Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 16
Application of Three Different Types of Pedicled Latissimus Dorsi Flaps in Repairing Nearby Deep Soft Tissue Defects: Cosmetic Result and Shoulder Functionality Evaluation in One Center
Authors Yu MW, Wang M, Wang Q, Chen Y, Yuan SM
Received 15 November 2022
Accepted for publication 17 March 2023
Published 31 March 2023 Volume 2023:16 Pages 793—801
DOI https://doi.org/10.2147/CCID.S397618
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Anne-Claire Fougerousse
Ming-Wei Yu,1,2,* Min Wang,1,* Qian Wang,1 Yong Chen,1 Si-Ming Yuan1
1Department of Plastic Surgery, Jinling Hospital, Nanjing, Jiangsu, 210002, People’s Republic of China; 2Department of Plastic Surgery, Wuhan Third Hospital, Wuhan, Hubei, 430000, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Si-Ming Yuan, Department of Plastic Surgery, Jinling Hospital, Nanjing Medical University, Nanjing, Jiangsu, 210002, People’s Republic of China, Email [email protected]
Objective: The latissimus dorsi (LD) flap has generally been considered a workhorse flap in clinics. However, the impairment of shoulder function and the dramatic appearance in the donor site are the major problems associated with traditional latissimus dorsi myocutaneous flap (LDMF). Here, we analyzed the reliability of three types of LD flaps in repairing deep soft tissue defects in the upper limbs, shoulder, back, and chest wall.
Methods: From December 2016 to December 2020, 21 patients from our center underwent reconstruction of deep soft tissue defects using different types of LD flaps. The distribution of the thoracodorsal artery and the location of its branches were confirmed by imaging examination. Based on the defects, traditional LDMF, thoracodorsal artery perforator flap with capillary perforators (TAPcp), or low-skin-paddle pedicled LDMF was selected and specifically designed for each patient. The appearance satisfaction and shoulder functional of daily life recovery were evaluated.
Results: A total of 12 traditional LDMF, 4 TAPcp, and 5 low-skin-paddle pedicled LDMFs were used. All flaps survived well. The donor site was sutured directly with satisfactory appearance (n = 7) or repaired using skin grafts (n = 14). Compared to traditional LDMF, TAPcp and low-skin-paddle pedicled LDMF have faster shoulder function of daily life recovery.
Conclusion: Based on the characteristics of defects, personalized design of different types of LD flaps is a reliable option to repair different defects.
Keywords: latissimus dorsi myocutaneous flap, thoracodorsal artery perforator flap with capillary perforators, low-skin-paddle pedicled flap, shoulder function
Introduction
The latissimus dorsi myocutaneous flap (LDMF) was first described in 1906.1 As a workhorse flap for soft tissue reconstruction, the LDMF can provide a large amount of soft tissue and had reliable blood supply and strong anti-infection ability.2 However, the major disadvantages of the LDMF are wide scar and impairment of shoulder function in donor site.3,4 Therefore, the technique has undergone constant improvements to reduce shoulder function impairment and improve donor site appearance.
The design of the LD flap has been variously improved, including the thoracodorsal artery perforator (TDAP) flap,5 thoracodorsal artery perforator flap with capillary perforators (TAPcp)6 and low-skin-paddle LDMF.7 Different types of LD flaps should be specifically designed and applied according to the characteristics of the soft tissue defect. Therefore, in this article, we describe our experience and outcomes with different deep soft tissue defects using three different types of LD flaps.
Patients and Methods
Patient Enrollment
The study was approved by the ethics committee of Jinling Hospital. Informed consent was obtained from all patients in accordance with the Declaration of Helsinki. From December 2016 to December 2020, a total of 21 patients who received LD flaps to repair deep soft tissue defects were enrolled in the study. Patients who were in good general condition and unobstructed thoracodorsal artery and tumors without distant metastasis were included. Patients who lost follow-up were excluded.
Preoperative Preparation
All patients underwent routine laboratory investigations. Computed tomographic angiography (CTA) or digital subtraction angiography (DSA) was performed in patients who had undergone traumatic injuries or radiotherapy to confirm the thoracodorsal artery patency. Patients with tumors or infected wounds underwent magnetic resonance imaging (MRI) to determine the extent of the defect. For patients who need to achieve sufficient pedicle length, angiographic visualization of the thoracodorsal artery was performed by DSA.
