Back to Journals » Infection and Drug Resistance » Volume 12
Antimicrobial resistance pattern and molecular genetic distribution of metallo-β-lactamases producing Pseudomonas aeruginosa isolated from hospitals in Minia, Egypt
Authors Farhan SM, Ibrahim RA , Mahran KM, Hetta HF , Abd El-Baky RM
Received 15 December 2018
Accepted for publication 17 April 2019
Published 16 July 2019 Volume 2019:12 Pages 2125—2133
DOI https://doi.org/10.2147/IDR.S198373
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Joachim Wink
Sara M Farhan,1 Reham A Ibrahim,2 Khaled M Mahran,3 Helal F Hetta4,5, Rehab M Abd El-Baky1,2
1Department of Microbiology and Immunology, Faculty of Pharmacy, Deraya University, Minia 11566, Egypt; 2Department of Microbiology and Immunology, Faculty of Pharmacy, Minia University, Minia 61519 Egypt; 3General Surgery and Laparoscopic Surgery, Faculty of Medicine, Minia University, Minia 61519 Egypt; 4Department of Medical Microbiology and Immunology, Faculty of Medicine, Assiut University, Assiut 71515, Egypt; 5Department of Internal Medicine, University of Cincinnati College of Medicine, Cincinnati, OH, USA
Background: Pseudomonas aeruginosa (P. aeruginosa) represents a great threat to public health worldwide, due to its high ability to acquire resistance to different antibiotic classes. Carbapenems are effective against multidrug resistant (MDR) P. aeruginosa, but their widespread use has resulted in the emergence of carbapenem-resistant strains, which is considered a major global concern. This study aimed to determine the prevalence of carbapenem resistance among P. aeruginosa strains isolated from different sites of infection.
Methods: Between October 2016 and February 2018, a total of 530 clinical specimens were collected from patients suffering from different infections, then processed and cultured. Isolates were tested for extended spectrum β-lactamase (ESBL) and metallo-β-lactamase (MBL) production using double-disk synergy test, modified Hodge tests, and disc potentiation test. PCR was used for the detection of selected OXA carbapenemases encoding genes.
Results: Of 530 samples, 150 (28.3%) P. aeruginosa isolates were obtained. MDR strains were found in 66.6% (100 of 150) of isolates. Of 100 MDR P. aeruginosa isolates, 54 (54%) were ESBL producers and 21 (21%) carbapenem resistant P. aeruginosa. MBL production was found in 52.3% (eleven) carbapenem-resistant isolates. CTX-M15 was found among 55.5% of ESBL- producing P. aeruginosa. Carbapenemase genes detected were blaIMP (42.8%, nine of 21), blaVIM (52.3%, eleven of 21), blaGIM (52.3%, eleven of 21), blaSPM (38%, 8/21). In addition, isolates that were positive for the tested genes showed high resistance to other antimicrobials, such as colistin sulfate and tigecycline.
Conclusion: Our study indicates that P. aeruginosa harboring ESBL and MBL with limited sensitivity to antibiotics are common among the isolated strains, which indicates the great problem facing the treatment of serious infectious diseases. As such, there is a need to study the resistance patterns of isolates and carry out screening for the presence of ESBL and MBL enzymes, in order to choose the proper antibiotic.
