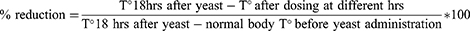Back to Journals » Journal of Experimental Pharmacology » Volume 15
Antipyretic Potential of 80% Methanol Extract and Solvent Fractions of Bersama abyssinica Fresen. (Melianthaceae) Leaves Against Yeast-Induced Pyrexia in Mice
Authors Tegegne BA , Alehegn AA
Received 25 December 2022
Accepted for publication 22 February 2023
Published 28 February 2023 Volume 2023:15 Pages 81—91
DOI https://doi.org/10.2147/JEP.S390825
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
Bantayehu Addis Tegegne,1 Agumas Alemu Alehegn2
1Department of Pharmacy, College of Health Sciences, Debre Markos University, Debre Markos, Ethiopia; 2Department of Pharmacy, Lumame Primary Hospital, Lumame, Ethiopia
Correspondence: Bantayehu Addis Tegegne, Email [email protected]
Introduction: Since fever is a complicated physiological reaction to an infection or aseptic stimulus, finding safer solutions that are more potent and derived from plants is essential to resolving this issue. Bersama abyssinica (Melianthaceae) is traditionally used to treat fever, though this has yet to be proven scientifically.
Objective: The present study aimed to assess the antipyretic potential of leaf extract and solvent fractions of B. abyssinica.
Methods: The antipyretic activities of crude extract and solvent fractions of B. abyssinica leaves were evaluated using a yeast-induced pyrexia model at three different dose ranges (100mg/kg, 200mg/kg, and 400mg/kg) methanol extract as well as chloroform, ethyl acetate, and aqueous fractions to mice showing an increase in temperature of ≥ 0.5 °C. The rectal temperature of each mouse was recorded using a digital thermometer. To analyze the data, SPSS version 20 and one-way ANOVA followed by Tukey’s HSD post hoc test to compare results between groups were utilized.
Results: The crude extract demonstrated significant antipyretic potential (P< 0.05 by 100 mg/kg and 200 mg/kg as well as P< 0.01 by 400 mg/kg), with a maximum of 95.06% reduction in rectal temperature at 400 mg/kg, comparable to 98.37% at 2.5 hours by the standard drug. Similarly, all doses of the aqueous fraction, as well as 200 mg/kg and 400 mg/kg doses of the ethyl acetate fractions, resulted in a significant (P< 0.05) reduction in rectal temperature when compared to the corresponding value of the negative control group.
Conclusion: Extracts of B. abyssinica leaves were found to have a significant antipyretic effect. Thus, the use of the plant for pyrexia in traditional settings has scientific ground.
Keywords: antipyretic activity, extract, fraction, B. abyssinica leaves, yeast-induced pyrexia
A Letter to the Editor has been published for this article.
A Response to Letter by Mrs Intan has been published for this article.
Introduction
When a person’s body temperature rises above the normal range (36.5–37.5°C) due to an infection, tissue damage, malignancy, transplant rejection, or other inflammatory disease conditions, they are said to have a fever.1 Up to 75% of extremely sick patients experience fever or pyrexia. It occurs in 19 to 30% of pediatric emergency visits, which creates tension among parents.2
The pyrogenic exogenous and endogenous substances are necessary for the initiation, manifestation, and management of the febrile reaction. Exogenous pyrogens, including interleukin-1b, enhance the creation and release of internal pyrogens, which causes it (IL-1b). On the other hand, endogenous pyrogens relocate to the hypothalamic organum vasculosum of the lamina terminalis (OVLT), where they induce the production of prostaglandins E2 (PGE2). Due to this, the thermostatic set point rises, which results in a feverish reaction.3,4
White blood cells extravasate into inflamed areas as a result of the inflammatory reaction brought on by microbial tissue invasion, which also activates local vascular endothelial cells and leukocytes. Activated leukocytes all produce pyrogenic cytokines, including IL-1b, Tumor necrosis factor (TNF), and IL-6. Endogenous pyrogens go through the bloodstream to the brain, where they induce the inducible cyclooxginous-2 (COX2) enzyme to increase the formation of PGE2 by vascular endothelial cells.5
E-prostanoid receptors are found in neurons in the pre-optic area of the anterior hypothalamus (POAH), the brain site of the primary thermoregulatory controller that compares and integrates central and peripheral thermal information. PGE2 then works by binding to the type 3 PGE2 receptor on glial cells, resulting in the production and release of cyclic adenosine monophosphate (cAMP) which acts as a neurotransmitter, activating thermosensitive neurons to raise the thermostatic set point from normothermic to fever levels, signaling efferent nerves, particularly sympathetic fibers that innervate peripheral blood vessels.6,7
On the other hand, currently available antipyretic nonsteroidal anti-inflammatory drugs (NSAIDs) have been linked to gastrointestinal side effects, renal and liver dysfunction, and a slew of other problems.5 Selective-cyclooxygenase-2 (COX-2) inhibitors like celecoxib, valdecoxib, and rofecoxib, can alleviate gastrointestinal side effects to some extent; however, these agents are toxic to hepatic cells, glomeruli, the cortex of the brain, and the heart muscles.
