exhibited; we found MMP1 and TNFRSF12A were the target of cancer drugs, as for the other hub genes, there was no drug targeting to them, which might be the promising target of new cancer drugs.
Discussion
OSCC is a common malignancy caused by abnormal squamous cells, which mainly occurs in older adults. Clinical symptoms are not often present in the early stages of the disease but are observed in the late stages of the disease. Its 5-year survival rate is 50%.23 In patients with high metastasized cancer, the 5-year survival rate drops to ~20%,24 which can seriously harm human health and life. Although the new and advanced medical methods have greatly improved the quality of life for patients with OSCC, the 5-year survival rate has not yet improved significantly. Therefore, it is of great help for the prevention and treatment of OSCC to study the development of OSCC at the molecular level and develop effective measures to prevent and suppress metastasis of OSCC. In the present study, we applied WGCNA to identify the key modules and hub genes in tumorigenesis of OSCC by R and identified two modules that were most significantly associated with the OSCC disease status (the turquoise module was positively correlated with OSCC status, and the brown module was negatively correlated with the disease status). Functional enrichment analysis of the two modules was performed by DAVID. A total of 10 hub genes with significant correlation with survival analysis in the two modules were identified and were further validated in another dataset and TCGA to confirm the reliability of the results. CBioPortal was used to show the correlation between hub genes and genetic alteration. The results provide novel insights that will help to explain the pathogenesis of OSCC at the molecular level, and the hub genes might serve as biomarkers as well as therapeutic targets for precise diagnosis and treatment of OSCC in the future.
In this study, we used a global approach to construct co-expression network of OSCC to predict clusters of genes involved in the pathogenesis and tumorigenesis of OSCC. We aimed to find new and critical biomarkers and to understand the molecular mechanism of OSCC, which might contribute to the diagnosis and treatment of the disease. In this study, WGCNA was performed by extracting co-expression networks of group genes from a large expression data.25 GSE30784 was downloaded from the GEO database, and 11 co-expression modules were obtained by WGCNA. Among the 11 modules, we found that the turquoise module and brown module were most significantly related to the OSCC status, in which the turquoise module was positively correlated and the brown module was negatively correlated. There are many genome-wide gene expressions of OSCC datasets available, and there are many opportunities for researchers to use these data to carry out multiple analyses. However, there are challenges in data analysis. For example, data from different platforms cannot be compared together, but WGCNA methods avoid this limitation by focusing on a group of genes rather than on individual genes. Besides, the cutoff criterion is not necessary for WGCNA, and hence, important information that is omitted when using other methods can be retrieved. Until now, WGCNA has been applied in many cancers such as pancreatic cancer,26 breast cancer27 and osteosarcoma.28 Although there were some reports on OSCC based on expression profiles,29 their disadvantages were obvious. There were no studies in which the expression profile of OSCC was analyzed by WGCNA. In this study, we used WGCNA to analyze OSCC from a new perspective.
From our research, the turquoise module and brown module identified by WGCNA were the most significantly related modules to OSCC disease status, indicating their importance in OSCC invasion, metastasis, migration and tumorigenesis. It has been reported that the epithelial–mesenchymal transition and the loss of cell adhesion are significant gene–molecular changes in the development and progression of OSCC.30 We found that the turquoise module played a role in the BPs and pathways, mainly in the regulation of ECM and collagen catabolic process, which play important roles in malignancy.31 The results were in accordance with previous reports32 that analyzed the DEGs between normal samples and OSCC in which the function of ECM was stressed and the MMP family and laminin family play an important role in tumorigenesis of OSCC.33 These findings revealed the function of the turquoise module in OSCC, and further research is required to identify the role of the genes in the turquoise module because they might have diverse functions in OSCC malignant phenotypes. The KEGG pathway analysis revealed similar results. These pathways were concerned with cell adhesion such as focal adhesion, PI3K–Akt signaling pathway and ECM–receptor interaction. ECM proteolysis confers cancer cells with invasive capability, and it influences migration in many types of cancers.34 It has been reported that COL11A1 promoted tumor progression in EOC via ECM–receptor interactions.35 FAK is an important mediator of proliferation, cell survival and cell migration; the development of malignancy is often associated with abnormal FAK activity.36 It has been reported in OSCC that focal adhesion is related to cell proliferation.37 PI3K–Akt pathway plays an important role in cancer cell cycle progression, apoptosis and neoplastic transformation38 and is involved in many of the mechanisms targeted by newer antitumor drugs.
