Back to Journals » Research and Reports in Urology » Volume 14
Application of Stem Cell in Human Erectile Dysfunction – A Systematic Review
Authors Siregar S, Novesar AR, Mustafa A
Received 28 May 2022
Accepted for publication 29 September 2022
Published 26 October 2022 Volume 2022:14 Pages 379—388
DOI https://doi.org/10.2147/RRU.S376556
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Panagiotis J Vlachostergios
Safendra Siregar, Aidil Rahman Novesar, Akhmad Mustafa
Urology Department, Hasan Sadikin Academic Medical Center Universitas Padjadjaran, Bandung, Indonesia
Correspondence: Safendra Siregar, Urology Department, Hasan Sadikin Academic Medical Center Universitas Padjadjaran, Bandung, Indonesia, Email [email protected]
Introduction: Erectile dysfunction is a health problem that arises from various conditions and causes an impaired quality of life with a significant health burden. Regenerative and stem cell therapies are some of the potential treatments for erectile dysfunction. This study aimed to review the available information in the literature regarding the use of stem cells in the treatment of erectile dysfunction.
Methods: This study is a systematic review conducted based on the PubMed, Google Scholar, Cochrane, and DOAJ databases. Literature searching was conducted in English and included articles from 2000 to 2020.
Results: The result was a total of 318 articles. Following the elimination process, 9 articles remained in the final analysis. The analyzed studies included 164 patients with erectile dysfunction with various medical conditions. Several stem cell types have been used for treating erectile dysfunction, including mesenchymal stem cell, placental matrix-derived stem cell, mesenchymal stem cell-derived exosome, adipose-derived stem cell, bone marrow-derived mononuclear stem cell, and umbilical cord blood stem cell. Generally, stem cell therapy showed a good efficacy and safety profile, although not enough studies on the protocol, dosage, and mechanism of action.
Conclusion: Stem cell therapy has a good therapeutic potential in erectile dysfunction, the available data from the literature could be the base of usage of stem cells in the treatment of erectile dysfunction although need more research for broader usage.
Keywords: erectile dysfunction, stem cells, regenerative medicine, systematic review
Introduction
Erectile dysfunction is a global male health issue, which causes psychosocial impacts and significant health burdens. Sexual function disturbances in men affect around 20–40% of couples who cannot produce children.1,2 Erectile dysfunction is a condition characterized by the inability to maintain an erection to achieve satisfactory sexual intercourse.3 Generally, erectile dysfunction can be caused by organic, psychologic, and neurogenic factors. Erectile dysfunction typically occurs in men aged above 40 years old and causes serious effects on the sexual life of the patient. In addition, erectile dysfunction can also be caused by hormonal disturbances and side effects of certain medications.4
Generally, the available treatment of erectile dysfunction aims to increase the ability of penile erection temporarily, although it does not provide permanent effects on the endothelial impairment or homeostasis of penile tissues. Commonly used drug classes are phosphodiesterase-5 inhibitors (PDE5i), intracorporal injection, vacuum device, and prosthesis implantation. However, these treatment options are limited by the high cost, side effects, pain, and unsatisfactory results.5
Material and Methods
This study was a systematic review aiming to identify and analyze the available clinical information in the literature regarding the use of stem cell or regenerative medicine therapies in treating erectile dysfunction. A systematic search was conducted in the PubMed, Google Scholar, Cochrane, and DOAJ databases. The search was conducted in English and with a publication year limit of 2000–2020. The keywords used were the combination between stem cells, regenerative medicine, and erectile dysfunction. The search was only limited to the study title and abstract to narrow the findings further. Keywords with negation related to irrelevant articles, including review, meta-analysis, and animal model, were used to exclude articles with inappropriate methodology. Cross-referencing was performed from the available systematic review articles, which were also included in the analysis.
The included articles were randomized controlled trials, prospective and retrospective cohorts, non-controlled before-and-after (NCBA), and controlled before-and-after (CBA) designs. Studies of animal models, review articles, systematic reviews, conference proceedings, correspondences, descriptive studies, and preclinical studies were excluded. Articles obtained from the initial search were then analyzed manually, and extracted in a summary Table 1 consisting of author, publication year, study design, study setting, total subjects, treatment protocols, and key findings of each study.
 |
Table 1 Summary of the Included Studies |
Results
Database searching resulted in a total of 318 articles from the four databases. After eliminating irrelevant articles, articles with inappropriate methodology, and duplicate from the four databases, we obtained 8 articles appropriate for analysis. A cross-reference from the available meta-analysis provided 1 extra article for analysis; therefore, a total of 9 articles were included in the final analysis. The elimination scheme is shown in Figure 1, and the summary of analyzed articles is shown in Table 1.
