Back to Journals » Cancer Management and Research » Volume 14
Application of Transurethral Prostate Resection Instrumentation for Treating Low Rectal Anastomotic Leakage: A Pilot Study
Authors Zhang Z, Hu Z, Qin Y, Qian J, Tu S, Yao J
Received 24 March 2022
Accepted for publication 10 June 2022
Published 16 June 2022 Volume 2022:14 Pages 1987—1994
DOI https://doi.org/10.2147/CMAR.S367039
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Supplementary video of "Transurethral prostate resection instrumentation' [ID 366836].
Views: 213
Zhenming Zhang,1,* Zhentao Hu,1,* Yujie Qin,2,* Jun Qian,3 Song Tu,1,3 Jiaxi Yao3,4
1Department of General Surgery II, Hexi University Affiliated Zhangye People’s Hospital, Zhangye, Gansu, 734000, People’s Republic of China; 2Department of Endoscopy Center, Hexi University Affiliated Zhangye People’s Hospital, Zhangye, Gansu, 734000, People’s Republic of China; 3Institute of Urology, Hexi University, Zhangye, Gansu, 734000, People’s Republic of China; 4Department of Urology, Hexi University Affiliated Zhangye People’s Hospital, Zhangye, Gansu, 734000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jiaxi Yao; Song Tu, Department of Urology, Zhangye People’s Hospital, affiliated to Hexi University, No. 67, Xihuan Road, Ganzhou District, Zhangye City, Gansu, 734000, People’s Republic of China, Tel +86-18093616382, Email [email protected]; [email protected]
Purpose: To determine an accurate method of inspecting low anastomotic leakages and application of transurethral prostate resection instrumentation for treating low rectal anastomotic leakage.
Patients and Methods: Clinical data of eight patients treated for anastomotic leakage after rectal cancer surgery at Zhangye People’s Hospital (affiliated to Hexi University), from August 2019 to November 2021, were retrospectively analyzed. Transanal prostate resection instrumentation was used to assess the leakage and surrounding conditions. Using prostate resection instrumentation, the presacral and perirectal residual cavities were washed and removed, and indwelling suprapubic presacral, transanal presacral, and rectal drainage tubes were placed. Continuous presacral saline irrigation and drainage and open negative-pressure suction in the rectal cavity were performed until the patients’ fistula healed.
Results: Of the eight patients with anastomotic leakages, one had grade B and seven had grade C International Study Group of Rectal Cancer anastomotic leakage classifications following Dixon operation. Transanal prostate resection instrumentation showed that the leakage of the one patient with grade B was less than a third of the circumference of the anastomosis. Among the seven patients with grade C, one leakage was less than a third of the anastomotic circumference. One patient had complete separation of the anastomosis and one distal colon necrosis, which necessitated immediate descending colostomy. Conservative treatment was successful in six patients; the conservative overall cure rate was 75%, and the median healing time was 43 (21– 68) days.
Conclusion: Transanal examination of rectal anastomotic leakage using prostate resection instrumentation is comprehensive, easy to perform, provides clear visualization, accurately guides catheter placement, and can be combined with continuous open negative-pressure drainage, which is a safe, convenient, and effective method for treating low rectal leakage.
Keywords: anastomotic leakage, prostate resection instrumentation, catheter, negative-pressure drainage
Introduction
Anastomotic leakage is a serious common complication of rectal cancer surgery; its incidence rate is approximately 10%.1–3 Anastomotic leakage leads to a series of infection-related complications and accounts for up to a third of all fatalities after rectal cancer surgery.4 When faced with an anastomotic leakage, many surgeons prefer intestinal bypass surgery, including temporary and permanent intestinal bypass surgery, for immediate control of infection.5,6 However, regardless of the surgical method, the patient will experience the negative impact of the second operation and its related complications. If permanent diversion surgery is adopted, the desire of the patients and doctors to preserve anal function and improve quality of life is often nullified.