Keywords: MDR; P. aeruginosa, ESBL, MBL, antimicrobial resistance
Introduction
Pseudomonas aeruginosa is an opportunistic pathogen that can cause outbreaks of hospital-acquired and life-threatening infections, especially among immunocompromised and critically ill patients.1 P. aeruginosa can cause respiratory tract, burn, wound infections and otitis media.2 P. aeruginosa infections are commonly associated with high mortality, attributed to its intrinsic resistance to many classes of antimicrobial agents and ability to acquire resistance by mutation and horizontal transfer of resistance determinants.3 The rapid emergence of penicillin and cephalosporin resistance among P. aeruginosa strains has become a serious clinical problem worldwide. Carbapenems (imipenem and meropenem), potent antipseudomonal drugs, have been used as the last resort for the treatment of infections associated with multidrug resistant (MDR) P. aeruginosa isolates.4 Resistance to carbapenems has developed through decreased permeability, overexpression of the efflux-pump system, alterations in penicillin-binding protein and carbapenem-hydrolyzing enzymes (carbapenemases).5
Carbapenemases represent three classes of β-lactamase (BL). Ambler class A and D (serine carbapenemases) and class B (zinc-dependent). These enzymes require zinc for their catalytic activity and are inhibited by metal chelators, such as EDTA and thiol-based compounds, and are called metallo-BLs (MBLs). MBL enzymes are able to hydrolyze all β-lactam antibiotics, with the exception of monobactams. The genes encoding these enzymes have found to be carried on highly mobile elements, which is the main cause of their dissemination in the hospital environment. MBLs are mainly plasmid-mediated and in some cases chromosomally mediated. The most common MBLs enzymes belong to the Verona integron–encoded MBL (VIM), imipenemase (IMP), São Paulo MBL (SPM), German imipenemase MBL (GIM), Seoul imipenemase MBL, and New Delhi MBL families.6
Infections caused by MBL-producing organisms are associated with high morbidity and mortality rate, especially in hospitalized and immunosuppressed patients.7 Recently, many studies reported the prevalence of P. aeruginosa strains harboring both extended-spectrum BL (ESBL) and MBL genes, which is considered a great challenge for antimicrobial therapy.8 In addition, it is difficult to detect ESBLs phenotypically.9 As such, molecular techniques are required to analyze the coexistence of carbapenemases and ESBLs in the same strain. The aim of this study was to study the prevalence and DR profile of carbapenem-resistant P. aeruginosa (CRPA) isolates obtained from hospitalized patients with various infections
Methods
Bacterial isolates
A total of 150 (28.3%) P. aeruginosa isolates were isolated from 530 samples collected from hospitalized patients with various infections as part of routine hospital-laboratory procedures. Samples were processed and cultured on blood agar at 37°C and 42°C for 24 hours. One colony was picked and subcultured on MacConkey agar plates and cetrimide agar. Isolated colonies were further identified according to colony morphology, lactose fermentation, and biochemical characteristics (oxidase, triple sugar iron, urease tests, sulphide–indole–motility). Colonies were able to grow on cetrimide agar, show positive reactions on catalase and oxidase tests, grow at 42°C (used to distinguish P. aeruginosa from other lactose nonfermenting Gram-negative rods), and show negative results in triple-sugar iron and glucose-fermentation tests.10,11 P. aeruginosa colonies were purified by streaking, and pure colonies were stored at 4°C.
Antimicrobial-susceptibility testing
Antimicrobial susceptibility was determined by Kurby–Bauer disk diffusion test.12 Results were assessed on the basis of Clinical and Laboratory Standards Institute criteria. The following antimicrobial disks (Oxoid, Basinstoke, UK) were used: azlocillin (75 µg), ciprofloxacin (5 µg), ampicillin–sulbactam (20 µg), levofloxacin (5 µg), cefepime (30 µg), meropenem (10 µg), aztreonam (30 µg), imipenem (10 µg), polymyxin B(300 µg), colistin sulfate (10 µg), tigecycline (15 µg), tobramycin (10 µg), ceftazidime (30 µg), amoxicillin–clavulanic (20/10 µg), carbenicillin (100 µg), amikacin (30 µg), gentamicin (10 µg), piperacillin (100 µg), and cefoperazone (75 µg).
Phenotypic detection of ESBL production
Detection of ESBL production by P. aeruginosa strains was performed by double-disk synergy test (DDST).13 Disks of ceftazidime, cefotaxime, aztreonam, and cefepime (30 µg each) were placed at a distance of 30 or 20 mm (center to center) from an amoxicillin 20 µg–clavulanic acid 10 µg disk. Increase in zones of inhibition toward amoxicillin–clavulanic acid antibiotic disks is indicative of the presence of ESBL.