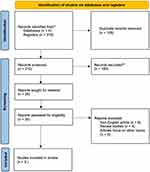 |
Figure 1 Study selection using PRISMA flow diagram. |
Included Studies
Of 9 included articles, 3 were randomized controlled trials, 3 prospective cohorts, 1 retrospective cohort, 1 controlled before-and-after, and 1 non-controlled before-and-after. All studies were multi-center study except for the study by Ory et al, which was a single-center study.
Study Population
Generally, all of the included studies were studies with small sample size. The study with the most subject was conducted by Zasieda et al and Ory et al, with 38 and 36 subjects, respectively. The total subjects of all included studies were 164, with various erectile dysfunction and medical conditions. The study by Bahk et al included 7 patients with type 2 diabetes mellitus who experienced erectile dysfunction after 6 months of PDE5i treatment.6 The study by Levy et al (2015) included 5 subjects with Peyronie disease who refused surgical treatment.7 Another study by Levy et al included 8 subjects with erectile dysfunction who could not tolerate oral PDE5i treatment and refused surgical treatment.8
The Etiology of Erectile Dysfunction
Several erectile dysfunction etiologies were described in the analyzed studies. Bahk et al reported the use of stem cells in patients with erectile dysfunction related to diabetes mellitus, similar to the study by Zasieda et al with erectile dysfunction subjects related to metabolic syndrome.6,9 Haahr et al and Yiou et al investigated the effect of stem cell therapy on erectile dysfunction in post-radical-prostatectomy patients.10,11 The study by Levy et al in 2015 included subjects with erectile dysfunction related to Peyronie disease, while the same author in 2016 did not use particular erectile dysfunction etiological inclusion criteria.7,8 Similarly, the study by Nasab and Protogerou et al also did not report particular erectile dysfunction etiologies.12–14
Study Duration
Generally, the analyzed studies investigated the effect of stem cells on erectile dysfunction after 6–12 months. The study by Bahk et al was conducted in the follow-up period of 9 months, with an evaluation every 3 months. Both of the studies by Levy et al were conducted for 6 months, with a follow-up evaluation on the sixth week, 3rd month, and 6th month. The studies by Haahr et al and Yiou et al were conducted for 6 months, with evaluation on the 1st, 3rd, and 6th months. Nasab et al conducted their study for 6 months, with evaluation on the 3rd and 6th months. Protogerou et al conducted their study for 12 months, with evaluation on the 1st, 3rd, 6th, and 12th months. The shortest study period was found in Zasieda et al, who conducted the intervention for 6 weeks.
A retrospective study by Ory et al reported the effect of stem cells in three clinical trials with different durations. The Comparison of Allogeneic vs Autologous Bone Marrow-Derived Mesenchymal Stem Cells Delivered by Transendocardial Injection in Patients With Ischemic Cardiomyopathy (POSEIDON) was conducted for 12 months with evaluation at 13 months; Transendocardial Mesenchymal Stem Cells and Mononuclear Bone Marrow Cells for Ischemic Cardiomyopathy (TAC-HFT) trials were conducted for 12 months with evaluation on the 1st, 2nd, 3rd, 4th, 5th, 6th, and 12th months; In contrast, the Dose Comparison Study of Allogeneic Mesenchymal Stem Cells in Patients With Ischemic Cardiomyopathy (TRIDENT) trial was conducted for 12 months with evaluation every 3 months.
