Back to Journals » Cancer Management and Research » Volume 10
AP-2α expression in papillary thyroid carcinoma predicts tumor progression and poor prognosis
Received 12 March 2018
Accepted for publication 30 May 2018
Published 13 August 2018 Volume 2018:10 Pages 2615—2625
DOI https://doi.org/10.2147/CMAR.S167874
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Lu-Zhe Sun
Hong Rong Wu, Jian Zhang
Department of Pathology, The Xiangya Hospital of Central South University, Changsha, Hunan, 410008, China
Background: The activator protein (AP)-2α is involved in a wide variety of biologic processes in tumor. However, little is known about the role of AP-2α in human papillary thyroid carcinoma (PTC).
Methods: The immunohistochemical method was used to detect AP-2α expression in 63 PTC cases. Western blotting was carried out to assess the change in expression of certain proteins. The bioinformatics analysis of 496 PTC samples comes from The Cancer Genome Atlas (TCGA). The Gene Set Enrichment Analysis (GSEA) was performed using TCGA data set. Cell transfection was used to induce related protein expression or to repress it by RNA interference procedures.
Results: Our results demonstrated that AP-2α expression was higher in tumor tissues than the corresponding adjacent nontumor tissues, the positive substances of AP-2α were observed mainly in the cytoplasm of PTC, and AP-2α was positively correlated with histologic type (P=0.026) of PTC patients. The high expression of AP-2α mRNA was associated significantly with tumor stages (P=0.011), histologic type (P=0.019), and independently predicted shorter overall survival (P=0.005) based on TCGA analysis. Patients with high AP-2α mRNA expression have shorter overall survival compared to those with low AP-2α mRNA expression, particularly in advanced tumor stages (III and IV) of PTC patients (P=0.011). Multivariate analysis suggested that AP-2α mRNA expression might be an independent prognostic indicator for the survival of patients with PTC (P=0.037). Moreover, the association between enhanced AP-2α expression and two pathways (notch signaling and focal adhesion) was revealed by GSEA, and then confirmed by cellular experiments.
Conclusion: Taken together, our findings suggest that AP-2α may be a potential prognostic molecular marker and therapeutic target for PTC patients.
Keywords: papillary thyroid carcinoma, AP-2α, prognosis, overall survival, notch signaling
Introduction
Thyroid cancer incidence increased, on average, 3.6% per year during 1974–2013, which was primarily associated with increases in papillary thyroid carcinoma (PTC) of 4.4% per year.1 PTC is the most common type of thyroid cancer, although the prevalence of different histologies and genetic profiles has changed over time.2 PTCs are usually curable with 5-year survival of over 95%.3 However, when they occasionally de-differentiate into more aggressive and lethal thyroid cancers, the five-year survival rate drops to 59% in the late stage.4 Therefore, identifying useful prognostic biomarkers to predict the risk of this devastating disease for PTC was urgent.
PTCs encompass several subtypes, including classical variant of papillary thyroid carcinoma (cPTC), follicular variant of PTC (fVPTC), and tall cell variant of PTC (tPTC). Other histologic variants included diffuse sclerosing, columnar, and others, according to the standardized criteria approved by the World Health Organization (WHO).5 Different histologic variants of PTC may have a different prognosis. For example, the tPTC was accepted as a variant with relatively aggressive behaviors such as invasion, metastasis, and recurrence.6
The activator protein 2 (AP-2) family of transcription factors includes five highly homologous members (AP-2α, AP-2β, AP-2γ, AP-2δ, and AP-2ε) known to be important in differentiation, cell growth, and apoptosis.7 AP-2α plays key roles in tumorigenesis and progression, such as glioma, lung cancer, and gastric adenocarcinoma, by regulating gene expression at the transcriptional levels.8–10 A group of previous studies have demonstrated that AP-2α was associated with hepatocellular carcinoma, breast cancer, acute myeloid leukemia, and bladder cancers.11–14 Therefore, I propose a hypothesis that AP-2α may be a useful prognostic biomarker for PTC.
Recent studies showed that AP-2α was correlated with tumor progression and prognosis, such as nasopharyngeal carcinoma, gastric adenocarcinoma prognosis, and epithelial ovarian cancer.10,15,16 However, to our knowledge, the role of AP-2α in PTC remains unclear. The aim of this study was to investigate the potential function of AP-2α in PTC and to determine its correlation with tumor progression and prognosis.
