Back to Journals » Infection and Drug Resistance » Volume 15
Aortic Involvement in Disseminated Tuberculosis – Challenges Beyond the Diagnosis
Authors Shi Y, Guo W, Hu W, Li X, Shangguan Y, Feng X, Hu M, Xia J, Wang S, Li J, Hong L, Lu B, Xu K
Received 4 February 2022
Accepted for publication 29 April 2022
Published 23 May 2022 Volume 2022:15 Pages 2633—2638
DOI https://doi.org/10.2147/IDR.S361168
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Suresh Antony
Yunzhen Shi,1,* Wanru Guo,2,* Wenjuan Hu,2 Xiaomeng Li,2 Yanwan Shangguan,2 Xuewen Feng,2 Ming Hu,2 Jiafeng Xia,2 Shuting Wang,2 Jingnan Li,1 Li Hong,1 Bin Lu,1 Kaijin Xu2
1Department of Infectious Diseases, Dongyang People’s Hospital, Dongyang, People’s Republic of China; 2State Key Laboratory for Diagnosis and Treatment of Infectious Diseases, National Clinical Research Center for Infectious Diseases, Collaborative Innovation Center for Diagnosis and Treatment of Infectious Diseases, The First Affiliated Hospital, College of Medicine, Zhejiang University, Hangzhou, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Kaijin Xu, Email [email protected]
Abstract: Tuberculous aortitis (TA) is a rare disease with a high mortality rate. Aortic pseudoaneurysm is the most common vascular pattern of TA, and isolated arterial wall thickening and arterial stenosis can also be seen in TA. We report two cases of disseminated tuberculosis involving the aorta with clinical improvement after treatment. One patient who had an aortic ulcer and intermural hematoma received anti-tuberculosis along with steroids therapy. The other patient, who developed a tubercular abdominal aortic pseudoaneurysm during anti-tuberculosis therapy, successfully received endovascular stent implantation. Clinicians should be aware that TA should be considered in patients with aortitis and active tuberculosis.
Keywords: tuberculosis aortitis, aortic pseudoaneurysm, anti-tuberculosis therapy, steroids, endovascular stent implantation
Introduction
Tuberculous aortitis (TA) is a rare disease that involves the aorta and is usually associated with disseminated tuberculosis. TA is related to the formation of atherosclerosis, stenosis, and aneurysms; and it may lead to fatal complications such as aortic wall perforation.1 The clinical manifestations of Mycobacterium infection of the aorta vary from asymptomatic, chest pain, and elevated blood pressure to haemorrhagic shock.2 Therefore, TA should be considered in patients with both aortitis and active tuberculosis. Early diagnosis is crucial, and timely anti-tuberculosis and appropriate surgical treatment are the keys to success. Here, we report two cases of disseminated tuberculosis involving the aorta: one with an aortic ulcer and intermural haematoma, and another with an aortic pseudoaneurysm. The patients were followed up dynamically, and the diagnosis and treatment process of the disease were recorded.