Types, Preparations, and Dosage of Stem Cell in the Treatment of Erectile Dysfunction
The analyzed studies used various stem cell types and stem cells derivate products from various strains. All studies, except for Ory et al, used the intracavernous injection method for treatment administration. Bahk et al used human umbilical cord blood stem cells with a 1.5×107 cell dose in one injection.6 In both the studies by Levy et al, the treatment used included placental matrix-derived mesenchymal stem cells (PM-MSC) diluted with isotonic NaCl with a ratio of 1:2. The diluted PM-MSC solution was injected with a dose of 1.5–2 mL once, and follow-up was conducted on the sixth week, 3 months, and 6 months post-injection.7,8
Two studies by Haahr et al and Protogerou et al used a one-time injection of adipose-derived stem cell (ADSC) with different preparation and dosage. In the study by Haahr et al, ADSC isolates with a mean total cell of 1.4×105 cells was injected directly into the corpus cavernosum,10 whereas the study by Protogerou et al used ADSC with a total cell of 9.5–51.4×106 cells diluted with 2 mL of platelet lysate plasma PLP, and injected with a volume of 1.8 mL.14 Yiou et al used autologous bone marrow mononuclear stem cells (BM-MNC) with a dose of 109 cells.11 The study by Zasieda et al was the only study that used more than one therapeutical dose. The study reported the use of mesenchymal stem cell-derived exosomes (MSC-DE) with a dosage of 5 mL, injected intravenously once per week for six weeks. Zasieda et al also combined MSC-DE injection with low-intensity shock wave therapy (LISWT) twice per week, 3000 strikes, and 3 Hz frequency.9
Ory et al analyzed the use of transendocardial human mesenchymal stem cell (hMSC) injection as a treatment in cardiomyopathy patients who experienced erectile dysfunction. The hMSC was autologous and allogeneic with varied doses of 20, 100, and 200 million cells.13 Finally, Nasab used an injection of stem cells isolated from the oral mucosal with a dose of 50–60 million cells intracavernous.12
Assessment and Efficacy Indicators of Stem Cell Therapy in Erectile Dysfunction
Most of the analyzed studies had used the International Index of Erectile Function (IIEF) score to assess the improvement of patients’ erectile function after stem cell therapy. The IIEF score was significantly increased compared to baseline in the studies by Nasab, Zasieda et al, Yiou et al, Haahr et al, and Ory et al9–13 Both of the studies by Levy et al reported a trend of increased IIEF score in post-treatment patients, although not statistically significant.7,8 Other than the IIEF score, the peak systolic velocity in the Doppler examination was also the assessed parameter in several studies. Both of the studies by Levy et al reported a significant post-therapy PSV increase.7,8 PSV was also shown to significantly increase in the study by Zasieda et al.9 Protogerou et al reported increased PSV after 1 month of treatment, although there was no statistical analysis result. The study by Nasab did not show a significant PSV increase after stem cell therapy. Another parameter used as an assessment indicator includes end diastolic-volume (EDV) reported by Levy (2016), Protogerou et al, Yiou et al, and Nasab. These four studies did not report any statistically significant EDV (end diastolic-volume) decrease at the end of their studies. Both of the studies by Levy et al also used penile length and girth as an indicator, although both of these did not increase significantly after stem cell therapy.7,8,11,12,14
Subjectively, patients reported improved morning erectile function and overall erectile function in the Haahr et al, Yiou et al, and Protogerou et al studies.9,11,14 In a study by Yiou et al, patients also reported improved sexual intercourse satisfaction post-treatment.11 The study by Haahr et al reported that erectile dysfunction patients with urinary incontinence post-radical prostatectomy incontinence were less likely to experience erectile function improvement.10 Finally, both the studies by Levy et al showed that stem cell therapy resulted in a significantly erectile function improvement without end-diastolic velocity, penile length, and girth changes.7,8
Objectively, each stem cell’s efficacy in erectile dysfunction was difficult to assess due to different duration and regimens in each analyzed study. A significant increase in IIEF-5 score was reported in the study by Haahr et al, Ory et al, Zasieda et al, Protogerou et al, Yiou et al, and Nasab.9–14 These studies used ADSC, BM-MNC, and MSC stem cells and reported a significant positive effect on a patient’s erectile functional status. Of these studies, the study by Protogerou et al that used ADSC reported the most significant increase in IIEF-5 score at the end of the study, with an increase between 2 and 8 points.
From Table 2, IIEF increasing was 15.3± 8.1 vs 18.1 ± 7 from a total of 10 samples, and the IIEF-5 increasing score was 14 to 20 from 54 samples. Mean PSV was 25.5 to 66.7 cm/s in 6 months. The stretched penile girth was statistically not significant from a total of 13 samples.
 |
Table 2 Indicator and Result |
Side Effects
There were no reports of significant side effects. Haahr et al reported erythematous and swelling on the injection area and hematoma in the penile and scrotum around the injection area. The study also reported abdominal tenderness, although these side effects were likely caused by liposuction procedure to isolate ADSC.10 The studies by Protogerou et al and Yiou et al, which in particular evaluated the safety of stem cell therapy, did not report any side effects of stem cell therapy in erectile dysfunction,11,14 while other studies did not report a therapeutic safety profile.