Flap Design
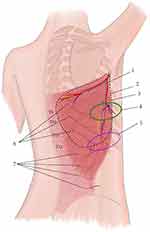
For each patient, the location, size, and extent of the lesion were evaluated. Based on the above information, the flap was designed and applied. The traditional LDMF was used to cover defects with exposure of bone or internal fixator, especially huge deep soft tissue defects. TAPcp was designed for defects involving only deep fascia. The low-skin-paddle LDMF was designed to get sufficient pedicle length to repair the farther distance defect, such as the upper limb, median back, etc.
Operative Technique
At first, debridement and tumor resection were performed. After severe infected debridement, negative-pressure therapy was applied for 1 or 2 weeks until the condition of the wound was suitable for reconstruction.
A line was drawn from the middle of the iliac crest to the bifurcation point of the thoracodorsal artery, at approximately 2 cm from the anterior edge of the LD muscle and 2.5 cm from the posterior axillary fold. For the traditional LDMF, a line was incised as the central axis. The anterior edge of the LD muscle was identified and exposed. The flap was incised to the deep surface of the LD muscle. The avascular plane deep to the muscle is entered and gently elevated with blunt instruments, permitting visualization of the thoracodorsal vessels that run along the muscle’s deep surface. After verifying the vessels’ location, a medial incision delineates the skin paddle to be harvested, ensuring the paddle overlies the vessels.
TAPcp flap was in transverse or slightly oblique locations. The lateral edge of the skin paddle was applied 2–3 cm lateral to the anterior border of the LD muscle. The width of the muscle strip included in this flap ranged from to 4–5 cm to ensure maximal perfusion from the capillary perforators of the thoracodorsal artery.
A low-skin-paddle LDMF was obtained from the outer lower margin of the muscle and middle-lower back, with a vascular pedicle of sufficient length. In case the length of the vascular pedicle still proved insufficient, the horizontal branch of the thoracodorsal artery was ligated for further separation from the root of the artery. During harvesting of the TAPcp and low-skin-paddle LDMF, particular attention was paid to protect the thoracodorsal nerve to preserve muscle function.
The flap donor sites were closed primarily when possible. We attempted to place the scar in the “bra strap” line, which allows the patient to expose the back without showing their scars. When the flap width was larger than 8 cm, the donor site needed to be covered by skin grafts.
Figure 1 illustrates the design of the three flaps.
Evaluation
All patients underwent at least one-year follow-up. The evaluation included the shoulder function in daily life and appearance satisfaction. The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire was used for the evaluation of shoulder function of daily life, which was performed 1 day before and 3, 6, 12 months after surgery. In the last follow-up, patient satisfaction with appearance was assessed using visual analog scale (VAS).
The Disabilities of the Arm, Shoulder, and Hand (DASH) questionnaire is a validated method for measuring arm, shoulder, and hand function.8 The DASH questionnaire consists of 30 questions assessing the impact of upper limb disability on activities of daily living, with scores ranging from 0 (full function) to 100 (no function). To obtain the final scores, the arithmetic means of at least 27 of the 30 items were adjusted utilizing the following formula:  .
.
Appearance satisfaction was assessed using an 11-point VAS questionnaire. Patients rated their appearance of recipient and donor sites on a numeric rating scale ranging from 0 to 10. Patients recording a score ≥8 were considered satisfied appearance, and those recording a score <8 were considered less satisfied.
Statistical Tools
Statistical analysis was performed using version 25.0 of the SPSS software (IBM, Chicago, IL, USA). The normal distribution of the data was determined by one-sample Kolmogorov–Smirnov test. Independent-Samples T text or Wilcoxon–Mann–Whitney U text was used to compare the differences between the two groups. If there were statistically significant differences, a post-unplanned comparison test was performed. Statistical significance was set at P < 0.05.
Results
The age of the patients ranged from 21 to 75 years. The causes of defects included tumor resection (n = 12), trauma (n = 1), radiation-induced ulcer (n = 7), and scar (n = 1). The defects were located in the upper limbs (n = 1), shoulder (n = 3), back (n = 4), and chest wall (n = 13). 17 patients had bone or internal fixator exposure and the muscularis propria exposure in 4. Patients were categorized according to the three types of LD flaps utilized for their reconstruction: traditional LDMF (Group 1; n = 12), TAPcp (Group 2, n = 4), and low-skin-paddle LDMF (Group 3, n = 5) (Table 1).
 |
Table 1 General Conditions and the VAS Scores and the Changes in the DASH Scores |
The size of the traditional LDMF ranged from 12 × 8 cm2 to 28 × 20 cm2, that of the TAPcp from 10 × 7 cm2 to 13 × 8 cm2, and that of the low-skin-paddle LDMF from 9 × 9 cm2 to 15 × 8 cm2. The donor site was sutured directly (n = 7) or repaired using a skin graft (n = 14). All flaps and skin grafts survived well. In one case, the donor site had a hematoma, and two cases presented with a seroma. All patients expressed satisfaction with their recipient site. The directly sutured donor sites had a good appearance (n = 7), while the majority of skin graft donor sites did not (n = 13) (Table 1).