Phenotypic detection of MBL production
Imipenem–EDTA combined disk synergy testing was used for identification of MBL-producing isolates according to Lee et al.14,15 A solution of 0.5 M EDTA (pH 8) was prepared by dissolving 18.61 g of EDTA in 100 mL distilled water and adjusting its pH to 8 using NaOH, then, autoclaving. The tested organisms were cultured on the surface of Müller–Hinton agar plates. Two 10 µg imipenem disks or two 10 µg meropenem disks were placed on the surface of agar plates and 5 µL EDTA solution added to one imipenem and one meropenem disks. Zones of inhibitions around discs with EDTA were examined after 16–18 hours' incubation at 35°C and compared to those without EDTA. An increase in zone diameter of at least 7 mm around the imipenem–EDTA disc and meropenem–EDTA disks were considered positive results.
Amplification of ESBL-CTX-M15 and MBL genes
Boiling was used to prepare DNA templates of genes. Specific primers — cefotaximase (blaCTX-M15), blaVIM, blaIMP, blaGIM, and blaSPM (Table 1) — were used for PCR amplification of the genes. PCR amplification was done using 25 µL reaction mixture containing 0.2 µL Taq polymerase 5 U/µL 1 pmol of each forward and reverse primer, 2.5 µL dNTP mix (2 Mm), 3 µL DNA template, and 14.8 µL DNase-free and RNase-free water. PCR reactions were performed using a Mastercycler personal 5332 (Eppendorf, Hamburg, Germany). Amplified products were analyzed by electrophoresis in 2% agarose gel at 80 V for 45 minutes in Tris–Borate–EDTA buffer containing ethidium bromide under ultraviolet irradiation.16,17
 |
Table 1 PCR primers used for detection of ESBL-CTX-M15 and MBL genes in Pseudomonas aeruginosa |
Results and discussion
P. aeruginosa is commonly associated with hospital-acquired infections. With regard to the specimen site, of 530 samples, 150 (28.3%) were positive for P. aeruginosa, which was similar to results reported by Al-Haik et al18 and Mansour et al19 and fewer than Gad et al.20 P. aeruginosa isolates were isolated from 65 of 332 (19.5%) wound swabs, 39 of 57 (68.4%) ear swabs, five of 26 (19.2%) burn swabs, six of 30 (20%) urine samples, eight of 12 (66.6%) sputum samples, eight of 35 (22.8%) stool samples, 19 of 38 (50%) of patients admitted to the intensive-care unit (ICU). Our results showed high incidence (68.4%) of P. aeruginosa among samples collected from patients suffering from otitis media, which was higher than reported by Umar et al,21 who found that 23.2% of samples of otitis media were positive for P. aeruginosa. The distribution of isolates across major hospitals in Minia Governorate was analyzed. High incidence of P. aeruginosa was observed among samples collected from the chest hospital, while all samples obtained from Minia General Hospital were negative for P. aeuginosa (Figure 1)
 |
Figure 1 Prevalence of Pseudomonas aeruginosa isolated from different hospitals in Minia. |
P. aeruginosa possesses MDR against a wide variety of antibiotics. Resistance of P. aeruginosa is usually accompanied by the production of many BLs, active expulsion of antibiotics by efflux pump, and alteration of outer-membrane protein expression.9,22 Resistance to variety of β-lactam antibiotics is a growing problem, due to their continuous mutation, which makes BLs production the most common cause of DR and antimicrobial therapy failure.23 Among BLs, ESBLs are widely distributed among Enterobacteriaceae members. They are also found in Acinetobacter baumannii and P. aeruginosa. At first, TEM-type ESBLs and SHV-type ESBLs were the most dominant among Gram negative isolates in Europe and other regions. Since the last decade, CTX-M type ESBL has became the most prevalent.