The Advantages of the Study
The advantage of this systematic review was the selection of the analyzed articles, which only included clinical studies with human subjects. This study reported nine clinical studies with RCT, prospective cohort, retrospective cohort, and before–after study designs with similar subject selection, and there were no animal model studies or studies with nonspecific disease included.
Discussion
Stem cells are cells that are able to proliferate and regenerate after tissue damage. Cells can be classified as stem cells if they have some characteristics, including “undifferentiated”, self-replicate, and differentiating into more than one cell type.15,16 Stem cells form a self-maintaining cell population that produces several functional differentiated derivates.17 Stem cells are widely explored as a potential cell-based regenerative treatment for a wide spectrum of diseases. The use of stem cells as a treatment for various urological disorders is growing, including the use of stem cells to treat erectile dysfunction.18
Interest in stem cell technology for erectile dysfunction recovery is increasing. The underlying mechanism of stem cell benefits is still not entirely elucidated. Mesenchymal stem cells (MSCs) are multipotent stem cells capable of self-renewal and can differentiate into several tissues, including muscles, cartilages, bones, and fats. The mesenchyme, an embryonic connective tissue, arises from the mesoderm. MSCs can be isolated from the germ layer or mature adult organs. Therefore, MSCs can be obtained from different tissues, resulting in the wide availability of this therapy.19 MSCs-mediated regeneration involves many cellular mechanisms. The positive effect of stem cell use is not due to cell differentiation and direct integration in the target tissue. In contrast, immunomodulation is achieved through a paracrine effect whereby the secretion of cytokines and growth factors reduces inflammation and promotes healing. The efficacy of stem cell therapy is potentially associated with the acuity of treatment. Theoretically, if inflammation reduction occurs close to the inflammatory triggering condition, normal wound healing may be facilitated.20
From the database search, a total of 318 articles from the four databases were obtained. Of the 9 included articles, 3 were randomized controlled trials, 3 were prospective cohorts, 1 retrospective cohort, 1 controlled before-and-after, and 1 non-controlled before-and-after. Generally, all of the included studies were studies with a small sample size. Several etiologies of erectile dysfunction were described in the analyzed studies. Bahk et al reported stem cell therapy in patients with diabetes mellitus-related erectile dysfunction, similar to Zasieda et al with metabolic syndrome-related erectile dysfunction patients. Generally, the analyzed studies investigated the effect of stem cells on erectile dysfunction after 6–12 months.
The analyzed studies used various stem cell types and their derivates from various strains. All studies, except for Ory et al, used the intracavernous injection method for administration. Bahk et al used human umbilical cord blood stem cells with a 1.5×107 cell dose in one injection. In patients who had not experienced complete improvement of erectile dysfunction, the study added PDE5i administered before sexual intercourse.6 In both of the studies by Levy et al, the treatment used included placental matrix-derived mesenchymal stem cells (PM-MSC) diluted with isotonic NaCl with a ratio of 1:2. The diluted PM-MSC solution was injected with a dose of 1.5–2 mL once, and follow-up was conducted on the sixth week, 3 months, and 6 months post-injection.7,8 The study by Zasieda et al was the only included study that used more than one therapeutical dose. The study reported the use of mesenchymal stem cell-derived exosomes (MSC-DE) with a dosage of 5 mL, injected intravenously once per week for six weeks. Zasieda et al also combined MSC-DE injection with low-intensity shock wave therapy (LISWT) twice per week, 3000 strikes, and 3 Hz frequency.9 Most of the analyzed studies had used the International Index of Erectile Function (IIEF) score to assess the improvement of patients’ erectile function after stem cell therapy. The IIEF score was significantly increased compared to baseline in the studies by Nasab, Zasieda et al, Yiou et al, Haahr et al, and Ory et al9–13 Both of the studies by Levy et al reported a trend of increased IIEF score in post-treatment patients, although not statistically significant.7,8 Other than IIEF score, the peak systolic velocity in the Doppler examination was also the assessed parameter in several studies. Both of the studies by Levy et al reported a significant post-therapy PSV increase.7,8
The authors should answer more questions before starting stem cell therapy as a reliable treatment method. The first question that arises may be determining which stem cell population has the greatest therapeutic potential. The efficacy of stem cells depends on the balance between associated risk, side effects, and implant costs. To date, no study has directly compared the efficacy of 2 or more stem cell lineage in the animal model on the treatment of ED. However, it is safe to say that there is strong evidence to consider BM-MSC, ADSC, and MDSC as the most superior stem cells. Moreover, the convenience of obtaining ADSCs and MDSCs from patients makes them superior to BM-MSCs.18