A prostate resectoscope is a commonly used surgical equipment in urology; thus, it is widely available in basic hospitals. It is a straight hard mirror, with good directionality and high definition. The resectoscope is a dual-sheath device that allows continuous circulation of irrigation fluid, with inlet and outlet channel, and the pressure is easy to control. Meanwhile, a flexible rectoscope is extremely inconvenient to operate and has poor directionality, and the intestinal contents easily contaminate the mirror surface, resulting in unclear visualization. Compared with flexible proctoscopy, a prostate resectoscope is easier to progress to the fistula to observe the anastomosis and surrounding conditions, and it is easier to remove the accumulated intestinal contents and foreign bodies in the fistula. Furthermore, the operation technique is easier to master than flexible proctoscopy, and the learning curve is shorter.
This study aimed to determine an accurate method of inspecting low anastomotic leakages and application of transurethral prostate resection instrumentation for treating low rectal anastomotic leakage.
Materials and Methods
General Information
From August 2019 to November 2021, eight patients developed anastomotic leakages following Dixon’s surgical procedure for rectal cancer at Zhangye People’s Hospital (affiliated to Hexi University). Three were men and five were women, and the average age was 64.4±9.0 (range, 51–72) years. One underwent open surgery, whereas seven underwent laparoscopic surgery; all patients had postoperative indwelling presacral drainage tube passing through the pubic bone. The extent of anastomotic leakage was determined intraoperatively during reintervention and classified according to the International Study Group of Rectal Cancer (ISREC) classification.
Surgical Method
When postoperative rectal anastomotic leakage is detected, accurate assessment using transanal prostate resection instrumentation should be performed urgently, and patients who are suitable for conservative treatment should undergo prostate resection instrumentation–guided catheter drainage. We used a plasma resectoscope (Zhuhai Simai Technology Co., Ltd, China.), which enables continuous entry and discharge of the irrigating liquid; the maximum outer diameter of the mirror sheath was 8.3 mm, and the working length was 19 cm. There were biopsy forceps and foreign body forceps for tissue biopsy, foreign body removal, and drainage tube adjustment. The cutting power was set to 280 W, and the coagulation power to was set to 160 W. The rinse solution was 0.9% sterile sodium chloride solution. The patient’s head was high and the feet were low at 30°, and gravity was used to prevent the irrigation fluid from overflowing the pelvic cavity and causing inflammation to spread to the abdomen. The flushing water pressure was 40 cm water column. The flow of flushing fluid was reduced as much as possible as long as the field of view under the microscope was sufficiently clear.
Under the guidance of transanal examination with prostate resection instrumentation, the following were assessed: size of the leak; retraction distance of the intestinal canal at both ends of the anastomosis; presence/absence of ischemic necrosis of the proximal intestinal canal; combination with rectovaginal leakage, rectovesical fistula, or rectourethral leakage; infection around the leak, and so forth (Figure 1). The mirror body extended through the leak to check the presacral and perirectal residual cavities. Combined with the classification of rectal anastomotic leakage to determine whether to perform proximal colostomy or catheter drainage, patients who can be treated conservatively with catheter drainage were evaluated. Under the guidance of prostate resection instrumentation, the rectal contents were thoroughly flushed to remove the rectal contents and intestinal contents in the presacral and perirectal residual cavity (Video 1).