ESBL production is widely spread among Enterobacteriaceae, especially P. aeruginosa. Our study showed that all P. aeruginosa isolates were completely resistant to azlocillin and amoxicillin–clavulanic acid. Of 150 P. aeruginosa isolates, 100 (66.6%) were MDR and 21 (21%) of these were CRPA (eleven isolates were imipenem-resistant and ten meropenem-resistant). Figure 2 shows that 46%, 28.7%, and 28% of P. aeruginosa were resistant to polymyxin B, colistin sulfate, and tigycycline, respectively.
 |
Figure 2 Resistance pattern of Pseudomonas aeruginosa isolates to different antimicrobial agents. |
In this study, it was found that 54 (54%) isolates of MDR P. aeruginosa were ESBL producers. Similarly high production of ESBL was reported by Ahmad et al,24 who reported that ESBL production by P. aeruginosa isolates was 61.6%, while lower incidence (27.33%) was reported by Dutta et al.25 In addition, our results showed that eleven (11%) isolates were MBL-producing P. aeruginosa. Furthermore, MBL-producing strains represented 52.3% (eleven of 21) of CRPA isolates. Coexistance of ESBL and MBL was found among 5% of MDR P. aeruginosa and five of 21 (23.8%) CRPA isolates. Antibiotic-resistance patterns of ESBL-producing strains revealed that all ESBL producers were completely resistant to azlocillin, amoxicillin–clavulanic acid, ampicillin/sulbctam and cefipime. Co-resistance with other antibiotics was observed including colistine sulfate, tigecycline, and polymyxin B (Table 2). Also, MBL-producing strains showed high resistance to cefipime and carbenicillin (72.7% each), but lower resistance was observed against ciprofloxacin, colistin sulfate, and levofloxacin (36.3% each). Ilyas et al26 showed higher incidence of antibiotic resistance exhibited by MBL- and ESBL-producing P. aeruginosa. They reported that ESBL- and MBL-producing P. aeruginosa isolates were completely resistant to amoxicillin–clavulanic acid, ceftriaxone, ciprofloxacin, and cefepime. Also, they showed higher incidence of MBL-producing P. aeruginosa (25.7%) and lower incidence of ESBL production (8.5%). Mirsalehian et al27 found that all MBL-producing P. aeruginosa were colistin-sensitive and 37.5% were resistant to aztreonam, while in the present study low incidence of resistance to colistin, ciprofloxacin, and levofloxacin (36.4%) and a resistance rate of 54.5% were reported against azetreonam. Bashir et al28 reported that all MBL- producing P. aeruginosa isolates were resistant to gentamicin, ceftazidime, carbenicillin, tobramycin, ceftriaxone, ofloxacin, cefoperazone, cefoperazone–sulbactum, and ceftazidime–clavulanic acid and low resistance to polymyxin B.
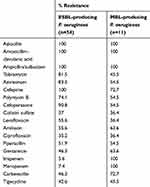 |
Table 2 Antibiotic-resistance patterns of ESBL- and MBL-producing strains |
The rapid spread and the emergence of MBL- and ESBL-producing P. aeruginosa isolated from hospitals is of great concern and threat. In addition, differences in resistance patterns among strains isolated from different countries may be attributed to antibiotic use, horizontal gene transfer, and environmental conditions. Therefore, it is important to test isolates for MBL and ESBL production and to test for antibiotic susceptibility before antimicrobial therapy.
Table 3 shows that the highest incidence of ESBL production was observed among MDR P. aeruginosa samples isolated from ear infections (80%), followed by those isolated from chest infections (75%), and ICU patients (70%). The highest incidence of MBL production was observed among MDR P. aeruginosa samples isolated from wound infections (19%) followed by those isolated from ear infections (14.3%). Nithyalakshmi et al29 reported that the frequency of occurrence of ESBL among P. aeruginosa isolates was 21.96%, and most ESBL producers were obtained from urine samples (27.7%), followed by respiratory infection (23.68%), and wound infection (22.95%).
 |
Table 3 Distribution of ESBL- and MBL-producing isolates among MDR Pseudomonas aeruginosa isolates from different clinical specimens |
All MDR P. aeruginosa isolates were tested for CTX-M15 and carbapenem-resistance genes: blaIMP, blaVIM, blaGIM, and blaSPM. It was found that 55.5% (30 of 54) of ESBL- producing P. aeruginosa isolates were harboring CTX-M15, which was higher than another study17 reporting that out of 200 MDR P. aeruginosa isolates, 19 were positive for CTX-M15, of which 64.28% were ESBL- positive. Although carbapenem resistance was found among 21 P. aeruginosa isolates, only eleven were found to harbor MBL genes. Of 21 carbapenem-resistant strains, 42.8% (nine of 21) were positive for blaIMP, 52.3% (eleven of 21) positive for blaVIM, 52.3% (eleven of 21) positive for blaGIM, and 38% (eight of 21) positive for blaSPM.