Objectively, the efficacy of each stem cell preparation in erectile dysfunction was challenging to assess due to different durations and regimens in each analyzed study and no significant side effects from all the analyzed studies. There are lots of questions regarding stem cell therapy for erectile dysfunction. Uncertainty regarding immunogenicity and whether allogeneic or autologous cells are superior suggests the need for future studies and discussions. Autologous stem cells would reduce immunogenic problems; acquiring this cell type is more intensive than allogeneic cells. There are possibilities that several cell types might result in adverse effects. The optimal cell amount may be different based on the type of stem cells. The optimal cell concentration for injection remains to be determined. It is unknown whether the dosage should be based on weight or relative standard concentration to the target tissue, as various studies reported mixed reports. The exact dosing schedule has not been established, and it would be very efficient to determine whether a single injection could be adequate or if several injections at several points in time would prove beneficial. Furthermore, it is important to determine the mechanism of stem cell-mediated therapeutic effect in detail, as each source of stem cells has different repair mechanisms. Determining whether stem cells can help treat other comorbid conditions such as Peyronie’s disease could help generate widespread use due to improvement on blood sugar in diabetic patients which is of paramount importance.20
The stem cell types used in various experimental studies of erectile dysfunction include the bone marrow, adipose cells, skeletal muscle, embryonic, endothelial progenitor, and umbilical cord blood stem cells. Personal preferences or the presence of certain conditions is the most important factor in normal scientific selection.
Before the discovery of the PDE5 inhibitor, which is administered orally, the intracavernous injection was one of the most effective treatments for erectile dysfunction. However, patients who are unresponsive or unable to receive PDE5 inhibitors may still be given an intracavernous injection. A study by Ching et al reported a trial of stem cell injection intracavernous, which could treat erectile dysfunction due to cavernous nerve damage.21
Stem cells are believed to be capable of differentiating into various cell types, including ECs, SMCs, Schwann, and neuron cells. Moreover, stem cell therapy is believed to be an erectile dysfunction therapy based on a hypothesis that stem cell therapy in the penis through intracavernous injection might replace the ECs and/or CSMCs damage. On the other hand, there is a consideration that stem cell transplantation intracavernous might improve the regeneration of ECs and CSMCs or reverse the interaction between ECs and CSMCs. In other words, the action of paracrine, which is the opposite of cellular differentiation, is responsible for the effectiveness of stem cell therapy as the main mechanism.21
Conclusion
In conclusion, stem cell therapy is a technology undergoing rapid development in modern medical science and has a significant therapeutic potential for various medical conditions. Stem cells or stem cells derivate products have been used in several clinical studies in the treatment of erectile dysfunction with a fairly good efficacy and safety profile. Advancement of the research on the field of stem cell therapy should be the optimization of ED treatment in the future. However, there are limited data regarding the type, preparation, dosage, and concomitant therapy, implicating the lack of certainty regarding the therapeutic regimen. Some of the studies included in this review also had low level of evidence. Other studies with high quality, comprehensive protocol design, and larger sample size, are required to support the use of stem cells in erectile dysfunction.
Abbreviation
ADSC, adipose-derived stem cell; BM-MNC, bone marrow mononuclear stem cell; CBA, controlled before-and-after; EDV, end diastolic-volume; IIEF, International Index of Erectile Function; LISWT, low-intensity shock wave therapy; MSC-DE, mesenchymal stem cell-derived exosomes; NCBA, non-controlled before-and-after; PDE5i, phosphodiesterase-5 inhibitors; PM-MSC, placental matrix-derived mesenchymal stem cells.
Data Sharing Statement
All data used in the research are available as part of articles and no additional source are needed to disclose.
Ethics Approval and Consent to Participate
There is no need for ethical clearance for this study that we did a review article.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was provided to support this project.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Kumar N, Singh AK. Trends of male factor infertility, an important cause of infertility: a review of literature. J Hum Reprod Sci. 2015;8(4):191–196. doi:10.4103/0974-1208.170370
2. Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. J Reprod Biol Endocrinol. 2015;13(1):1–9.
3. Muneer A, Kalsi J, Nazareth I, Arya M. Erectile dysfunction. BMJ. 2014;348. doi:10.1136/bmj.g129
4. Protogerou V, Chrysikos D, Karampelias V, Spanidis Y, El Bisari S, Troupis T. Erectile dysfunction treatment using stem cells: a review. Medicines. 2021;8(1):2.