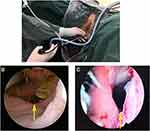 |
Figure 1 (A) 26.5-Fr (diameter, ~8 mm) prostate resectoscope was inserted into the rectum through the anus (A). Anastomotic fistula (yellow arrow) seen under prostate resectoscope (B, C). |
The indwelling trans-sacral presacral common single-lumen drainage tube (Figure 2A) was replaced with a 22-Fr three-lumen balloon catheter (tube 1); then, the tube was pulled out through the leakage to the anus, and the head was connected to the transanal presacral drainage tube (tube 2). Next, the trans-anorectal drainage tube (tube 3) was connected to tube 2 in parallel. Under the monitoring and guidance of prostate resection instrumentation, tube 1 was pulled back, and the head ends of tubes 1 and 2 were placed in the presacral residual cavity, outside the leak, to form a hedging drainage pattern. Tube 1 was sutured to the skin. Tube 3 was placed in the intestinal lumen for continuous negative-pressure suction. Both tubes 2 and 3 were multi-hole tubes; the lateral hole of tube 2 spanned the leak, and the lateral hole of tube 3 spanned the anastomosis. The auxiliary cavity of tube 1 was connected to normal saline for continuous low-flow flushing, and the drip rate was controlled at 20 drops/min. Tube 2 was connected to the drainage bag for normal-pressure drainage, and tube 3 was connected to negative pressure for continuous irrigation and suction. The negative pressure was controlled at 0.1 to 0.2 kPa (Figure 2B). To standardize the operation, we performed the “1-2-3-4 tube placement method”; 1 represents the balloon (ie, the catheter balloon), which acts as the suprapubic drainage tube; 2 represents the two drainage directions, the suprapubic presacral drainage and the transanal presacral drainage; 3 represents the three drainage tubes, the suprapubic sacral drainage and the two transanal drainage tubes; 4 represents the four lumens of the drainage tube, namely the main lumen, the auxiliary lumen of the suprapubic presacral drainage tube, and the transanal presacral drainage lumen, which drains the lumen through the anorectal cavity.
In the early stage of drainage, intravenous nutrition support should be given first, and transition should be made to enteral nutrition as soon as possible. During treatment, routine blood and biochemical parameters were easily monitored, and abdominal imaging examination was performed. If the patient has no symptoms (such as fever, abdominal pain, or abdominal distension), the laboratory indicators are normal, and the abdominal ultrasound and computed tomography examinations confirmed absence of pelvic abscess, the drainage tube can be removed in sequence. When intestinal content flows through the anastomosis, most drainage is performed by tube 3, and part of the intestinal content that enters the presacral space through the leak can be removed by irrigation and drainage of tubes 1 and 2, so as to keep the presacral space clean to the greatest extent. The presacral residual cavity gradually shrinks until no intestinal content or dirt spreads to the pelvic cavity (as confirmed by angiography), thus creating an environment conducive to tissue growth. Tube 2 was removed when the leakage was downgraded to grade A. Tube 3 was removed when there was no infection spread for 1 week. During this time, tube 1 was adjusted for continuous flushing and drainage until the leak ceased.
Results
Eight patients with low rectal cancer underwent suprapubic indwelling presacral tube drainage after Dixon’s operation, as low rectal anastomotic leakage occurred after operation. The median time for diagnosis of anastomotic leakage was 6.5 (range, 4–13) days. Examination through transanal prostate resection instrumentation revealed ISREC grades B and C in one case and seven cases, respectively. The anastomotic leakage in the one case with grade B was less than a third of the anastomotic circumference, and there was no ischemic necrosis near or distal to the anastomosis. In the seven cases of grade C, one case of anastomotic leakage was less than one third of the circumference of the anastomosis; hence, catheter drainage was placed under the guidance of prostate resection instrumentation. One case of leakage was larger than a third of the circumference of the anastomosis; the anastomotic stoma was completely separated and the distal end of the colon was necrotic; thus, a descending colostomy was performed. One case had rectovaginal leakage, and the patient had complications of massive bleeding from the leak; a large amount of blood clots accumulated in the pelvic cavity and lower abdominal-intestinal space with secondary bacterial infection. In the end, conservative treatment failed, and the patient underwent colostomy. In six patients, the rectal anastomotic leakages were cured after drainage tube extubation. The conservative overall cure rate was 75%; of the seven patients with ISREC grade C, five were cured, with a cure rate of 71.4%. The median healing time was 43 (range, 21–68) days (Table 1).