In the evaluation of shoulder function of daily life, one patient with upper limb trauma was excluded. Compared to preoperative baseline values, the changes in the DASH scores are shown in Table 1. The traditional LDMF caused significant impairment of shoulder function of daily life at 6 months after operation (P < 0.05). On the other hand, TAPcp and low-skin-paddle LDMF caused a statistically significant functional impairment of the shoulder at 3 months after operation (P < 0.05). All in all, compared to the traditional LDMF, TAPcp and low-skin-paddle LDMF have less functional impairment and faster postoperative recovery.
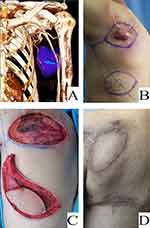
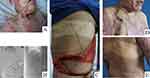
Figures 2–4 introduced the characteristics of three patients that received the traditional LDMF, TAPcp, and low-skin-paddle LDMF, respectively.
Discussion
Deep soft tissue defects in the upper limb, shoulder, back, or chest wall caused by trauma, tumor resection, and radiotherapy are common clinical problems. However, skin grafts for deep soft tissue defects are not suitable due to their poor blood supply.9 For these defects, musculocutaneous flaps perfused by large vessels, can increase the blood supply to the defects, fill the cavity, and cover the wounds.10 Among these, the LD flap has been greatly applied.
The anatomical study has shown11 that the LD muscle is a typical V-shaped muscle and is supplied by the branches of the thoracodorsal artery, the 10th and 11th intercostal arteries, and the 1st and 2nd lumbar arteries. The LD muscle is divided into different vascular territories, with an abundant vascular network connecting them. This provides the possibility of designing different types of flaps.
In other words, LD muscle can provide large amounts of soft tissue and rich blood supply. As a result, LD flaps are widely used in plastic and reconstruction surgery. The advantage of using “LD flaps with muscle” is that such procedures can fill large deep soft tissue defects and performed immediately in cases requiring urgent vascularized soft tissue coverage.12 To fill the defects and promote healing reliably, we prefer the flap with muscle to cover defects with extensive exposure to bone and internal fixation. As a result, traditional LDMF is irreplaceable role in clinical applications.
Due to poor back tissue mobility, when the width of traditional LDMF was more than 8cm, it could not be sutured directly to close the donor sites. In our study, we observed that the donor site was sutured directly with satisfactory appearance. In order to repair large deep soft tissue defects while obtaining a good appearance of the donor site, many improvements of traditional LDMFs have been proposed, such as a multilobed LDMF3 and pre-expanded LDMFs.13 In addition, several studies have reported that perforator propeller flaps can be utilized to correct the LDMF donor site defects.14
The main functions of the LD muscle are internal contraction, internal rotation, and abduction of the shoulder joint. A number of studies8,15,16 had shown that the function of the LD muscle can be partially replaced by the synergic muscle through exercise, and traditional LDMF grafting has little impact on the patients’ daily life. However, large-scale or even total muscle damage had a significant impact on physical activity and movement, with the shoulder joint flexion and extension of shoulder, and the impact of upper extremity strength are significant. The functional impairment and donor site scarring caused by harvesting large size traditional LDMF, which would sacrifice most of the muscles, have been a subject of debate.3,17 An increased focus on donor site morbidity and preservation of function has also influenced views on traditional LDMF designs.
To reduce muscle injury and protect the function, the TDAP flap was first described as the “latissimus dorsi flap without muscle” in 1995.18 Since then, TDAP flap has been widely used in plastic surgery.19 However, the major difficulty with the TDAP flap is the variability and anatomical difficulty of the perforators that harvesting requires a complex pedicle dissection in operation.20 In 2001, Schwabegger et al introduced the design of a muscle-sparing latissimus dorsi flap (MSLDF) to ensure a reliable blood supply to the flap without specifically dissecting out the perforators of thoracodorsal artery, instead carrying a small piece of muscle.21 In 2010, thoracodorsal artery perforator flap with capillary perforators (TAPcp) had been described, which is perfused by capillary perforators.6 The design concepts of the MSLD flap and TAPcp flap were similar, but with different nomenclature perspectives. The TAPcp flap was named for the source of blood supply, whereas the preserved MSLD flap advocated preserving most of the muscle at the time of flap harvesting. Compared to the TDAP flap, TAPcp with a small amount of muscle around the vascular pedicle without dissecting a specific perforator are relatively more robust, reliable, and easier to harvest.22 The skin paddle location is designed along the axis of the descending branch of the thoracodorsal artery whatever the perforator location. In other words, TAPcp introduces further improvements such as increased robustness, reliability, and easy harvesting compared to the TDAP flap. Compared with the bulky traditional myocutaneous flap, this flap was thinner and was suitable for repairing deep fascial defects is more suitable for repairing deep fascial defects. We also preferred to name the flaps based on the source of blood supply. Therefore, in this study, we used the naming of “thoracodorsal artery perforator flap with capillary perforators”.