As a result, research into the discovery of new antipyretic agents that are safe, more effective, and less expensive, particularly from natural products, is encouraged.5,8,9 A large portion of the global healthcare industry is comprised of medicinal plants and herbal treatments. At least 80% of people now rely on herbal remedies and dietary supplements in some capacity for their basic healthcare, a dramatic growth in usage over the previous three decades.10,11
B. abyssinica (Melianthaceae) leaves, seeds, roots, stem barks, and other parts of this plant have been used to treat malaria and fever.12–17 The leaf decoction is used to treat feverish pains, loss of appetite, and other ailments.18 The leaves, stem barks, and roots of the plant have also been used to treat abdominal pain, colic, diarrhea, cholera, intestinal worms, amoebiasis, dysentery, and mycobacterial infections.19,20 Furthermore, It is empirically used to treat diseases such as headaches, diabetes, typhoid, and hemorrhoid.21
Phytochemical screening tests showed the presence of phenols, alkaloids, flavonoids, glycosides, tannins, steroids, anthraquinones, triterpene, polysterols, and coumarins.22 Terpenes, flavonoids, steroids, vitamins, carotenoids, and saturated and unsaturated fatty acids with anti-microbial, insecticidal, antiseptic, preservative, anti-tumor, and antioxidant activities were discovered.23 The antibacterial,22,24,25 anti-mycobacterial,26 antifungal,25 antiviral /anti-HIV-1 HIV-2,27 antioxidant,28 and antispasmodic29 activities of the plant material have been confirmed through different methods.
Materials and Methods
Material/Drugs/Chemicals
Chemicals, reagents, drugs, equipment, and supplies utilized in this study were: normal saline solution (Medsol pharmaceuticals manufacturing, Addis Ababa, Ethiopia), methanol (Alpha Chemika, India), ethyl acetate (Atico, India), chloroform (Atico, India), distilled water, trisodium citrate (Sheba pharmaceuticals, Addis Ababa, Ethiopia), digital electronic balance (EPH-400 Abron Exports), Acetylsalicylic acid (Bayer Schering Pharma AG, Germany), yeast extract powder (Titan Biotech ltd, India), micro-hematocrit centrifuge (Medit-Medizin Technik, Germany), digital thermometer (Infiniti Medlab instruments group co., ltd, Germany), qualitative Whatman filters paper No. and oral feeding tube.
Plant Sample Collections and Deposition of a Voucher Specimen
In December 2018, B. abyssinica leaves were collected from Tara Gedam, South Gondar zone, Amhara region, 85 kilometers south of Gondar city. Mr. Abiyou Enyew, a botanist from the biology department at the University of Gondar, verified the authenticity of the plant material collected, and a voucher specimen (voucher no. 001AAA) was deposited in the biology department’s herbarium for future use.