 |
Table 1 Clinical Characteristics of Patients |
Discussion
Rectal cancer is a common malignant tumor of the digestive tract, and low rectal cancer accounts for approximately 70% of all rectal cancers.7 Radical surgery is still primarily used in the treatment of rectal cancer, with laparoscopic low anterior resection being the most widely used.8,9 In the past three decades, owing to the popularization and promotion of total mesorectal excision, the development of laparoscopic technology, use of endoluminal cutting and stapler, and continuous exploration and innovation of surgeons, the limits of anus preservation have been continuously surpassed, and the survival period and quality of life after surgery for low rectal cancer have greatly improved.10,11 The increase in the rate of sphincter-preserving surgery means that more patients with rectal cancer will undergo anastomosis at a lower position, thus increasing the number of cases with anastomotic leakage accordingly.12,13 There are many factors that cause anastomotic leakage after rectal cancer surgery, which are related to the patient’s physiological function, tumor growth, surgical technique, and postoperative management.14,15 Preventing or reducing anastomotic leakage after rectal cancer surgery has always been the focus of surgeons. Once anastomotic leakage occurs, it causes varying degrees of harm to patients, pelvic and abdominal bacterial infections, and even septic shock and death; some patients even require surgery again. Therefore, accurate and timely treatment of anastomotic leakage is a challenge that surgeons need surmount.
There are many methods for grading anastomotic leakage after rectal cancer surgery; however, there is no international consensus yet. Currently, the ISREC standard is more commonly used. Anastomotic leakage is defined as a defect in the integrity of the intestinal wall of the colorectal or colon-anal anastomosis, resulting in communication between the internal and external spaces of the intestinal lumen.16–19 Anastomotic leakage is divided into three grades according to the degree of damage to the patient’s body, clinical symptoms, and treatment needed:18 grade A, no obvious clinical symptoms after leakage occurs, and no active treatment is required; grade B, with clinical symptoms that can be cured with drainage and drug treatment, and active treatment is required; grade C, anastomotic leakage requires repeat laparotomy, usually permanent descending colostomy or temporary ileostomy. However, this grading method only takes into account the clinical manifestations of the patient’s abdominal symptoms and systemic conditions and emphasizes whether to choose to perform fecal diversion. The size of the leak, blood supply of the bowel wall near and distal to the anastomosis, retraction of the bowel, occurrence of leak, and time of diagnosis are not fully considered; thus, we believe that this grading method is not comprehensive. Because different medical institutions or doctors have different understanding, experience, and treatment methods on anastomotic leakage, the choice of whether to perform relaparotomy differs. For patients with grade B leakage, secondary surgery may be done earlier, and some patients with grade C leakage may be downgraded to grade B leakage after appropriate irrigation and drainage. With advancements in minimally invasive technology, increasingly more people need to consider the use of laparoscopy, transanal endoscopy, and the combination of the two to detect, diagnose, and treat anastomotic leakages successfully and accumulate certain experience. Although a second operation is performed, it is not an open operation in the traditional sense. Some scholars suggested that this condition be classified as a grade B leakage, and we agree with this view.
From our experience, once an anastomotic leakage occurs, the local anastomotic condition should be checked as soon as possible, including the size of the leak, retraction of the intestine at both ends of the anastomosis, presence of ischemic necrosis of the proximal intestinal canal, infection around the leakage opening, combination with rectovaginal leakage, rectal bladder fistula, rectal and urethral leakage, and so forth. The decision to perform drainage or reoperate is based on these conditions and the patient’s symptoms. If the leak is smaller than a third of the circumferential diameter, no ischemic necrosis of the bowel is present, the retraction of the broken end does not exceed 1 cm, and there is no peritonitis or pelvic abscess, drainage should be performed first, and if the disease cannot be controlled, a temporary ostomy should be performed. If the leak exceeds a third of the circumferential diameter, the broken end retracts more than 1 cm, the broken end of the intestinal canal is avascular and necrotic, and there is concomitant complicated leakage, a permanent distal colostomy should be performed as soon as possible.