As for the brown module, GO analysis revealed that it is involved in the regulation of epidermis development and epithelial cell differentiation, which oppose OSCC EMT. The brown module decreased in those BP that promotes the OSCC EMT, resulting in invasion and metastasis of tumor cells.39 This illustrated an important role of the brown module as an antitumor.
Owing to the importance of turquoise and brown module in OSCC tumorigenesis, we screened the hub genes in the two modules. We found MMP1, TNFRSF12A, PLAU, FSCN1 and PDPN in the turquoise module and KRT78, EVPL, GGT6, SMIM5 and CYRST1 in the brown module. They were significantly related to survival analysis results and were validated by other data at transcriptional and translational levels. Many studies have reported that some of the 10 hub genes were cancer-related genes, which function in tumorigenesis and malignant phenotypes such as MMP1, TNFRSF12A and FSCN. As for PLAU, PDPN, KRT78, EVPL, GGT6, SMIM5 and CYSRT1, there were few reports implicating them in cancers. MMP1 encodes for a member of the peptidase M10 family of MMPs. It is involved in the breakdown of ECM in normal physiological processes, such as embryonic development, reproduction and tissue remodeling, as well as in disease processes, such as arthritis and metastasis. It was shown that MMP1 associated with Ras, thereby initiating malignant tumor formation and leading to aberrant regulation of cell survival, proliferation and mobilization.40 TNFRSF12A encodes receptor for TNFSF12/TWEAK. It can promote angiogenesis and modulate cellular adhesion to matrix proteins. Knockdown of TNFRSF12A would lead to inhibition of cell proliferation and migration in hepatocellular carcinoma,41 but no report has shown that it is linked to OSCC. PLAU encodes for a secreted serine protease that converts plasminogen into plasmin. Mutations in this gene may be associated with Quebec platelet disorder and late-onset Alzheimer’s disease. Studies on PLAU were mainly focused on Alzheimer’s disease, but several reports have shown that PLAU played an important role in breast cancer cell line aggression and metastasis.42 PLAU mutation occurs more or less in many cancer types, but there was no study that reported the effects of PLAU mutation in cancers. However, in Quebec platelet disorder, tandem duplication of a 78 kb region of chromosome 10 containing PLAU (the uPA gene) would lead to increasing production of normal PLAU transcripts.43 FSCN1 encodes for a member of the fascin family of actin-binding proteins, which plays a critical role in cell migration, motility, adhesion and cellular interactions. It has been reported that overexpression of this gene may play a role in the metastasis of multiple types of cancer by increasing cell motility. For instance, in esophageal cancer, tumor-suppressive miRNAs target FSCN1 and directly control oncogenic FSCN1 gene.44 FSCN1 gene alteration was rarely reported, but there was a report showing that alteration of FSCN1 increased when using demethylating agent, resulting in drug resistance of gastric cancer.45 However, there are no reports implicating it in OSCC at present. PDPN encodes for type I integral membrane glycoprotein, which has diverse distribution in human tissues. The specific function of this protein has not been determined, but it has been proposed to be a marker of lung injury. The role of PDPN in cancers has not been studied at present. Alteration of PDPN was reported to be associating with keratocystic odontogenic tumors’ neoplastic invasion.46 EVPL encodes for a member of the plakin family of proteins that forms a component of desmosomes and the epidermal cornified envelope. It has been reported that EVPL was underexpressed in esophageal squamous cell carcinoma compared to normal tissue, and hence, it may be used for early detection of ESCC.47 The EVPL gene mutation was closely linked to the tylosis esophageal cancer locus in sporadic OSC and could be the target gene responsible for OSC.48 OSCC was similar to OSC, which means the EVPL mutation also plays an important role in OSCC. As for KRT78, GGT6, SMIM5 and CYSRT1, they are relatively new molecules with only few reports regarding their role in OSCC at present. Nevertheless, they played an important role in OSCC tumorigenesis and were significantly different between normal samples and OSCC. Further research is required to fully explore their roles in OSCC.