Materials and methods
Patients and samples
A total of 63 formalin-fixed, paraffin-embedded PTC specimens (with 38 adjacent nontumor tissues [ANTs]) were obtained from the Xiangtan City Hospital China between 2015 and 2017. The diagnosis of PTC was established histologically according to the WHO classification. The diagnoses were confirmed histologically in all cases, based mainly on the examination of sections stained with hematoxylin and eosin. Table 1 shows the clinicopathologic features of these patients. Tumors were staged based on the guidelines of the American Joint Committee on Cancer.17 No local or systemic treatment was conducted in these patients before surgery. Our study was approved by the Research Ethics Committee of Central South University, China. Written informed consent was obtained from all participants.
Histology and immunohistochemistry
Immunohistochemical (IHC) staining was performed on 4 µm sections of paraffin-embedded specimens with the use of rabbit anti-human AP2 alpha monoclonal antibody (1:400; Abcam, Boston, MA, USA). Briefly, tissues were fixed in 10% formaldehyde solution (pH 7.0) and paraffin-embedded, paraffin sections are dewaxed in xylene and then rehydrated in a graded alcohol series. The endogenous peroxidase activity was quenched by 3% hydrogen peroxide for 10 minutes. EDTA was used as a buffer (pH =8), with antigen retrieval at high temperature and pressure for 2.5 minutes. Nonspecific protein binding was blocked with 5% goat serum incubated at room temperature for 10 minutes, and the appropriate proportion (1:350) of diluted primary antibody was dropped against AP-2α (Abcam) incubated at 4°C for overnight. Subsequently, secondary horseradish peroxidase antibodies were incubated for 30 minutes at room temperature and visualized using 3,3’-diaminobenzidine peroxidase chemistry (DAKO K6438). Finally, the slices are then mounted on slides, dehydrated using alcohol washes of increasing concentrations (50%, 75%, 90%, 95%, 100%), and cleared using a detergent like xylene before being imaged under a microscope. These judgments were made by two independent pathologists. As a negative control, duplicate sections were immunostained without exposure to primary antibodies.
The IHC scores of the sections was made using the immunoreactivity scoring (IRS) system.18 This IRS system considers both the percentage of positive cells and the intensity of the observed staining. Staining intensity was graded as 0 (negative), 1 (weak), 2 (moderate), and 3 (strong); percentage of positive cells examined was scored as 0 (negative), 1 (<10%), 2 (11%–50%), 3 (51%–80%), and 4 (>80%). Percentage (P) and intensity (I) of AP-2α expression were multiplied to generate a numerical final score (IRS = P×I). The IRS (values from 0 to 12) was determined: 0–1 as negative, values 2–3 as weak, values 4–8 as moderate, and multiplication values 8–12 as strongly positive. “IRS >0” was defined as positive or high AP-2α expression, while “IRS =0” was considered as negative or low AP-2α expression.
Cell culture
Human PTC cell lines (BCPAP and TPC-1) were obtained from iCell Bioscience Inc. (Shanghai, China), and one normal human thyroid epithelial cell line (Nthy-ori3-1) was obtained from yipu biotechnology Co., Ltd (Wuhan, China). The cell lines were cultured in Roswell Park Memorial Institute (RPMI)-1640 (Gibco, Invitrogen, NY, USA) supplemented with 10% fetal bovine serum at 37°C in 5% CO2.
Cell transfection
Transfection was performed by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s instruction. First, one day before transfection, cells were plated in 0.5 mL of growth medium (without antibiotics); 100 pmol siRNA was diluted in 250 µL Opti-MEM I (Invitrogen catalog number 31985070) without serum (or other medium without serum), and mixed gently. After 5 minutes of incubation, the diluted siRNA was combined with the diluted Lipofectamine 2000 (total volume is 100 µL), mixed gently, and incubated for 20 minutes at room temperature to allow the siRNA: Lipofectamine 2000 complexes to form: siRNA-AP-2α (sense, GUUACCCUGCUCACAUCACdTdT; antisense, GUGAUGUGAGCAGGGUAACdTdT). The siRNAs against AP-2α were synthesized by Generalbiolsystems Co., Ltd. (Anhui, China).