Among the measures to treat anastomotic leakage, drainage is consistent, and its effect determines the development of the disease.20–22 If the drainage effect is good, some patients can be exempted from receiving secondary diversion surgery, thus preserving anal function. The drainage path can be through the pelvis, through the anus, and through both the pelvis and anus. In this study, seven patients were classified as having ISREC grade C and required surgical treatment according to the previous standard. After our treatment plan, except for one patient with rectovaginal fistula complicated with bleeding and the patient with complete colonic separation, the remaining five patients had successful conservative treatment, and the fistula was gradually downgraded from grade C to grade B to grade A, and the patients finally recovered. With our treatment plan, many patients can avoid secondary surgery injuries and achieve anal-preserving treatment effect.
There are various drainage methods, including continuous pelvic irrigation and drainage, continuous irrigation and drainage through the anal catheter, combined pelvic irrigation and drainage through anus, and endoscopic transanal rectal closure with negative-pressure drainage.23–27 These drainage methods all focus on the design of the drainage tube and the selection of the drainage method. Few people assess the anastomosis and leakage before drainage. The placement of the drainage tube is mostly done blindly, ignoring the precise placement of the drainage. Endoscopic trans-anorectal closure negative-pressure drainage technology uses flexible endoscopy to check the anastomosis and guide placement of suction sponges.27 However, because of difficulty in the operation of the flexible mirror as well as poor directionality, intestinal contents easily contaminate the mirror surface, causing poor visualization. In addition, during colonoscopy, gas needs to be injected to expand the bowel, and the pressure is not easy to control. The gas can overflow into the pelvic and abdominal cavities through the leak and the intestinal contents can lead to the spread of infection.
We use prostate resection instrumentation for examination and catheter drainage through the anus. The mirror is a straight rigid mirror with good directionality and high definition, allowing continuous circulation of irrigation fluid, and the pressure is easy to control. In addition to the precise observation of the anastomotic stoma and surrounding conditions, the extra-rectal and presacral conditions can be further observed from the leak. Compared with flexible endoscopy, prostate resection instrumentation facilitates easier removal of the accumulated intestinal contents and foreign bodies in the pelvis as well as comprehensive and accurate decision-making when determining if bypass surgery is required. At the same time, various drainage tubes can be placed in ideal positions with the help of foreign body forceps and biopsy forceps under the guidance of the prostate resectoscope. The catheter balloon was inserted into the presacral fistula, and 5 mL of sterile saline was injected; the catheter was fixed to the abdominal skin to prevent prolapse. The auxiliary cavity of the suprapubic drainage tube was continuously flushed with low-flow sterile saline, and the anal presacral drainage tube was connected to the bag for drainage. Because these two tubes are connected head to head, the drainage of the irrigation fluid, presacral exudate, and pus is ensured. It can fully drain and create a clean presacral microenvironment, making it easy to flush and dredge when the tube is blocked. Continuous negative-pressure suction through the anorectal drainage tube gives the rectum a negative-pressure state, allowing the rectum to pass through the leak and the presacral space to form a pressure difference between the inner low and the outer high, thus preventing intestinal contents from overflowing into the presacral space and causing further pollution, which is conducive for inflammation. The presacral porous drainage tube through the anus connects the presacral space and rectum. When the rectal drainage tube is suctioned by negative pressure, the presacral residual cavity also indirectly appears to have negative pressure, which is conducive for the growth of granulation tissue, thus accelerating the growth of presacral tissue and healing of anastomotic fistula.
In this study, eight patients with anastomotic leakage after low anterior rectal resection were examined by transanal prostate resection instrumentation immediately after a diagnosis was made. In one case, the anastomotic stoma was completely severed and retracted, and permanent bypass surgery was immediately performed. In another case, pelvic and intestinal space hematoma infection occurred because of rectovaginal fistula and bleeding, and permanent diversion surgery was done. Six patients who met the indications for conservative treatment were all cured under the monitoring and guidance of resectoscope with catheter drainage.
Conclusion
We believe that transanal prostate resection instrumentation examination and accurately guides catheter placement, is a safe, convenient, and effective method for treating low rectal anastomotic leakage, making it worthy of clinical application.
Abbreviation
ISREC, International Study Group of Rectal Cancer.