Case 1
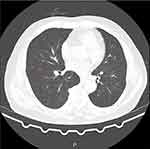
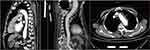
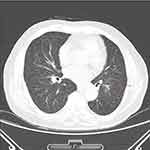
A 78-year-old man was admitted due to recurrent fever and fatigue for past one year. Two weeks prior to admission, his fatigue intensified, and the highest temperature reached 39.5℃. He had lost 5kg over the past year and had a history of cerebral infarction and hypertension. On examination, the patient was febrile at 38℃. His C-reactive protein (CRP) (62.49 mg/dl), procalcitonin (0.213ng/mL) and erythrocyte sedimentation rate (84 mm/h) were elevated. Computed tomography (CT) of the chest revealed diffusely distributed nodules in both lungs, bilateral bronchiolitis, and tuberculosis could not be excluded. The aortic arch was thickened and the local calcified plaque moved inward, which indicated further aortic computed tomography angiography (CTA) examination (Figure 1). Echocardiography revealed no vegetation in the mitral and aortic valve areas. The chest CTA confirmed multiple atherosclerosis and ulcers, and intermural hematoma in the aortic arch and thoracic aorta. Combined with the medical history, the involvement of tuberculosis in the aorta should be highly suspected (Figure 2). Re-examination of lung CT revealed bronchiole lesions and multiple miliary foci in both lungs, which were larger than one week prior, with pleural effusion, and slightly thickened pleura, suggestive of miliary tuberculosis (Figure 3). The T cell spot test, sputum culture, and sputum GeneXpert test were positive for Mycobacterium tuberculosis and GeneXpert indicated sensitivity to rifampicin. Since disseminated tuberculosis was definite and the patient’s disease potentially involved the major thoracic vessels, anti-tuberculosis and steroids were initiated immediately. Methylprednisolone was administered at 40mg/day for 3 days, 20mg/day for 3 days, 8mg/day for 3 days, 4mg/day for 7 days, and then discontinued. Due to liver dysfunction, with alanine aminotransferase of 161U/L and aspartate aminotransferase of 203U/L, a regimen consisting of meropenem 1 g every 8 h, linezolid 0.6 g once daily, amikacin 0.4 g once daily, and levofloxacin 0.5 g once daily was administered. Surgical consultation concluded that no immediate surgical intervention was needed because the aortic wall was not ruptured. On normalisation of liver function, the anti-tuberculosis regimen was changed to isoniazid 0.3 g once daily, rifapentine 0.45 g twice a week, linezolid 0.6 g once daily, ethambutol 0.75 g once daily, levofloxacin 0.5 g once daily. After two weeks of treatment, the patient’s body temperature returned to normal, and a re-examination of the lung CT indicated that the lesion was partially absorbed (Figure 4). The patient was discharged and continued to receive oral medications. We planned the follow-up to be weekly initially, gradually transitioning to every two weeks, and then monthly after stabilisation. Routine blood, liver and kidney functions were examined at each follow-up visit. Sputum smears and cultures were performed monthly. Pulmonary CT was re-examined two months later, and aortic CTA was re-examined four months later. The patient tolerated and was compliant with anti-tuberculosis drugs.
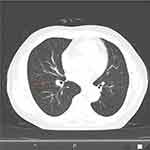 |
Figure 4 Chest CT showing: partially absorption of the miliary foci, suggesting that anti-tuberculosis treatment is effective. |
Case 2
A 61-year-old woman presented with intermittent low back pain that persisted for past one month. The pain worsened within one week and became unbearable. Three months previously, the patient was admitted to a local hospital for productive cough. Lung CT showed suspicious TB lesions, and T-SPOT was positive. After excluding other diseases, the local hospital started diagnostic anti-tuberculosis treatment consisting of HRZE. The patient took the medication irregularly because of gastrointestinal reaction. A physical examination revealed no abnormalities. Laboratory results were white blood cells, 7.22×109/L; hemoglobin, 122 g/L; platelets, 216 ×109/L; CRP, 32.97 mg/dl; and procalcitonin, 0.213ng/mL. The sputum GeneXpert test result was positive and sensitive to rifampicin. Multiple infections and diffuse miliary nodules in both lungs suggested tuberculosis. Thoracic and abdominal aortic CTA examination showed a 51×28 mm2 pseudoaneurysm arising from the lower abdominal aorta, surrounded by a patchy shadow and lymphadenopathy (Figure 5). Combined with the patient’s medical history, tuberculous aortic pseudoaneurysm was considered. Emergency digital subtraction angiography was performed after admission, and two coated stents were implanted in the abdominal aorta and iliac artery. Angiography showed that the stents were well- positioned, blood flow was smooth, and a pseudoaneurysm did not develop. The patient was discharged from the hospital 2 days post-operation and received anti-tuberculosis drug treatment, including isoniazid 0.6 g once daily, rifapentine 0.45 g twice a week, ethambutol 0.75 g once daily, pyrazinamide 0.5 g three times daily, and moxifloxacin 0.4 g once daily. Abdominal aortic CTA performed two weeks later revealed that the aneurysm did not recur (Figure 6). Our follow-up plan was consistent with that of the patient in case 1. The patient tolerated the anti-tuberculosis drugs well.