We acknowledge there were some limitations and shortcomings in this study. First, this study was mainly focused on the data mining and data analysis, which were based on methodology and the results were not validated by experiments. Further experiments are needed to better confirm the findings of this study. Second, the clinical parameters and prognosis were not well analyzed in this study because of the availability of data. Last, the datasets we could obtain were limited. We could only find one dataset that contained normal samples, dysplasia samples and OSCC samples. If there would have been another dataset that contained these three or more statuses of OSCC, it would be much better. Besides, we only validated our findings in one GEO dataset and TCGA data; more datasets should be involved to obtain a solid result.
Conclusion
We applied WGCNA to construct co-expression network for exploring the development of OSCC (from normal to dysplasia and normal to OSCC) for the first time and found two modules (turquoise module and brown module) and 10 hub genes (MMP1, TNFRSF12A, PLAU, FSCN1, PDPN, KRT78, EVPL, GGT6, SMIM5 and CYRST1), which played an important role in OSCC tumorigenesis. The turquoise module plays a positive role in OSCC tumorigenesis, which includes functions of cell migration, cell adhesion and ECM, and the brown module plays a negative role in tumorigenesis, which functions opposite to the turquoise module. The two modules may provide a better understanding of the mechanisms of tumorigenesis in OSCC patients. Moreover, the 10 hub genes we newly found might serve as prognostic biomarkers and therapeutic targets in the future.
Acknowledgment
This study was partially supported by the National Nature Science Foundation of China (Grant Nos 81470719 and 81611140133) to Li M.
Disclosure
The authors report no conflicts of interest in this work.
References
Mignogna MD, Fedele S, Lo Russo L. The World Cancer Report and the burden of oral cancer. Eur J Cancer Prev. 2004;13(2):139–142. | ||
Hosokawa S, Takahashi G, Okamura J, et al. Risk and prognostic factors for multiple primary carcinomas in patients with head and neck cancer. Jpn J Clin Oncol. 2018;48(2):124–129. | ||
Jacobs CD. Etiologic considerations for head and neck squamous cancers. Cancer Treat Res. 1990;52:265–282. | ||
Falk RT, Pickle LW, Brown LM, Mason TJ, Buffler PA, Fraumeni JF. Effect of smoking and alcohol consumption on laryngeal cancer risk in coastal Texas. Cancer Res. 1989;49(14):4024–4029. | ||
Choi S, Myers JN. Molecular pathogenesis of oral squamous cell carcinoma: implications for therapy. J Dent Res. 2008;87(1):14–32. | ||
Marioni G, Staffieri A, Lionello M, et al. Relationship between anti-apoptotic proteins survivin and Bcl-2, and response to treatment in patients undergoing post-operative RT for laryngeal cancer: a pilot study. J Oral Pathol Med. 2013;42(4):339–344. | ||
Fu S, Guo Y, Chen H, et al. MYCT1-TV, a novel MYCT1 transcript, is regulated by c-Myc and may participate in laryngeal carcinogenesis. PLoS One. 2011;6(10):e25648. | ||
Jalali MM, Heidarzadeh A, Zavarei MJ, Sarmast H. p53 overexpression impacts on the prognosis of laryngeal squamous cell carcinomas. Asian Pac J Cancer Prev. 2011;12(7):1731–1734. | ||
Boonyaphiphat P, Pruegsanusak K, Thongsuksai P. The prognostic value of p53, Bcl-2 and Bax expression in laryngeal cancer. J Med Assoc Thai. 2012;95(10):1317–1320. | ||