Preparation and Extraction of Plant Material
After cleaning with tap water and drying at room temperature, the leaves were ground into powder and kept safely till extraction commenced. In April 2019, a total of 1500 grams of powdered plant materials were macerated in 15 liters of 80% methanol at room temperature with occasional shaking. After three days, the extract was separated from the residue using gauze followed by Whatman filter paper No. 1. The residue was macerated for an additional three days using an equal volume of solvent each time. The filtrates were dried at a temperature of 40°C to obtain the crude extract. By freeze-drying, the extract was further concentrated using a Lyophilizer. The percentage yield was calculated by measuring the weight of the dry extract and expressing it as a percentage of the total mass of dry plant matter.22
Solvent Fractionation Methods
The sequential solvent partitioning technique was used to further fractionate the crude extract by different solvents of increasing polarity. Using a separatory funnel, 90 grams of the crude extract was diluted in 400 mL of distilled water. To get the chloroform fraction, the aqueous portion was suspended three times with 400 mL of chloroform. Then, the aqueous residue was further suspended three times with 400 mL of ethyl acetate to get the ethyl acetate fraction. Lastly, the aqueous portion was collected as the third fraction. A hot air oven of 40 °C was used to concentrate the chloroform and ethyl acetate fractions, while the aqueous portion was frozen in the refrigerator overnight and then, dried using a lyophilizer. Finally, the percentage yield of the fractions was calculated.30
Experimental Animals
The Ethiopian Public Health Institute (EPHI) provided 100 healthy Swiss Albino mice of either sex (25±5 g 6 to 8 weeks old). The mice were caged per international guidelines (Organization for Economic Cooperation and Development (OECD) and Institute for Laboratory Animal Research (ILAR)).31,32 Before conducting the actual research, the animals were allowed to acclimatize to the laboratory condition for a week.
Acute Oral Toxicity Testing
The crude extract’s acute oral toxicity was tested using five female albino mice (6–8 weeks) per the organization for economic cooperation and development (OECD-425) guidelines. The mice fasted for three hours before and one hour after receiving the extract. The first animal was given a limited dose of 2 g/kg and no mortality or severe toxicity was observed. Then the limit dose was also given for the four additional animals based on the result from the first animal. The mice were placed in a separate cage and observed for signs of behavioral and physical toxicity. Over a single day, the mice were observed continuously for the first 30 minutes and intermittently for the next 4 hours.33
Antipyretic Activities of Crude Extract and Solvent Fractions
According to Shibru T et al (2015), the yeast-induced fever (pathogenic fever) in the mice model is the most commonly used model for investigating the antipyretic potentials of unknown substances,34 was adopted for this study to investigate the antipyretic activity of the crude extract and solvent fractions. Before the experiment, mice were fasted overnight and given free access to water. Each mouse’s initial basal rectal temperature was measured with a digital thermometer by inserting a thermistor probe about 1 cm into the rectum. Pyrexia was then induced in all mice by subcutaneously injecting a 30% w/v yeast extract powder suspension in 0.9% normal saline at a dose of 3 g/kg below the nape of the neck. After 18 hours of yeast administration, the rectal temperature of each mouse has been measured again to coincide with the stable or peak phase of the fever, indicating an appropriate time to test the antipyretic activity of drugs.35 The experiment used only mice that showed a temperature increase of ≥ 0.5 °C after yeast injection.
Animal Grouping for the Crude Extract
There were five groups: Group I: served as the negative control, receiving 10 mL/kg of distilled water; Groups II, III, and IV were given 100 mg/kg, 200 mg/kg, and 400 mg/kg crude extract, respectively. While group V was given the standard drug (ASA 100 mg/kg).
Animal Grouping for the Solvent Fractions
There were eleven different groups (Figure 1) for each fraction (chloroform, ethyl acetate, and aqueous fraction): Group, I served as the negative control, receiving 10 mL/kg of distilled water; group II–IV were given 100, 200, and 400 mg/kg doses of Aqes fraction; Group V–VII were given 100, 200, and 400 mg/kg doses of ethyl fraction, and group VIII–X were given 100, 200, and 400 mg/kg doses of chloroform fraction. While group XI was given the ASA 100 mg/kg). Finally, each mouse’s temperature was recorded at 0.5, 1, 1.5, 2, 2.5, and 3 hours following dosing. The following formula was used to compute the percentage reduction in rectal temperature (antipyresis).36
Data Management and Statistical Analysis
The results of each group were determined using Microsoft Excel 2013, while the statistical evaluation and significance of various treatments were calculated using a one-way Analysis of Variance (ANOVA) followed by Tukey’s HSD post hoc test. All results were expressed as mean ± Standard error of the mean for each group and were considered statistically significant at a 95% confidence level and a P-value of 0.05. SPSS version 20 was used to analyze the data.