The distribution of carbapenem-resistance genes and blaCTX-M15 among MDR P. aeruginosa-producing ESBL and/or MBL isolates were tested (Table 4). Of eleven MBL-producing MDR P. aeruginosa, three (27.2%) were CTX-M15, nine (81.8%) positive for blaIMP, four (36.3%) for blaVIM, five (45.4%%) for blaSPM and six (54.5%) for blaGIM. Lower incidence was found by Zubair et al30 who reported that among 22 isolates positive for MBL production phenotypically, only five were harboring MBL genes. Furthermore, they reported that blaVIM was the predominant gene, and none of the other genes were detected.
 |
Table 4 Distribution of different groups of carbapenem-resistance genes in phenotypically positive ESBL- and MBL-producing Pseudomonas aeruginosa isolates |
Also, It was found that 55.1% of ESBL/non-MBL-producing MDR P. aeruginosa isolates were positive for CTX-M15, while none of these strains was found to harbor blaIMP. On the other hand, blaVIM was the most common carbapenem-resistance gene (14.2%). Rafiee et al31 and Laudy et al32 showed that all ESBL-producing isolates were negative for CTX-M gene, while Ahmed et al33 reported a lower incidence of blaCTX-M production (10.7%) among P. aeruginosa strains isolated from Makkah hospitals. All MBL/non-ESBL-producing P. aeruginosa harbored blaIMP-like genes and 50% were positive for blaGIM, while 33.3% only were positive for both blaVIM and blaSPM (Table 4). Similar findings were shown by Abiri et al34. In contrast, Mirsalehian et al27 reported that blaVIM was the most prevalent carbapenemase gene among MBL-producing P. aeruginosa, while 25% of MBL isolates were positive for blaIMP and all MBL isolates negative for blaGIM and blaSPM. Our results showed that five isolates of MDR P. aeruginosa were ESBL and MBL coproducers. Three isolates (60%) were found to have blaCTX-M15, blaIMP, blaGIM, and bla-SPM, and two (40%) were positive for blaVIM.
MDR P. aeruginosa samples were classified into seven groups according to the number of carbapenem-resistant genes harbored by MBL-producing P. aeruginosa isolates, in order to study their demographic, phenotypic, and genotypic features: group A comprised MBL-producing P. aeruginosa isolates harboring two genes (bla-IMP and bla-GIM), group B isolates positive for blaIMP, blaVIM, and blaSPM, group C including those which were positive for blaIMP, group D isolates positive for blaIMP and blaSPM, group E isolates positive for blaIMP, blaGIM, and blaSPM, Group Fisolates positive for blaIMP and blaVIM, group G MBL-producing P. aeruginosa isolates positive for blaVIM, blaGIM, and blaSPM, and group H including isolates positive for blaVIM and blaGIM (Table 5).