Zhang B, Horvath S. A general framework for weighted gene co-expression network analysis. Stat Appl Genet Mol Biol. 2005;4:Article17. | ||
Ivliev AE, ‘t Hoen PA, Sergeeva MG. Coexpression network analysis identifies transcriptional modules related to proastrocytic differentiation and sprouty signaling in glioma. Cancer Res. 2010;70(24):10060–10070. | ||
Clarke C, Madden SF, Doolan P, et al. Correlating transcriptional networks to breast cancer survival: a large-scale coexpression analysis. Carcinogenesis. 2013;34(10):2300–2308. | ||
Presson AP, Yoon NK, Bagryanova L, et al. Protein expression based multimarker analysis of breast cancer samples. BMC Cancer. 2011;11:230. | ||
Xiang Y, Zhang CQ, Huang K. Predicting glioblastoma prognosis networks using weighted gene co-expression network analysis on TCGA data. BMC Bioinformatics. 2012;13(Suppl 2):S12. | ||
Wang L, Tang H, Thayanithy V, et al. Gene networks and microRNAs implicated in aggressive prostate cancer. Cancer Res. 2009;69(24):9490–9497. | ||
Huang Daw, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37(1):1–13. | ||
Huang Daw, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4(1):44–57. | ||
Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: a web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45(W1):W98–W102. | ||
Bardou P, Mariette J, Escudié F, Djemiel C, Klopp C. jvenn: an interactive Venn diagram viewer. BMC Bioinformatics. 2014;15:293. | ||
Uhlén M, Fagerberg L, Hallström BM, et al. Proteomics. Tissue-based map of the human proteome. Science. 2015;347(6220):1260419. | ||
Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6(269):pl1. | ||
Cerami E, Gao J, Dogrusoz U, et al. The cBio cancer genomics portal: an open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012;2(5):401–404. | ||
Scott SE, Grunfeld EA, Mcgurk M. The idiosyncratic relationship between diagnostic delay and stage of oral squamous cell carcinoma. Oral Oncol. 2005;41(4):396–403. | ||
Smith RA, Cokkinides V, Eyre HJ. American Cancer Society guidelines for the early detection of cancer, 2006. CA Cancer J Clin. 2006;56(1):1149–2550 quiz. | ||
Langfelder P, Horvath S. WGCNA: an R package for weighted correlation network analysis. BMC Bioinformatics. 2008;9:559. | ||
Zhou Z, Cheng Y, Jiang Y, et al. Ten hub genes associated with progression and prognosis of pancreatic carcinoma identified by co-expression analysis. Int J Biol Sci. 2018;14(2):124–136. | ||
Zhao Q, Song W, He DY, Li Y. Identification of key gene modules and pathways of human breast cancer by co-expression analysis. Breast Cancer. 2018;25(2):213–223. | ||
Liu X, Hu AX, Zhao JL, Chen FL. Identification of Key Gene Modules in Human Osteosarcoma by Co-Expression Analysis Weighted Gene Co-Expression Network Analysis (WGCNA). J Cell Biochem. 2017;118(11):3953–3959. | ||
Fountzilas E, Kotoula V, Angouridakis N, et al. Identification and validation of a multigene predictor of recurrence in primary laryngeal cancer. PLoS One. 2013;8(8):e70429. | ||
Chung CH, Parker JS, Ely K, et al. Gene expression profiles identify epithelial-to-mesenchymal transition and activation of nuclear factor-kappaB signaling as characteristics of a high-risk head and neck squamous cell carcinoma. Cancer Res. 2006;66(16):8210–8218. | ||
Wu JS, Sheng SR, Liang XH, Tang YL. The role of tumor microenvironment in collective tumor cell invasion. Future Oncol. 2017;13(11):991–1002. | ||