Data Sharing Statement
The data generated in the present study are included in the figures of this manuscript.
Ethics Approval and Informed Consent
Ethical ratification was authorized by the Ethics Committee of Zhangye People’s Hospital (affiliated to Hexi University), with approval number B2021-012. The study was approved by our institutional review board and followed the Helsinki Declaration. The patients provided written informed consent.
Consent for Publication
Consent was obtained for publication of the patient’s data/images in this manuscript.
Acknowledgments
We would like to thank Editage for English language editing.
Funding
This study was funded by grants from NHC Key Laboratory of Diagnosis and Therapy of Gastrointestinal Tumor (NLDTG2020015) and Gansu Province Science and Technology Planning Project (20JR10RG310).
Disclosure
The authors report no conflicts of interest in this work.
References
1. Vasiliu EC, Zarnescu NO, Costea R, Neagu S. Review of risk factors for anastomotic leakage in colorectal surgery. Chirurgia. 2015;110(4):319–326.
2. Sciuto A, Merola G, De Palma GD, et al. Predictive factors for anastomotic leakage after laparoscopic colorectal surgery. World J Gastroenterol. 2018;24(21):2247–2260. doi:10.3748/wjg.v24.i21.2247
3. Gessler B, Eriksson O, Angenete E. Diagnosis, treatment, and consequences of anastomotic leakage in colorectal surgery. Int J Colorectal Dis. 2017;32(4):549–556. doi:10.1007/s00384-016-2744-x
4. Borly L, Ellebaek MB, Qvist N. Leakage after surgery for rectum cancer: Inconsistency in reporting to the Danish Colorectal Cancer Group. Surg Res Pract. 2015;2015:376540. doi:10.1155/2015/376540
5. Chi P, Huang S. [Anastomotic leakage after rectal cancer surgery: classification and management]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21(4):365–371. Chinese.
6. Meyer J, Naiken S, Christou N, et al. Reducing anastomotic leak in colorectal surgery: the old dogmas and the new challenges. World J Gastroenterol. 2019;25(34):5017–5025. doi:10.3748/wjg.v25.i34.5017
7. Wilkinson N. Management of rectal cancer. Surg Clin North Am. 2020;100(3):615–628. doi:10.1016/j.suc.2020.02.014
8. Mathew R. Radical surgery versus organ preservation for early-stage rectal cancer. Lancet Gastroenterol Hepatol. 2021;6(4):263. doi:10.1016/S2468-1253(21)00015-7
9. Dulskas A, Miliauskas P, Tikuisis R, Escalante R, Samalavicius NE. The functional results of radical rectal cancer surgery: review of the literature. Acta Chir Belg. 2016;116(1):1–10. doi:10.1080/00015458.2015.1136482
10. Rullier E, Denost Q, Vendrely V, Rullier A, Laurent C. Low rectal cancer: classification and standardization of surgery. Dis Colon Rectum. 2013;56(5):560–567. doi:10.1097/DCR.0b013e31827c4a8c
11. Hawkins AT, Albutt K, Wise PE, et al. Abdominoperineal resection for rectal cancer in the twenty-first century: indications, techniques, and outcomes. J Gastrointest Surg. 2018;22(8):1477–1487. doi:10.1007/s11605-018-3750-9
12. Sun R, Dai Z, Zhang Y, Lu J, Zhang Y, Xiao Y. The incidence and risk factors of low anterior resection syndrome (LARS) after sphincter-preserving surgery of rectal cancer: a systematic review and meta-analysis. Support Care Cancer. 2021;29(12):7249–7258. doi:10.1007/s00520-021-06326-2
13. Zhuang CL, Liu Z, Zhang FM, Wang Z, Liu Q, Liu ZC. [Surgical key points of precision functional sphincter-preserving surgery]. Zhonghua Wei Chang Wai Ke Za Zhi. 2020;23(6):597–600. Chinese. doi:10.3760/cma.j.cn.441530-20200403-00182
14. Foppa C, Ng SC, Montorsi M, Spinelli A. Anastomotic leak in colorectal cancer patients: new insights and perspectives. Eur J Surg Oncol. 2020;46(6):943–954. doi:10.1016/j.ejso.2020.02.027
15. Tao K, Gao J. [Risk factors for anastomotic leakage after rectal cancer surgery]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21(4):384–387. Chinese.