Results
The Yield of Crude Extract and Fractions
As shown in Table 1, the aqueous fraction provided the highest percentage yield.
 |
Table 1 Yields of 80% Methanol Crude Extract and Solvent Fractions |
Acute Toxicity Test of Crude Extract
There was no mortality observed during the acute toxicity study at a limited dose of 2 gm/kg. During the observation periods, no obvious physical or behavioral toxicity was observed. This indicates that the crude extract has a lethal dose (LD50) greater > than 2 g/kg.
Antipyretic Activity of the Crude Extract
Table 2 and Figure 2 show the antipyretic effect of B. abyssinica leaf crude extract. After 18 hours, all yeast suspension-injected animals developed fevers with rectal temperatures ranging from 38.03±0.26 to 38.68±0.42 °C. At a dose of 200 mg/kg, the crude extract significantly reduced yeast-induced fever (P < 0.01 at 2, 2.5, and 3 hours) when compared to the same group’s 0-hour rectal temperature. The 400 mg/kg dose of the crude extract also resulted in a significant reduction in fever (P < 0.05 at 1.5, 2, 2.5, and 3 hours) when compared to the same group’s 0-hour rectal temperature. When compared to the control group, all doses of B. abyssinica crude extract resulted in a significant (p < 0.05 with 100 mg/kg and 200 mg/kg, and p < 0.01 with 400 mg/kg) reduction in rectal temperature. The percentage reduction in rectal temperature increases with time for the crude extract and standard drug. The antipyretic effects of the standard drug and the 400 mg/kg dose of crude extract began as early as 1.5 hours after administration, whereas the antipyretic effects of the 200 mg/kg and 100 mg/kg doses of crude extract began as early as 2 hours after administration and progressed throughout the experiment. These effects were most noticeable at the 2nd and 2.5th hours after treatment. The maximum antipyretic effect (95.06%) was observed after 2.5 hours of treatment with crude extract at 400 mg/kg, which is comparable to the standard drug’s 98.37% at 2.5 hours (Figure 2).
 |
Table 2 Effect of B. abyssinica Crude Extract on Yeast-Induced Pyrexia in Mice |
 |
Figure 2 Percent reduction in rectal temperature by crude extract on yeast-induced pyrexia in mice. n=6. Abbreviations: DW, distilled water; ASA, Acetylsalicylic acid; h, hour. |
Antipyretic Activities of Solvent Fractions
The results of the antipyretic activities of solvent fractions of the leaves of B. abyssinica were summarized in Table 3 and Figures 3-5. The aqueous fraction at 200 mg/kg (p < 0.001 at 1.5, 2, 2.5, and 3 hours) and ethyl acetate fraction at 200 and 400 mg/kg (p < 0.01 at 1.5, 2, 2.5, and 3 hours) were able to significantly reduce yeast induced fever as compared to the 0-hour rectal temperature of the same group. On the other hand, all doses of the aqueous fraction as well as 200 and 400 mg/kg doses of the ethyl acetate fractions showed significant (P < 0.05) lowering of rectal temperature as compared to the corresponding value of the control group. Like the crude extract, the percentage reductions in rectal temperature after the administration of all doses of the fractions increases with time as shown in Figures 3-5. The antipyretic effects of the standard drug, AQ 100 mg/kg, and EA 200 and 400 mg/kg started as early as 1.5 hours while the antipyretic effects of the aqueous fraction at 200 and 400 mg/kg started as early as 2 hours after administration. The 100 mg/kg dose of the ethyl acetate fraction and all doses of the chloroform fraction didn`t show any significant antipyretic effects as compared to the 0-hour rectal temperature as well as the corresponding value of the control group. Among the fractions, the aqueous fraction at 400 mg/kg showed the maximum (93.83%) percent reduction followed by the ethyl acetate fraction at 400 mg/kg (92.31%) after 2 hours of treatment.