Western blot analysis
Cells (BCPAP, TPC-1, Nthy-ori3-1) were washed twice with cold PBS and lysed on ice in RIPA buffer (150 mM NaCl, 1% NP-40, 0.1% sodium dodecyl sulfate [SDS], 5 mM EDTA, 50 mM Tris–HCl (pH 8.0), 0.5% sodium deoxycholate, supplemented with 50 µM leupeptin,100 µM orthovanadate, and 1 mM sodium fluoride) with protease inhibitors. All Western blotting procedures were carried out at room temperature with agitation except when stated otherwise. After centrifugation (13,000 rpm) for 20 minutes at 4°C, quantification of the total protein concentrations of AP-2α was determined using a bicinchoninic acid assay (Santa Cruz Biotechnology Inc., Dallas, TX, USA). Equal amounts of proteins were separated using SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membranes. After blocking with 5% nonfat milk in TBST buffer for 60 minutes, the membranes were incubated for 90 minutes with the indicated primary antibodies overnight at 4°C in a dilution of 1:1,000 for AP-2α (Abcam), 1:1,000 for Notch1 (Abcam), 1:600 for MMP2 (Abzoom Biolab Inc., Dallas, TX, USA), and 1:500 for N-cadherin (Abzoom). β-actin (1:2000) was used as the endogenous control. After washing, the membranes were incubated with goat peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin G and the signal was detected using a commercial enhanced chemiluminescence detection system. Protein levels were evaluated through spectrophotometry.
The Cancer Genome Atlas (TCGA) databases
Data of all 510 patients (as included in the database on February 2016) with PTC were directly downloaded from the TCGA Data Portal at https://tcga-data.nci.nih.gov/tcga/. The RNA-Seq gene expression level 3 data contain counts, which are simply the number of reads overlapping a given gene. The total number of reads for a given transcript is proportional to the expression level of the transcript, and the corresponding clinical data files were downloaded from TCGA. The exclusion criteria for patients with AP-2α mRNA were as follows: i) history of any other malignancy; ii) not primary tumor sample. The RNA-Seq gene expression level 3 data of 496 patients with PTC and clinic data were retained and further analyzed.
Gene Set Enrichment Analysis (GSEA) assay
GSEA is a computational method that determines whether an a priori defined set of genes shows statistically significant, concordant differences between two biologic states.19 TCGA Data can be ordered in a ranked list, according to their differential expression between the phenotype. AP-2α mRNA expression level was dichotomized as low and high categories to annotate phenotype, and PTC-related gene sets from MSigDB C2.cp.keggv6.0.symbols.gmt (curated) were used.
Statistical analysis
All the analyses were completed by R version 3.4.3 or Graphpad prism 5 software, with P <0.05 being considered statistically significant. Data are presented as mean ± SD. Comparisons of continuous variables were made using Student’s t-test. The Mann–Whitney U-test is a nonparametric test used to assess for significant differences in a scale variable by a single dichotomous independent variable. The association between AP-2α mRNA and the clinical pathologic features was analyzed by the Pearson’s chi-squared test or Fisher’s exact test. The Kaplan–Meier method was implemented for the relationship between AP-2α mRNA expression and overall survival (OS) in PTC patients. The cutoff value of AP-2α mRNA expression was determined by its mean value. Cox proportional hazards model was utilized for analysis of prognostic factors.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (the Xiangya Hospital of Central South University Changsha China) and with the 1964 Declaration of Helsinki and its later amendments.
Results
AP-2α is upregulated in PTC tissues and cell lines
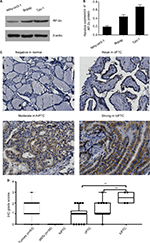
Our Western blotting analysis demonstrated that PTC cell lines (BCPAP and TPC-1) exhibited higher level of AP-2α protein expression as compared to that in Nthy-ori3-1 (Figure 1 A and B). To further explore the differences in AP-2α expression levels between tumor and nontumor tissues, we used IHC method to detect AP-2α expression level in PTC tissue specimens. Figure 1C showed that the positive substances of AP-2α were observed mainly in the cytoplasm of PTC tissues, and the representative four categories of immunostaining intensity of AP-2α were observed. Figure 1D showed that AP-2α expression was higher in tumor tissues than the corresponding ANTs.
Association of AP-2α protein and mRNA expression with clinicopathologic characteristics
The correlations between clinicopathologic parameters of PTC and AP-2α protein expression are shown in Table 1; high expression of AP-2α correlated significantly with histologic type (P<0.05). As shown in Figure 1D, AP-2α protein expression levels were higher in the tall cell or diffuse sclerosing variants of PTC (tcPTC) than in the cPTC and fvPTC based on IHC scores (P<0.01, P<0.001, separately), but not with the other clinicopathologic variables, such as the age, gender, tumor size, lymph nodes metastasis, and distant metastasis of PTC patients.