 |
Table 5 Demographic, phenotypic and genotypic features of MBL-producing Pseudomonas aeruginosa |
Our study showed that all MBL-producing P. aeruinosa isolates in groups A–H were obtained from Minia University Hospital except one isolate that had been obtained from a chest hospital. Of eleven MBL-producing P. aeruginosa, five were ESBL producers and obtained from surgery and ICU units of Minia University Hospital. Of these, three (two from surgery unit and one from ICU) were positive for CTX-M15 gene. The isolate obtained from the ICU unit showed resistance to meropenem, polymyxin B, tigecycline, gentamicin, amikacin, and ceftazidime, which represents a great challenge for antimicrobial therapy patients. The other two isolates (surgery unit) showed resistance to gentamicin, ceftazidime, meropenem, imipenem, tigycycline, and colistin sulfate. Furthermore, the isolate obtained from the chest hospital belonged to group H, was positive for ESBL but negative for CTX-M15, and showed resistance to ceftazidime, cefoperazone, gentamicin, amikacin, tigycycline, polymxin B, and meropenem. Chaudhary et al35 found that the frequency of blaIMP and blaVIM among MBL- producing strains was 28.73% and 47.12%, respectively. Coexistence of MBL and ESBL was found among 14.3% of isolates, of which 17.5% were positive for TEM and IMP genes and 14.8 positive for AMP-C and VIM. Also, they found that isolates coproducing ESBL and MBL were highly resistant to cefepim, piperacillin–tazobactam, ceftazidime, meropenem, and imipenem.
Our study showed the prevalence of ESBL- and MBL-producing P. aeruginosa with limited sensitivity to antibiotics among the isolated strains, which indicates the great problem in the treatment of serious infectious diseases. In addition, there is a need to study resistance pattern of isolates and carry out screening for the presence of ESBL and MBL enzymes, in order to choose the proper antibiotic.
Study limitations and future recommendations
We detected the distribution of genes only among resistant strains. Quantitative PCR assays are recommended for future studies, and should be performed to verify expression differences of different resistance genes in MDR P. aeruginosa.
Conclusion
Using carbapenems in clinical practice was initially the solution to treatment of serious bacterial infections caused by β-lactam-resistant bacteria. Due to their widespread use, the emergence of MBL-producing strains and strains coproduce both ESBL and MBL was observed. As found in our study, strains showed high resistance to the commonly used antibiotics, which emphasizes the need to know the resistance patterns and testing for the coexistence of these enzymes, in order to design newer policies for antimicrobial chemotherapy.
Abbreviations list
MHT, modified Hodge Test; MDR, multidrug-resistant; ESBL, extended-spectrum β-lactamase; MBL, metallo-β-lactamase.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Santajit S, Indrawattana N. Mechanisms of antimicrobial resistance in ESKAPE pathogens. Biomed Res Int. 2016;2016:2475067. doi:10.1155/2016/2475067
2. Lister PD, Wolter DJ, Hanson ND. Antibacterial-resistant Pseudomonas aeruginosa: clinical impact and complex regulation of chromosomally encoded resistance mechanisms. Clin Microbiol Rev. 2009;22(4):582–610. doi:10.1128/CMR.00040-09
3. Potron A, Poirel L, Nordmann P. Emerging broad-spectrum resistance in Pseudomonas aeruginosa and Acinetobacter baumannii: mechanisms and epidemiology. Int J Antimicrob Agents. 2015;45(6):568–585. doi:10.1016/j.ijantimicag.2015.03.001
4. Pitout JD, Gregson DB, Poirel L, McClure JA, Le P, Church DL. Detection of Pseudomonas aeruginosa producing metallo-beta-lactamases in a large centralized laboratory. J Clin Microbiol. 2005;43(7):3129–3135. doi:10.1128/JCM.43.7.3129-3135.2005
5. Kaleem F, Usman J, Hassan A, Khan A. Frequency and susceptibility pattern of metallo-beta-lactamase producers in a hospital in Pakistan. J Infect Dev Ctries. 2010;4(12):810–813.
6. Owlia P, Saderi H, Karimi Z, Rad A, Bagher SM, Bahar MA. Phenotypic detection of Metallo-beta-Lactamase producing Pseudomonas aeruginosa strains isolated from burned patients. Iran J Pathol. 2008;3(1):20–25.