 |
Table 3 Activity of B. abyssinica Solvent Fractions on Yeast-Induced Pyrexia in Mice |
Discussion
The current study investigated the acute toxicity and antipyretic activities of B. abyssinica 80% methanolic leaf extract and solvent fractions against yeast-induced pyrexia in mice. In acute oral toxicity investigation, the crude extract did not cause any toxicity or death in this study up to a dose of 2000 mg/kg. Additional physical and behavioral observations revealed no evidence of acute toxicity with the same dose. In general, this substance is a good candidate for further research because its LD50 is 20 times greater than the minimum effective dose tested (100 mg/kg), which meets the minimum requirements.37
In a yeast-induced pyrexia model, the subcutaneous injection of 30% brewer’s yeast suspension significantly increased the rectal temperature of the mice 18 hours after administration, owing to the release of pro-inflammatory cytokines, which stimulate the synthesis of PGE2 in the vicinity of the hypothalamic thermoregulatory centers once they reach circulation.38,39
All doses of the crude extract (Table 2) and aqueous fraction as well as ethyl acetate fraction at a dose of 200 and 400mg (Table 3), showed a statistically significant reduction in rectal temperature as compared to the negative control group. However, all doses of the chloroform fraction (100mg, 200mg, and 400mg) and ethyl acetate fraction at 100mg dose didn`t show a significant reduction in rectal temperature. This could be explained by the polar nature of the active principle responsible for antipyretic activity. Furthermore, higher doses may contain more active and synergistic principles responsible for antipyretic activity than lower doses.
The antipyretic effects of the crude extract (400 mg), an aqueous fraction (100 mg), ethyl acetate fraction (200 and 400 mg), and the standard drug (ASA 100mg) were observed as early as 1.5 hours post-treatment and the effect was maintained for three hours after single oral administration, suggesting that the plant has a reasonable kinetic profile in terms of onset and duration of action. The current study’s findings agreed with other studies on the antipyretic potential of medicinal plants in animal models. Similar work by Bhowmick et al40 showed antipyretic effects of Litsea glutinosa leaves in yeast-induced pyrexia in Swiss albino mice.
The most likely mechanism for lowering the temperature in yeast-induced pyrexia is to reduce the synthesis of prostaglandins within the brain, particularly PGE2, secondary to inhibiting the enzymes responsible for prostaglandin production, possibly through inhibition of cyclooxygenase enzyme or stimulation of the system’s antipyretic substances, as seen with vasopressin and arginine.41
There are several mediators of pyrexia, and inhibiting any of these mediators may result in antipyresis.5 Although there is no direct evidence that B. abyssinica interferes with PGE2 synthesis in the hypothalamus, the anti-inflammatory and immunomodulatory activities of various phyto-compounds identified in methanolic fractions of the plant’s leaves can support the result.23
Steroids, tannins, and flavonoids are major inhibitors of PGE2 synthetase and cyclooxygenase or lipoxygenase, which aids in the inhibition of pyrexia.42 Flavonoids have been shown to interfere with prostaglandins, and related flavonoid compounds have been shown to inhibit arachidonic acid peroxidation, resulting in lower prostaglandin levels and thus a reduction in fever.43 Another possible antipyretic mechanism of the extract is the mediation of superficial blood vessel dilation, which results in improved heat loss from the resetting of the hypothalamic thermostat.41
Zekeya et al studied different phyto-components from the methanolic fraction of the leaves of B. abyssinica such as; Gibberellic acid (a pentacyclic diterpene); Hexa-t-butylselenatrisiletane; 3,7,11,15-tetramethyl-2 hexadecenol (aterpenealcohol); 7,8 epoxylanostan-11-ol,3 acetoxy (alcoholic compound); vitamin E; 2 furan carboxaldehyde, 5-(hydroxymethyl); 1,2,3-benzenetriol (apyrogallol); dasycarpidan-1- methanol; acetate (ester); capric ether (a fatty acid) and dodecanol 2-methyl-,(S) were identified to be most likely responsible for the antimicrobial, anti-inflammatory, antioxidant, immunomodulatory activities of the plant.23 The components found in this extract may contribute to the observed antipyretic effect in one or more ways too.