16. Ye Y, Liu F. [Definition and diagnostic criteria of anastomotic leakage after sphincter-preserving surgery for rectal cancer]. Zhonghua Wei Chang Wai Ke Za Zhi. 2018;21(4):361–364. Chinese.
17. Qiu XY, Li YH, Lin GL, et al. [Protective colostomy and protective ileostomy for the prevention of anastomotic leak in patients with rectal cancer after neoadjuvant chemoradiotherapy and radical surgery]. Zhonghua Wei Chang Wai Ke Za Zhi. 2021;24(6):523–529. Chinese. doi:10.3760/cma.j.cn.441530-20210304-00100
18. Tzu-Liang Chen W, Fingerhut A. Minimal access surgery has its place in the treatment of anastomotic leakage after anterior resection: suggestion for a modification of the International Study Group of Rectal Cancer (ISREC) classification. Surgery. 2021;170(1):345–346. doi:10.1016/j.surg.2021.02.044
19. Allaix ME, Rebecchi F, Famiglietti F, Arolfo S, Arezzo A, Morino M. Long-term oncologic outcomes following anastomotic leak after anterior resection for rectal cancer: does the leak severity matter? Surg Endosc. 2020;34(9):4166–4176. doi:10.1007/s00464-019-07189-9
20. Zhao S, Zhang L, Gao F, et al. Transanal drainage tube use for preventing anastomotic leakage after laparoscopic low anterior resection in patients with rectal cancer: a randomized clinical trial. JAMA Surg. 2021;156(12):1151–1158. doi:10.1001/jamasurg.2021.4568
21. Tan X, Zhang M, Li L, Wang H, Liu X, Jiang H. Retrospective study of active drainage in the management of anastomotic leakage after anterior resection for rectal cancer. J Int Med Res. 2021;49(12):3000605211065942. doi:10.1177/03000605211065942
22. Wang Z, Liang J, Chen J, Mei S, Liu Q. Effectiveness of a transanal drainage tube for the prevention of anastomotic leakage after laparoscopic low anterior resection for rectal cancer. Asian Pac J Cancer Prev. 2020;21(5):1441–1444. doi:10.31557/APJCP.2020.21.5.1441
23. Han Z, Yang C, Wang Q, Wang M, Li X, Zhang C. Continuous negative pressure drainage with intermittent irrigation leaded to a risk reduction of perineal surgical site infection following laparoscopic extralevator abdominoperineal excision for low rectal cancer. Ther Clin Risk Manag. 2021;22(17):357–364. doi:10.2147/TCRM.S306896
24. Kaneko T, Funahashi K, Ushigome M, et al. Incisional negative pressure wound therapy to reduce perineal wound infection after abdominoperineal resection. Int Wound J. 2021;18(1):103–111. doi:10.1111/iwj.13499
25. Kirat HT, Remzi FH, Shen B, Kiran RP. Pelvic abscess associated with anastomotic leak in patients with ileal pouch-anal anastomosis (IPAA): transanastomotic or CT-guided drainage? Int J Colorectal Dis. 2011;26(11):1469–1474. doi:10.1007/s00384-011-1272-y
26. Allen-Mersh TG, Sprague DB, Mann CV, Turner MJ. Pelvic drainage after anterior resection of the rectum. Dis Colon Rectum. 1989;32(3):223–226. doi:10.1007/BF02554533
27. Ferko A, Vana J, Adamik M, et al. Mucosa plication reinforced colorectal anastomosis and trans-anal vacuum drainage: a pilot study with preliminary results. Updates Surg. 2021;73(6):2145–2154. doi:10.1007/s13304-021-01105-4
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.