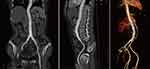 |
Figure 6 Abdominal aortic CTA showing: no recurrence of the abdominal aortic aneurysm. |
Discussion
Our case highlights the different manifestations of TA along with its diagnosis and treatment processes. Disseminated tuberculosis is a critical tuberculosis, caused by the entry of M. tuberculosis into the blood circulation, resulting in lung and systemic lesions in multiple organs.3 A normal aorta is generally immune to infection. The risk factors include atherosclerosis, stent implantation, surgery, diabetes, tumours, immunosuppressive therapy, etc.4 In addition, poor compliance and irregular use of anti-TB drugs increase the risk of aortic involvement. Tuberculosis that invades the aorta is a very rare and life-threatening condition. Mycobacteria can invade the aortic wall in three ways: (1) direct extension from adjacent lesions, (2) direct implantation on the inner surface of the vascular wall, and (3) spread from distant lesions through blood circulation, the first being the most common.2
Aortitis generally presents with no specific symptoms. Therefore, aortitis with systemic symptoms and diseases can be easily ignored, especially in elderly patients or patients at high risk of open surgery, where a clear diagnosis of TA is often challenging. The median delay between the first onset of symptoms and start of anti-tuberculosis treatment was 18 months.5 M. tuberculosis culture is negative in most cases, and clinicians should be aware that aortic lesions may be caused by M. tuberculosis even in the absence of microbiological confirmation. Imaging examination can be used to evaluate the aortic wall and lumen to aid in the diagnosis and monitoring of outcomes; however, calcification of the aortic wall cannot be used to distinguish infection from atherosclerosis.6 Infectious aortic disease is characterised by fever; fatigue; and elevated levels of leukocytes, neutrophils, ESR, CRP, and procalcitonin. The patient in Case 1 was elderly with a long history of hypertension and diabetes, and had an atherosclerotic basis and risk factors for aortic infection. In addition, the patient was confirmed to have miliary tuberculosis. Furthermore, chest CTA showed multiple aortic ulcers combined with intermural haematoma, supporting the diagnosis of tuberculosis involving the aorta, which may lead to rupture of the aorta, bleeding, and shock if not treated in a timely manner. Studies have shown that early use of steroids may be useful in reducing inflammation and thickness of the aortic wall.1,7 Our patient was started on steroids while receiving anti-tuberculosis therapy. His clinical condition improved and he will be followed up for a long time. We recommend the follow-up frequency to gradually transition from weekly to biweekly to monthly. Patients should monitor body temperature, heart rate, and blood pressure daily at home, and complete blood routine, urine routine (to determine whether the renal artery is involved), liver function, renal function, and D-dimer levels during every follow-up. If symptoms are stable, CTA or DSA can be reviewed after 3–4 months to understand the lesions condition, and if symptoms worsen, medical attention should be sought immediately.
TA is involved in aneurysm formation in approximately half of the cases.8 The high death rate from this disease is associated with haemorrhagic shock from aortic rupture, because most tuberculous aortic aneurysms are not diagnosed until they rupture.9 Tuberculous aneurysms progress rapidly and rupture at a much higher rate than atherosclerotic aortic aneurysms owing to the tissue damage caused by infection. It may occur during anti-tuberculosis therapy, and the emergence of new symptoms or sudden deterioration of the patient’s general condition during treatment are important clues.10,11 As in Case 2, the patient developed low back pain during anti-tuberculosis treatment, which prompted us to perform further aortic CTA examination, leading to the identification of aortic aneurysms. Tuberculous aneurysms are rare and fatal, and there are no randomised, double-blind, controlled studies or unified treatment standards. The conventional treatment for tuberculous aortic aneurysms is surgery combined with anti-tuberculosis drugs, which should be continued until the tuberculosis foci are cleared.12 In recent years, endovascular stent implantation has been reported to have good therapeutic effects.13 The patient in Case 2 received minimally invasive endovascular treatment and achieved clinical improvement. Considering that tuberculosis infection may persist, the duration of anti-tuberculosis treatment should be determined according to the patient’s condition. Further research is needed to explore faster diagnostic and best treatment methods.