Conclusion
According to the findings of this study, the plant extract is found to be safe for mice. The crude extract was found to have significant antipyretic activity in a yeast-induced pyrexia model. The study’s overall findings suggest that B. abyssinica leaf extracts could be used as a new source for the development of new plant-based antipyretic agents. In general, current pharmacological evidence support the folklore claim of B. abyssinica leaves being an antipyretic agent. Nevertheless, further research is needed to understand the molecular mechanism underlying antipyretic activity.
Abbreviations
COX2, Cyclooxygenase-2; POAH, Pre-optic area of the anterior hypothalamus; PGE2, Prostaglandin-E2; SEM, Standard Error of the Mean; OECD, Organization for Economic Cooperation and development; ILAR, Institute for Laboratory Animal Research.
Data Sharing Statement
The manuscript contains all pertinent facts. On request, the data used to support the findings of this study can be obtained from the corresponding author.
Ethical Considerations
Since the University of Gondar is fully stocked with supplies, laboratory animals, and lab equipment, this experimental study was conducted there. The experiment was carried out in compliance with the guidance for the care and use of laboratory animals, and the study protocol was submitted to and authorized by the department of pharmacology at the University of Gondar.44 On March 13, 2019, a letter of clearance with reference number Sop/107/11 was acquired.
Acknowledgments
We are pleased to acknowledge the Amhara National Regional State Health Bureau for funding this study. We would also want to express our gratitude to the University of Gondar’s Department of Pharmacology for providing us with the necessary materials and allowing us to use the entire laboratory setup.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest.
References
1. Walter EJ, Hanna-Jumma S, Carraretto M, Forni L. The pathophysiological basis and consequences of fever. Crit Care. 2016;20(1):200. doi:10.1186/s13054-016-1375-5
2. Sullivan JE, Farrar HC. Clinical report—fever and antipyretic use in children. Pediatrics. 2011;3:2010–3852.
3. Ogoina D. Fever, fever patterns and diseases called ‘fever’–a review. J Infect Public Health. 2011;4(3):108–124. doi:10.1016/j.jiph.2011.05.002
4. El-Radhi AS. Pathogenesis of Fever. Clin Manual Fever Children. 2019;53–68. doi:10.1007/978-3-319-92336-9_3
5. Aronoff DM, Neilson EG. Antipyretics: mechanisms of action and clinical use in fever suppression. Am J Med. 2001;111(4):304–315. doi:10.1016/S0002-9343(01)00834-8
6. Blomqvist A, Engblom D. Neural Mechanisms of Inflammation-Induced Fever. Neuroscientist. 2018;24(4):381–399. doi:10.1177/1073858418760481
7. Eskilsson A. Inflammatory Signaling Across the Blood-Brain Barrier and the Generation of Fever. Linköping University Electronic Press; 2020.
8. Sun SX, Lee KY, Bertram CT, Goldstein JL. Withdrawal of COX-2 selective inhibitors rofecoxib and valdecoxib: impact on NSAID and gastroprotective drug prescribing and utilization. Curr Med Res Opin. 2007;23(8):1859–1866. doi:10.1185/030079907X210561
9. Alves FS, Cruz JN, de Farias Ramos IN, et al. Evaluation of Antimicrobial Activity and Cytotoxicity Effects of Extracts of Piper nigrum L. and Piperine. Separations. 2023;10(1):21.