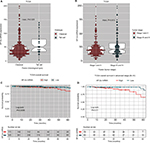
To further understand the relationship between AP-2α and clinicopathologic features, a total of 496 PTC samples with AP-2α mRNA expression data across all patient characteristics were downloaded from TCGA. As shown in Table 2, similarly, AP-2α mRNA expression was correlated with tumor stages (P=0.011) and histologic type (P=0.019). The expression of AP-2α mRNA level was higher in stage III–IV tissues than in stage I–II tumors (P=0.013, Figure 2B). Elevated AP-2α mRNA expression levels correlated well with tcPTC (P=0.025, Figure 2A). The P-value was calculated using the Pearson’s chi-squared test, whereas there was no significant correlation in patients’ age, gender, tumor size, lymph nodes metastasis, and distant metastasis.
Elevated expression of AP-2α predicts tumor progression and poor prognosis in patients with PTC
To determine the prognostic value of AP-2α, the OS rate of PTC patients (n=496) in TCGA databases was analyzed using Kaplan–Meier survival curves and the log-rank test. The result revealed that high expression of AP-2α mRNA was inversely associated with OS (log-rank test, P<0.006, Figure 2C). For patients with advanced stage (III, IV) PTC, the survival analysis showed that high AP-2α mRNA expression was associated with poorer OS rate (log-rank, P=0.011; Figure 2D), but there was no significant relationship between the AP-2α mRNA expression and the OS rate of patients with early stage (I, II) PTC or disease-free survival (data not shown, log-rank P>0.05).
Univariate analysis showed that AP-2α mRNA expression (P=0.010) and tumor stage (P=0.000) were significantly associated with the OS of PTC (Table 3). Multivariate analysis revealed that AP-2α mRNA expression was determined as independent prognostic factors in PTC (Table 3).
AP-2α-related pathways through GSEA
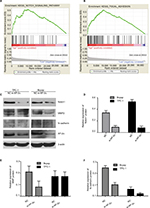
To investigate biologic properties shared by the different AP-2α expression levels, we performed GSEA assay. GSEA reveals significant differences (false discovery rate <0.25, nominal P-value <0.05) in enrichment of KEGG pathways between low-AP-2α-expression tumors and high-AP-2α-expression tumors. The top seven significant pathways for upregulated gene sets in the significance order (size of NES values) are listed in Table 4. Figure 3A and B shows that FOCAL ADHESION and NOTCH SIGNALING PATHWAY are differentially enriched in AP-2α high-expression phenotype.
AP-2α expression was associated with focal adhesion and notch signaling
To confirm the function of AP-2α on focal adhesion and notch signaling, we did Western blotting experiment using siRNA to knock down AP-2α in PTC cell lines (TPC-1, BCPAP) to detect related protein changes. As shown in Figure 3 (C–F), the results indicated that the protein expression of Notch1, MMP2, and N-cadherin was decreased in AP-2α knockdown PTC cell groups. However, no association was found between MMP2 and AP-2α in TPC-1 cell line.
Discussion
Previous studies have shown the role of transcription factor AP-2α in thyroid tumorigenesis; the PTC cell lines studied, NPA and TPC, exhibited an increase in AP-2α expression, and AP-2α mRNA expression was upregulated in the two papillary thyroid cancer cell lines (NPA, TPC).20However, the expression of AP-2α and its potential prognostic impact on PTC have not yet been explored, and the potential role of AP-2α in PTC was the focus in the present study.
The current study first investigated the clinicopathologic significance and the potential function of AP-2α in PTC with multiple methods including IHC assay, Western blot analysis, bioinformatical assay, and cellular experiments. Our IHC results demonstrated that AP-2α expression was higher in tumor tissues than the corresponding ANTs, the positive substances of AP-2α were observed mainly in the cytoplasm of PTC tissues, and the high expression of AP-2α in cytoplasm was significantly correlated with histologic type of PTC. A similar association has also recently been shown between increased AP-2α expression and subtype in ovarian cancer.16,21 We found that the upregulation of AP-2α mRNA was correlated with the advanced stage (III, IV), and histologic type of PTC using datasets obtained from the TCGA databases. AP-2α mRNA expression in PTC or in advanced stage PTC shows a significant correlation with patient survival. The multivariate analysis revealed that high AP-2α mRNA expression was an independent poor prognostic factor. GSEA results demonstrated the association between high AP-2α expression and notch signaling or focal adhesion. Further cellular experiments confirmed that Notch1, MMP2, and N-cadherin were decreased in knockdown AP-2α in PTC cell lines.