Due to the limitations imposed by the pedicle length, traditional LDMF and TAPcp cannot repair distal defects. In cases where a longer vascular pedicle is necessary, low-skin-paddle pedicled LDMF can be applied.7 The low-skin-paddle pedicled LDMF is named as such because of the position of the skin island. This flap is supplied by the complex anastomotic branches among the thoracodorsal artery, the 10th and 11th intercostal arteries, and the 1st and 2nd lumbar arteries. It is necessary to identify the location and distribution of the thoracodorsal artery and its branches when these flaps were designed. In our cases, we observed that the descending branch of the thoracodorsal artery could extend to the 9th rib or lower by DSA. Therefore, we designed these flaps along the middle-lower back, between the 9th and 11th ribs, that is, 15–20 cm above the iliac crest. Therefore, the distal part of the skin paddle can be harvested without damaging the LD muscle or fascia. This flap has a lower position, longer vascular pedicle, and wider arc of rotation, so it can be utilized to repair distal defects without tension.
In this study, both the TAPcp flap and low-skin-paddle pedicled LDMF preserved most of the LD muscle at the time of flap harvesting; therefore, we believed that these two types of flaps could be classified as “MSLDF”. Regardless, both TAPcp and low-skin-paddle pedicled LDMF offer the possibility of preserving the LD muscle, thus reducing donor site morbidity. Our study showed that TAPcp and low-skin-paddle pedicled LDMF preserved the function of the donor area to the greatest extent and result in a faster postoperative recovery.
These improvements achieve primary donor site closure and greatly improve the versatility and capability of the LDMF in the reconstruction of large defects. Unfortunately, these methods have their own limitations, and the suitable method for widespread clinical application has not been found. We are continuously exploring better myocutaneous flap designs to repair large size defects while closing the donor site in one stage.
In conclusion, for deep defects with exposed bones or internal fixation stents, particularly in large areas, we continue to prefer the flap with muscle to cover defects. Compared with flaps without muscle, myocutaneous flaps are stronger and more reliable, and can better fill the defect cavity and promote wound healing. In addition, the TAPcp flap or low-skin-paddle LDMF ensure significantly lower shoulder disability compared with the traditional LDMF and faster recovery. Patient satisfaction of flaps was very high in this study, confirming that these three types of flaps provide excellent soft tissue reconstruction in patients with diverse lesions.
Acknowledgments
All authors declare that (i) this was supported by the Special Fund for the Key Medical Specialty of Nanjing, Jiangsu, China (No. SZDZK202001); (ii) there are no other relationships or activities that could appear to have influenced the submitted work.
Disclosure
Ming-Wei Yu and Min Wang are co-first authors of this study. The authors report no conflicts of interest in this work.