10. Ekor M. The growing use of herbal medicines: issues relating to adverse reactions and challenges in monitoring safety. Front Neuro. 2014;2014:548. doi:10.3389/fphar.2013.00177
11. Muzammil S, Neves Cruz J, Mumtaz R, et al. Effects of Drying Temperature and Solvents on In Vitro Diabetic Wound Healing Potential of Moringa oleifera Leaf Extracts. Molecules. 2023;28(2):710. doi:10.3390/molecules28020710
12. Suleman S, Tufa TB, Kebebe D, et al. Treatment of malaria and related symptoms using traditional herbal medicine in Ethiopia. J Ethnopharmacol. 2018;213:262–279. doi:10.1016/j.jep.2017.10.034
13. Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. Scientific World J. 2013;2013:1–16. doi:10.1155/2013/162750
14. Karunamoorthi K, Tsehaye E. Ethnomedicinal knowledge, belief and self-reported practice of local inhabitants on traditional antimalarial plants and phytotherapy. J Ethnopharmacol. 2012;141(1):143–150. doi:10.1016/j.jep.2012.02.012
15. Nondo RS, Zofou D, Moshi MJ, et al. Ethnobotanical survey and in vitro antiplasmodial activity of medicinal plants used to treat malaria in Kagera and Lindi regions, Tanzania. J Med Plants Res. 2015;9(6):179–192. doi:10.5897/JMPR2014.5685
16. Guédé NZ, N’guessan K, Dibié TE, Grellier P. Ethnopharmacological study of plants used to treat malaria, in traditional medicine, by Bete Populations of Issia (Côte d’Ivoire). J Pharm Sci Res. 2010;2(4):216–227.
17. Okello S, Nyunja R, Netondo GW, Onyango JC. Ethnobotanical study of medicinal plants used by Sabaots of Mt. Elgon Kenya. Af J Traditional Complementary Alternative Med. 2010;7:1. doi:10.4314/ajtcam.v7i1.57223
18. Edeoga H, Okwu D, Mbaebie B. Phytochemical constituents of some Nigerian medicinal plants. Af j Biotechnol. 2005;4(7):685–688. doi:10.5897/AJB2005.000-3127
19. Amit L, Vikas G, Vaibhav T, Vikash K, Siddhartha G, Lather A. Phytochemistry and pharmacological activities of Bersama engleriana Guerke-An overview. Sexually Transmitted Dis. 2010;11:12.
20. Lemilemu F, Girmay S, Shenkute K, Endale M. Development S. Antibacterial steroids from roots of Bersama abyssinica. Ethiopian J Sci Sustainable Dev. 2020;7(1):27–34.
21. Schmelzer G, Gurib-Fakim A. Plant Resources of Tropical Africa 11 (1). Medicinal Plants 1. Wageningen/Leiden: PROTA Foundation. Backhuys Publishers; 2008.
22. Mathewos Anza FW, Libsu S, Mamo F. Milkyas Endale Phytochemical Screening and Antibacterial Activity of Leaves Extract of Bersama abyssinica. J Adv Botany Zool. 2015;3(2). doi:10.15297/JABZ.V3I2.07
23. Zekeya N, Chacha M, Shahada F, Kidukuli A. Analysis of phytochemical composition of Bersama abyssinica by gas chromatography-mass spectrometry. J Pharmacognosy Phytochemistry. 2014;3(4):246–252.
24. Bolou G, Bagré I, Ouattara K, Djaman A. Evaluation of the antibacterial activity of 14 medicinal plants in Côte d’Ivoire. Trop J Pharm Res. 2011;10:3. doi:10.4314/tjpr.v10i3.3
25. Zekeya N, Shahada F, Chacha M. In vitro Antibacterial and Antifungal Activity of Tanzanian Bersama abyssinica. Internat J Sci Res. 2014;3:1150–1153.
26. Mwambela NZ, Chacha M, Shahada F. Investigation of antimycobacterial and cytotoxicity activity of Bersama abyssinica Fresen extracts from Tanzania. Int J Life Sci Biotechnol Pharma Res. 2014;3(4):23.