Thus, we believe that the PTC patients with different levels of AP-2α may have a differential prognosis, and patients with high expression of AP-2α have a poorer prognosis. Based on this view, we can predict and evaluate prognosis of patients with PTC. AP-2α may have the potential to play a preventive role in the progression and deterioration of the PTC patients and may be a promising therapeutic target for PTC.
AP-2α is commonly known as a suppressor gene by regulation of the protein levels of Blc-2 and ErbB2, phosphorylated extracellular signal-regulated protein kinase, β-catenin, p53, epithelial–mesenchymal transition (EMT), and CD133 expression in tumors, such as lung cancer, hepatocellular carcinoma, human glioma, and gastric cancer.8,9,11,22 However, there is accumulating evidence supporting that elevated level of AP-2α showed a significant expression in cancer and activated multiple growth factor signaling pathways, correlated with cancer progression and decreased survival.13,23,24 Thus, the role of AP-2α potentially acting as an oncogene is context-dependent. Using Western blot analysis and IHC analysis, we found that AP-2α had higher expression in PTC cell lines and PTC tissues as compared to normal cell line and tissues, suggesting an oncogene role. To our knowledge, this should be the fourth case report to show such effects.
One accepted explanation is that dual roles of AP-2α may be due to differences in promoter activity.25 However, our results may not support this view. It is generally accepted that transcription factors, for their effective functioning, need to be in the nucleus. But AP-2α protein expression in PTC tissues was observed in the cytoplasm. Thus, we believe that AP-2α may exert additional biochemical functions in the cytoplasm.
Some studies have showed that AP-2α was found in both the nucleus and cytoplasm, such as hepatocellular carcinoma, melanoma, ovarian cancer, stomach cancer, and breast cancer. The past research found that difference in subcellular localization of AP-2α expression may be associated with prognoses and progression. Ramona Britto et al21 found that decreased nuclear expression was indicative of a better prognosis, and observed the shift in localization of this protein from the nucleus to the cytoplasm with increasing tumor grade, pointing to a crucial role for this transcription factor in the progression of astrocytoma. But there are some different views for other researchers on the shift in localization, and MA Anttila16 found that the high cytoplasmic AP-2α expression favored the OS. In contrast, the nuclear AP-2α expression combined with low cytoplasmic expression increased the risk of dying of ovarian cancer. But the present study shows that the subcellular localization of AP-2α expression in PTC was almost entirely in the cytoplasm, having very little in nucleus. This may imply a novel function of AP-2α in contrast to previous accepted view of transcriptional regulation.
To explore the mechanism of AP-2α in PTC, we perform the GSEA assay. GSEA results demonstrated the association between high AP-2α expression and focal adhesion or notch signaling. Further cellular experiments confirmed that Notch1, MMP2, and N-cadherin were decreased in knockdown AP-2α in PTC cell lines. These protein markers are associated with EMT. We conclude that AP-2 may affect PTC prognosis through EMT. Further experimental validation should be performed to prove the biologic mechanism of AP-2α.
In conclusion, we provide evidence that AP-2α predicts the prognosis of PTC patients. AP-2α might serve as an independent prognostic factor in PTC patients through EMT, and it may help clinical oncologists to render more rational and efficient treatment for PTC patients.
Acknowledgments
The results shown here are in whole or part based upon data generated by the TCGA Research Network: http://cancergenome.nih.gov/.
This study was funded by the Technology Project of Changsha, China (grant number: kq1701096) and the School Independent Innovation Project of Central South University (grant number: 2016zzts130).
Disclosure
The authors report no conflicts of interest in this work.