References
1. Maxwell GP. Iginio Tansini and the origin of the latissimus dorsi musculocutaneous flap. Plast Reconstr Surg. 1980;65(5):686–692. doi:10.1097/00006534-198005000-00027
2. Kuriyama M, Yano A, Yoshida Y, et al. Reconstruction using a divided latissimus dorsi muscle flap after conventional posterolateral thoracotomy and the effectiveness of indocyanine green-fluorescence angiography to assess intraoperative blood flow. Surg Today. 2016;46(3):326–334. doi:10.1007/s00595-015-1181-8
3. Zhang YX, Messmer C, Pang FK, et al. A novel design of the multilobed latissimus dorsi myocutaneous flap to achieve primary donor-site closure in the reconstruction of large defects. Plast Reconstr Surg. 2013;131(5):752e–758e. doi:10.1097/PRS.0b013e3182865bcc
4. Rindom MB, Gunnarsson GL, Lautrup MD, et al. Shoulder-related donor site morbidity after delayed breast reconstruction with pedicled flaps from the back: an open label randomized controlled clinical trial. J Plast Reconstr Aesthet Surg. 2019;72(12):1942–1949. doi:10.1016/j.bjps.2019.07.027
5. Elgohary H, Nawar AM, Zidan A, et al. Outcome of pedicled thoracodorsal artery perforator flap in the surgical treatment of stage II and III hidradenitis suppurativa of axilla. Ann Plast Surg. 2018;81(6):688–693. doi:10.1097/SAP.0000000000001658
6. Koshima I, Narushima M, Mihara M, et al. New thoracodorsal artery perforator (TAPcp) flap with capillary perforators for reconstruction of upper limb. J Plast Reconstr Aesthet Surg. 2010;63(1):140–145. doi:10.1016/j.bjps.2008.07.020
7. Maia M, Oni G, Wong C, et al. Anterior chest wall reconstruction with a low skin paddle pedicled latissimus dorsi flap: a novel flap design. Plast Reconstr Surg. 2011;127(3):1206–1211. doi:10.1097/PRS.0b013e318205f2f7
8. Garusi C, Manconi A, Lanni G, et al. Shoulder function after breast reconstruction with the latissimus dorsi flap: a prospective cohort study – combining DASH score and objective evaluation. Breast. 2016;27:78–86. doi:10.1016/j.breast.2016.02.017
9. Li X, Zhang F, Liu X, et al. Staged treatment of chest wall radiation-induced ulcer with negative pressure wound therapy and latissimus dorsi myocutaneous flap transplantation. J Craniofac Surg. 2019;30(5):e450–e453. doi:10.1097/SCS.0000000000005514
10. Groth AK, Pazio ALB, Kusano LDC, et al. Thoracic wall reconstruction: surgical planning in extended malignant resections. Ann Plast Surg. 2020;85(5):531–538. doi:10.1097/SAP.0000000000002298
11. Watanabe K, Kiyokawa K, Rikimaru H, et al. Anatomical study of latissimus dorsi musculocutaneous flap vascular distribution. J Plast Reconstr Aesthet Surg. 2010;63(7):1091–1098. doi:10.1016/j.bjps.2009.05.042
12. Maselli AM, Mella JR, Guo L. Refining the latissimus dorsi flap: a useful tool to salvage complex breast defects. Ann Plast Surg. 2019;83(4S):S11–S16. doi:10.1097/SAP.0000000000002092
13. Xiao Y, Xiao H, Wan H, et al. Pre-expanded latissimus dorsi myocutaneous flap for total scalp defect reconstruction. J Craniofac Surg. 2020;31(2):e151–e153. doi:10.1097/SCS.0000000000006084
14. Liu Y, Zang M, Wu L, et al. Improving the versatility of the latissimus dorsi myocutaneous flap using the perforator propeller flap concept. Ann Plast Surg. 2020;84(6):689–696. doi:10.1097/SAP.0000000000002299
15. Koh E, Watson DI, Dean NR. Quality of life and shoulder function after latissimus dorsi breast reconstruction. J Plast Reconstr Aesthet Surg. 2018;71(9):1317–1323. doi:10.1016/j.bjps.2018.05.011
16. Spear SL, Hess CL. A review of the biomechanical and functional changes in the shoulder following transfer of the latissimus dorsi muscles. Plast Reconstr Surg. 2005;115(7):2070–2073. doi:10.1097/01.PRS.0000163329.96736.6A
17. Lee KT, Mun GH. A systematic review of functional donor-site morbidity after latissimus dorsi muscle transfer. Plast Reconstr Surg. 2014;134(2):303–314. doi:10.1097/PRS.0000000000000365
18. Angrigiani C, Grilli D, Siebert J. Latissimus dorsi musculocutaneous flap without muscle. Plast Reconstr Surg. 1995;96(7):1608–1614. doi:10.1097/00006534-199512000-00014
19. Kim SW, Sung IH, Kim YH. Reconstruction of severe atherosclerotic and obstructive diabetic feet using thoracodorsal artery perforator flaps with long vascular pedicles. Microsurgery. 2018;38(3):287–294. doi:10.1002/micr.30251
20. Vaucher R, Dast S, Bout-Roumazeilles A, et al. Harvesting method of thoracodorsal perforator flap: adaptation. Ann Chir Plast Esthet. 2019;64(3):266–270. doi:10.1016/j.anplas.2018.07.007
21. Schwabegger AH, Harpf C, Rainer C. Muscle-sparing latissimus dorsi myocutaneous flap with maintenance of muscle innervation, function, and aesthetic appearance of the donor site. Plast Reconstr Surg. 2003;111(4):1407–1411. doi:10.1097/01.PRS.0000049448.56511.23
22. Youssif S, Hassan Y, Tohamy A, et al. Pedicled local flaps: a reliable reconstructive tool for partial breast defects. Gland Surg. 2019;8(5):527–536. doi:10.21037/gs.2019.09.06
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.