5. Mobley DF, Khera M, Baum N. Recent advances in the treatment of erectile dysfunction. Postgrad Med J. 2017;93(1105):679–685.
6. Bahk JY, Jung JH, Han H, Min SK, Lee YS. Treatment of diabetic impotence with umbilical cord blood stem cell intracavernosal transplant: preliminary report of 7 cases. Exp Clin Transplant. 2010;8(2):150–160.
7. Levy JA, Marchand M, Iorio L, Zribi G, Zahalsky MP. Effects of stem cell treatment in human patients with Peyronie disease. Int J Osteopath Med. 2015;115(10):e8–e13.
8. Levy JA, Marchand M, Iorio L, Cassini W, Zahalsky MP. Determining the feasibility of managing erectile dysfunction in humans with placental-derived stem cells. Int J Osteopath Med. 2016;116(1):e1–e5.
9. Zasieda Y. Erectile dysfunction treatment with combination of mesenchymal stem cell derived exosomes and focused low-intensive shock wave therapy. Men Health Gender Psychosom Med. 2020;(1–2):70–78. doi:10.37321/UJMH.2020.1-2-08
10. Haahr MK, Jensen CH, Toyserkani NM, et al. Safety and potential effect of a single intracavernous injection of autologous adipose-derived regenerative cells in patients with erectile dysfunction following radical prostatectomy: an open-label Phase I clinical trial. EBioMedicine. 2016;5:204–210.
11. Yiou R, Hamidou L, Birebent B, et al. Intracavernous injections of bone marrow mononucleated cells for postradical prostatectomy erectile dysfunction: final results of the INSTIN clinical trial. Eur Urol Focus. 2017;3(6):643–645.
12. Nasab MAB. Clinical trial to evaluate the effect of stem cell intravenous injection treatment in the treatment of erectile dysfunction (sexual dysfunction), in comparison with the control group, in diabetic patients referred to Kerman Diabetes Clinic. Faculty of Medicine [Internet]. Kerman, Iran: Kerman University of Medical Sciences; 2020. Available from: http://eprints.kmu.ac.ir/35518/1/8113.pdf.
13. Ory J, Saltzman RG, Blachman-Braun R, et al. The effect of transendocardial stem cell injection on erectile function in men with cardiomyopathy: results from the TRIDENT, POSEIDON, and TAC-HFT trials. J Sex Med. 2020;17(4):695–701.
14. Protogerou V, Michalopoulos E, Mallis P, et al. Stem cell therapy for erectile dysfunction: preliminary results from a single-center pilot study in Greece. Hellenic Urol. 2018;29:3.
15. Damai Yanti DY, Wida Nengsih WN. An overview of traditional birth attendant knowledge about the role of its partnership with midwives in the working area of Rancaekek Health Center, Bandung Regency. J Kesehatan Masyarakat. Indonesian. 2019;5(1):1–8. doi:10.35329/jkesmas.v5i1.301
16. Indonesian Ministry of Health. Guidelines for antenatal care, childbirth, postpartum, and newborns in the era of adaptation to new habits. Indonesian Ministry of Health. Treasure EMENKES. 2020. Available from: http://rsdustira.com/atm/assets/buku/Pedoman_Pel_ANC,_Persalinan,_Nifas,_dan_BBL_di_Era_Adaptasi_Kebiasaan_Baru_(Revisi_2).pdf.
17. Bajada S, Mazakova I, Richardson JB, Ashammakhi N. Updates on stem cells and their applications in regenerative medicine. J Tissue Eng Regen Med. 2008;2(4):19–83.
18. Peak TC, Anaissie J, Hellstrom WJ. Current perspectives on stem cell therapy for erectile dysfunction. Sex Med Rev. 2016;4(3):247–256.
19. Gur S, Abdel-Mageed AB, Sikka SC, Hellstrom WJ. Advances in stem cell therapy for erectile dysfunction. Expert Opin Biol Ther. 2018;18(11):1137–1150.
20. Matz EL, Terlecki R, Zhang Y, Jackson J, Atala A. Stem cell therapy for erectile dysfunction. Sex Med Rev. 2019;7(2):321–328.
21. Lin C-S, Xin Z-C, Wang Z, et al. Stem cell therapy for erectile dysfunction: a critical review. Stem Cells Dev. 2012;21(3):343–351.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