27. Asres K, Bucar F, Kartnig T, Witvrouw M, Pannecouque C, De Clercq E. Antiviral activity against human immunodeficiency virus type 1 (HIV‐1) and type 2 (HIV‐2) of ethnobotanically selected Ethiopian medicinal plants. Phytotherapy Res. 2001;15(1):62–69. doi:10.1002/1099-1573(200102)15:1<62::AID-PTR956>3.0.CO;2-X
28. Bene K, Camara D, Kanga Y, Zirihi GN. Potentiel antiradicalaire des extraits de feuilles de Bersama abyssinica Fresen. (Melianthaceae). Int J Biol Chem Sci. 2017;11(6):2962–2970. doi:10.4314/ijbcs.v11i6.32
29. Makonnen E, Hagos E. Antispasmodic effect of Bersama abyssinica aqueous extract on Guinea‐pig ileum. Phytotherapy Res. 1993;7(2):211–212. doi:10.1002/ptr.2650070226
30. Ayaz M, Junaid M, Ahmed J, et al. Phenolic contents, antioxidant and anticholinesterase potentials of crude extract, subsequent fractions and crude saponins from Polygonum hydropiper L. BMC Complement Altern Med. 2014;14(1):145. doi:10.1186/1472-6882-14-145
31. Cooperation OOfE, Development. OECD Guidelines for the Testing of Chemicals: Repeated Dose 28-Day Oral Toxicity Study in Rodents. Organization for Economic Cooperation and Development Paris; 2008.
32. Garber JC, Barbee RW, Bielitzki JT, et al. Guide for the care and use of laboratory animals. National Acad Press Washington DC. 2011;8:220.
33. Toxicity–Up AO. The OECD guidelines for testing of chemicals, “Acute Oral Toxicity: up- and- Down procedure”. 2008. 1–27.
34. Tesema S, Makonnen E. In vivo analgesic and antipyretic activities of N-Butanol and water fractions of Ocimum suave. Ethiop J Health Sci. 2015;25(2):139–146. doi:10.4314/ejhs.v25i2.6
35. Dangarembizi R, Erlwanger KH, Mitchell D, Hetem RS, Madziva MT, Harden LM. Measurement of body temperature in normothermic and febrile rats: limitations of using rectal thermometry. Physiol Behav. 2017;179:162–167. doi:10.1016/j.physbeh.2017.06.002
36. Ahlawat S, Mishra P, Dalal K, Patra A. Antipyretic activity of roots of Argyreia speciosa (Burm. F.) Bojer. Int J of PharmTech Research. 2010;2(4):2165–2167.
37. Uwakwe A, Monago C. Antiplasmodial activity of methanolic stem bark extract of Anthocleista grandiflora in mice. Int J Applied. 2012;2:4.
38. Shashank Kumar AKP. Chemistry and Biological Activities of Flavonoids: an Overview. Sci World J. 2013;2013:16.
39. Chan GH, Fiscus RR. Exaggerated production of nitric oxide (NO) and increases in inducible NO-synthase mRNA levels induced by the pro-inflammatory cytokine interleukin-1β in vascular smooth muscle cells of elderly rats. Exp Gerontol. 2004;39(3):387–394. doi:10.1016/j.exger.2004.01.002
40. Bhowmick R, Sarwar MS, RahmanDewan SM, et al. In vivo analgesic, antipyretic, and anti-inflammatory potential in Swiss albino mice and in vitro thrombolytic activity of hydroalcoholic extract from Litsea glutinosa leaves. Bio Res. 2014;47(1):1–8.
41. Khan IA, Aziz A, Manzoor Z, et al. Study on antipyretic activity of Rumex vesicarius leaves extract in albino rabbits. Veterinary World. 2014;7:1. doi:10.14202/vetworld.2014.44-48
42. Narayana KR, Reddy MS, Chaluvadi M, Krishna D. Bioflavonoids classification, pharmacological, biochemical effects and therapeutic potential. Indian J Pharmacol. 2001;33(1):2–16.
43. Taïwe GS, Bum EN, Talla E, et al. Antipyretic and antinociceptive effects of Nauclea latifolia root decoction and possible mechanisms of action. Pharm Biol. 2011;49(1):15–25. doi:10.3109/13880209.2010.492479
44. Care IoLARCo, Animals UoL. Guide for the Care and Use of Laboratory Animals. US Department of Health and Human Services, Public Health Service, National; 1986.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.