References
Lim H, Devesa SS, Sosa JA, Check D, Kitahara CM. Trends in thyroid cancer incidence and mortality in the United States, 1974-2013. JAMA. 2017;317(13):1338–1348. | ||
Jung CK, Little MP, Lubin JH, et al. The increase in thyroid cancer incidence during the last four decades is accompanied by a high frequency of BRAF mutations and a sharp increase in RAS mutations. J Clin Endocrinol Metab. 2014;99(2):E276–E285. | ||
Hay ID, Thompson GB, Grant CS, et al. Papillary thyroid carcinoma managed at the Mayo Clinic during six decades (1940-1999): temporal trends in initial therapy and long-term outcome in 2444 consecutively treated patients. World J Surg. 2002;26(8):879–885. | ||
C.G.A.R. Network, Cancer Genome Atlas Research Network, Network CGAR. Integrated genomic characterization of papillary thyroid carcinoma. Cell. 2014;159(3):676–690. | ||
Delellis RA, Lloyd RV, Heitx PU. Pathology and Genetics of Tumors of Endocrine organs. in World Health Organization of Tumours. Lyon: IARC Press; 2004:73–6 p. | ||
Leung AK, Chow SM, Law SC. Clinical features and outcome of the tall cell variant of papillary thyroid carcinoma. Laryngoscope. 2008;118(1):32–38. | ||
Kerr CL, Zaveri MA, Robinson ML, Williams T, West-Mays JA. AP-2α is required after lens vesicle formation to maintain lens integrity. Dev Dyn. 2014;243(10):1298–1309. | ||
Su W, Xia J, Chen X, et al. Ectopic expression of AP-2α transcription factor suppresses glioma progression. Int J Clin Exp Pathol. 2014;7(12):8666–8674. | ||
Meng X, Meng C, Yang B, et al. AP-2α downregulation by cigarette smoke condensate is counteracted by p53 in human lung cancer cells. Int J Mol Med. 2014;34(4):1094–1100. | ||
Wang W, Lv L, Pan K, et al. Reduced expression of transcription factor AP-2α is associated with gastric adenocarcinoma prognosis. PLoS One. 2011;6(9):e24897. | ||
Huang W, Chen C, Liang Z, et al. AP-2α inhibits hepatocellular carcinoma cell growth and migration. Int J Oncol. 2016;48(3):1125–1134. | ||
Yan F, He Q, Hu X, et al. Direct regulation of caspase3 by the transcription factor AP2α is involved in aspirininduced apoptosis in MDAMB453 breast cancer cells. Mol Med Rep. 2013;7(3):909–914. | ||
Ding X, Yang Z, Zhou F, et al. Transcription factor AP-2α regulates acute myeloid leukemia cell proliferation by influencing Hoxa gene expression. Int J Biochem Cell Biol. 2013;45(8):1647–1656. | ||
Zhou J, Duan H, Xie Y, et al. MiR-193a-5p targets the coding region of AP-2α mRNA and induces cisplatin resistance in bladder cancers. J Cancer. 2016;7(12):1740–1746. | ||
Shi D, Xiao X, Tian Y, et al. Activating enhancer-binding protein-2α induces cyclooxygenase-2 expression and promotes nasopharyngeal carcinoma growth. Oncotarget. 2015;6(7):5005. | ||
Anttila MA, Kellokoski JK, Moisio KI, et al. Expression of transcription factor AP-2alpha predicts survival in epithelial ovarian cancer. Br J Cancer. 2000;82(12):1974–1983. | ||
Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC Cancer Staging Manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. | ||
Kaemmerer D, Peter L, Lupp A, et al. Comparing of IRS and Her2 as immunohistochemical scoring schemes in gastroenteropancreatic neuroendocrine tumors. Int J Clin Exp Pathol. 2012;5(3):187. | ||
Subramanian A, Tamayo P, Mootha VK, et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci U S A. 2005;102(43):15545–15550. | ||
Bhogal N, Wishnia S, Wang Y, Zeiger M, Libutti S, Rosen J. The role of transcription factor AP-2α in thyroid tumorigenesis. Cancer Res. 2007;67:1234–1234. | ||
Britto R, Umesh S, Hegde AS, et al. Shift in AP-2alpha localization characterizes astrocytoma progression. Cancer Biol Ther. 2007;6(3):413–418. | ||
Zeng C, Liu Z, Zhang J, et al. Functions of the AP-2α gene in activating apoptosis and inhibiting proliferation of gastric cancer cells both in vitro and in vivo. Arch Med Sci. 2017;13(6):1255. | ||
Shi D, Xie F, Zhang Y, et al. TFAP2A regulates nasopharyngeal carcinoma growth and survival by targeting HIF-1α signaling pathway. Cancer Prev Res. 2014;7(2):266–277. | ||
Bennett KL, Romigh T, Eng C. AP-2alpha induces epigenetic silencing of tumor suppressive genes and microsatellite instability in head and neck squamous cell carcinoma. PLoS One. 2009;4(9):e6931. | ||
Rietveld LE, Koonen-Reemst AM, Sussenbach JS, Holthuizen PE. Dual role for transcription factor AP-2 in the regulation of the major fetal promoter P3 of the gene for human insulin-like growth factor II. Biochem J. 1999;338(3):799–806. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.