Yang B, Chen Z, Huang Y, Han G, Li W. Identification of potential biomarkers and analysis of prognostic values in head and neck squamous cell carcinoma by bioinformatics analysis. Onco Targets Ther. 2017;10:2315–2321. | ||
Tanis T, Cincin ZB, Gokcen-Rohlig B, et al. The role of components of the extracellular matrix and inflammation on oral squamous cell carcinoma metastasis. Arch Oral Biol. 2014;59(11):1155–1163. | ||
Wolf K, Friedl P. Mapping proteolytic cancer cell-extracellular matrix interfaces. Clin Exp Metastasis. 2009;26(4):289–298. | ||
Wu YH, Chang TH, Huang YF, Huang HD, Chou CY. COL11A1 promotes tumor progression and predicts poor clinical outcome in ovarian cancer. Oncogene. 2014;33(26):3432–3440. | ||
Mclean GW, Carragher NO, Avizienyte E, Evans J, Brunton VG, Frame MC. The role of focal-adhesion kinase in cancer – a new therapeutic opportunity. Nat Rev Cancer. 2005;5(7):505–515. | ||
Abu-Ali S, Fotovati A, Shirasuna K. Tyrosine-kinase inhibition results in EGFR clustering at focal adhesions and consequent exocytosis in uPAR down-regulated cells of head and neck cancers. Mol Cancer. 2008;7:47. | ||
Chang F, Lee JT, Navolanic PM, et al. Involvement of PI3K/Akt pathway in cell cycle progression, apoptosis, and neoplastic transformation: a target for cancer chemotherapy. Leukemia. 2003;17(3):590–603. | ||
Lumerman H, Freedman P, Kerpel S. Oral epithelial dysplasia and the development of invasive squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1995;79(3):321–329. | ||
Uhlirova M, Bohmann D. JNK- and Fos-regulated Mmp1 expression cooperates with Ras to induce invasive tumors in Drosophila. Embo J. 2006;25(22):5294–5304. | ||
Wang T, Ma S, Qi X, et al. Knockdown of the differentially expressed gene TNFRSF12A inhibits hepatocellular carcinoma cell proliferation and migration in vitro. Mol Med Rep. 2017;15(3):1172–1178. | ||
Moquet-Torcy G, Tolza C, Piechaczyk M, Jariel-Encontre I. Transcriptional complexity and roles of Fra-1/AP-1 at the uPA/Plau locus in aggressive breast cancer. Nucleic Acids Res. 2014;42(17):11011–11024. | ||
Hayward CP, Liang M, Tasneem S, et al. The duplication mutation of Quebec platelet disorder dysregulates PLAU, but not C10orf55, selectively increasing production of normal PLAU transcripts by megakaryocytes but not granulocytes. PLoS One. 2017;12(3):e0173991. | ||
Kano M, Seki N, Kikkawa N, et al. miR-145, miR-133a and miR-133b: Tumor-suppressive miRNAs target FSCN1 in esophageal squamous cell carcinoma. Int J Cancer. 2010;127(12):2804–2814. | ||
Maeda O, Ando T, Ohmiya N, et al. Alteration of gene expression and DNA methylation in drug-resistant gastric cancer. Oncol Rep. 2014;31(4):1883–1890. | ||
Zhang X, Wang J, Ding X, et al. Altered expression of podoplanin in keratocystic odontogenic tumours following decompression. Oncol Lett. 2014;7(3):627–630. | ||
Hu N, Qian L, Hu Y, et al. Quantitative real-time RT-PCR validation of differential mRNA expression of SPARC, FADD, Fascin, COL7A1, CK4, TGM3, ECM1, PPL and EVPL in esophageal squamous cell carcinoma. BMC Cancer. 2006;6(1):33. | ||
Iwaya T, Maesawa C, Kimura T, et al. Infrequent mutation of the human envoplakin gene is closely linked to the tylosis oesophageal cancer locus in sporadic oesophageal squamous cell carcinomas. Oncol Rep. 2005;13(4):703–707. |
Supplementary materials
  | Figure S3 Survival analysis of the 10 hub genes using GSE41613 for validation. |
  | Table S2 KEGG pathway enrichment analysis of turquoise module and brown module |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.