Conclusion
TA can not only present as a pseudoaneurysm of the main artery, but also as isolated arterial wall thickening and arterial stenosis. Early diagnosis, and timely anti-tuberculosis and appropriate surgical treatments are keys to successful recovery.
Abbreviations
TA, tuberculous aortitis; CT, computed tomography; CTA, computed tomography angiography; CRP, C-reactive protein.
Ethics Approval and Consent for Publication
The study was approved by Ethics Committee of Dongyang People’s Hospital (2022-YX-007). The institutional has approved the publication of case details. Written informed consent was provided by the patients to allow the case details to be published.
Acknowledgments
We would like to thank the patients for their support.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Delaval L, Goulenok T, Achouh P, et al. New insights on tuberculous aortitis. J Vasc Surg. 2017;66(1):209–215. doi:10.1016/j.jvs.2016.11.045
2. Long R, Guzman R, Greenberg H, et al. Tuberculous mycotic aneurysm of the aorta: review of published medical and surgical experience. Chest. 1999;115(2):522–531. doi:10.1378/chest.115.2.522
3. Sharma SK, Mohan A, Sharma A, et al. Miliary tuberculosis: new insights into an old disease. Lancet Infect Dis. 2005;5(7):415–430. doi:10.1016/s1473-3099(05)70163-8
4. Mearelli F, Burekovic I, Zanetti M, et al. Disseminated tuberculosis in an immunocompetent patient. Int J Infect Dis. 2013;17(9):e784–e786. doi:10.1016/j.ijid.2013.02.026
5. Hsu YC, Luo CY, Tsai YS. Tuberculous aortitis associated with pleuritis and spondylitis. Heart Lung Circ. 2018;27(6):e84–e85. doi:10.1016/j.hlc.2017.12.010
6. Lin MM, Cheng HM. Images in cardiovascular medicine: tuberculous aortitis. Intern Med. 2012;51(15):1983–1985. doi:10.2169/internalmedicine.51.7690
7. Critchley JA, Young F, Orton L, et al. Corticosteroids for prevention of mortality in people with tuberculosis: a systematic review and meta-analysis. Lancet Infect Dis. 2013;13(3):223–237. doi:10.1016/s1473-3099(12)70321-3
8. Bukhary ZA, Alrajhi AA. Tuberculous aortitis. Ann Saudi Med. 2006;26(1):56–58. doi:10.5144/0256-4947.2006.56
9. Choi JB, Yang HW, Oh SK, et al. Rupture of ascending aorta secondary to tuberculous aortitis. Ann Thorac Surg. 2003;75(6):1965–1967. doi:10.1016/s0003-4975(02)05017-8
10. Park SC, Moon IS, Koh YB. Tuberculous pseudoaneurysm of the descending thoracic aorta. Ann Vasc Surg. 2010;24(3):
11. Choudhary SK, Bhan A, Talwar S, et al. Tubercular pseudoaneurysms of aorta. Ann Thorac Surg. 2001;72(4):1239–1244. doi:10.1016/s0003-4975(01)03002-8
12. Silbergleit A, Arbulu A, Defever BA, et al. Tuberculous aortitis: surgical resection of ruptured abdominal false aneurysm. JAMA. 1965;193:333–335. doi:10.1001/jama.1965.03090050009003
13. Dogan S, Memis A, Kale A, et al. Endovascular stent graft placement in the treatment of ruptured tuberculous pseudoaneurysm of the descending thoracic aorta: case report and review of the literature. Cardiovasc Intervent Radiol. 2009;32(3):572–576. doi:10.1007/s00270-008-9456-8
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.