7. Hong DJ, Bae IK, Jang IH, Jeong SH, Kang HK, Lee K. Epidemiology and characteristics of metallo-beta-lactamase-producing Pseudomonas aeruginosa. Infect Chemother. 2015;47(2):81–97. doi:10.3947/ic.2015.47.2.81
8. Bae IK, Suh B, Jeong SH, et al. Molecular epidemiology of Pseudomonas aeruginosa clinical isolates from Korea producing beta-lactamases with extended-spectrum activity. Diagn Microbiol Infect Dis. 2014;79(3):373–377. doi:10.1016/j.diagmicrobio.2014.03.007
9. Polotto M, Casella T, de Lucca Oliveira MG, et al. Detection of P. aeruginosa harboring bla CTX-M-2, bla GES-1 and bla GES-5, bla IMP-1 and bla SPM-1 causing infections in Brazilian tertiary-care hospital. BMC Infect Dis. 2012;12. doi:10.1186/1471-2334-12-166
10. Fazeli H, Akbari R, Moghim S, Narimani T, Arabestani MR, Ghoddousi AR. Pseudomonas aeruginosa infections in patients, hospital means, and personnel’s specimens. J Res Med Sci. 2012;17(4):332–337.
11. Tille P. Bailey & Scott’s Diagnostic Microbiology-E-Book. Philadelphia: Elsevier Health Sciences; 2015.
12. Wayne P. Clinical and Laboratory Standards Institute: performance standards for antimicrobial susceptibility testing: 20th informational supplement. CLSI document M100-S20. 2010.
13. Tzelepi E, Giakkoupi P, Sofianou D, Loukova V, Kemeroglou A, Tsakris A. Detection of extended-spectrum beta-lactamases in clinical isolates of Enterobacter cloacae and Enterobacter aerogenes. J Clin Microbiol. 2000;38(2):542–546.
14. Clinical and Laboratory Standards Institute (2007) Performance Standards for Antimicrobial Susceptibility Testing; Seventeenth Informational Supplement. CLSI Document M100-S17. Wayne, PA: Clinical and Laboratory Standards Institute; 2007.
15. Lee K, Lim YS, Yong D, Yum JH, Chong Y. Evaluation of the Hodge test and the imipenem-EDTA double-disk synergy test for differentiating metallo-beta-lactamase-producing isolates of Pseudomonas spp. and Acinetobacter spp. J Clin Microbiol. 2003;41(10):4623–4629. doi:10.1128/jcm.41.10.4623-4629.2003
16. Ellington MJ, Kistler J, Livermore DM, Woodford N. Multiplex PCR for rapid detection of genes encoding acquired metallo-beta-lactamases. J Antimicrob Chemother. 2007;59(2):321–322. doi:10.1093/jac/dkl481
17. Ullah W, Qasim M, Rahman H, et al. CTX-M-15 and OXA-10 beta lactamases in multi drug resistant Pseudomonas aeruginosa: first report from Pakistan. Microb Pathog. 2017;105:240–244. doi:10.1016/j.micpath.2017.02.039
18. Al-Haik WM, Al-Mahbash AA, Al-Mahdi AY, Mohamed MM, Al-Haddad A. Genotypic characteristics of clinical and non-clinical isolates of Pseudomonas aeruginosa: distribution of different antibiogram profiles and molecular typing. Jordan J Biol Sci. 2016;9:3.
19. Mansour SA, Eldaly O, Jiman-Fatani A, Mohamed ML, Ibrahim EM. Epidemiological characterization of P. aeruginosa isolates of intensive care units in Egypt and Saudi Arabia. East Mediterr Health J. 2013;19(1):71–80.
20. Gad GF, El-Domany RA, Zaki S, Ashour HM. Characterization of Pseudomonas aeruginosa isolated from clinical and environmental samples in Minia, Egypt: prevalence, antibiogram and resistance mechanisms. J Antimicrob Chemother. 2007;60(5):1010–1017. doi:10.1093/jac/dkm348
21. Umar JB, Ibrahim MM, Tom IM, Umoru AM, Isa T. Pseudomonas aeruginosa in otitis media. Int J Med. 2016;4(2):55–57. doi:10.14419/ijm.v4i2
22. Bonfiglio G, Laksai Y, Franchino L, Amicosante G, Nicoletti G. Mechanisms of beta-lactam resistance amongst Pseudomonas aeruginosa isolated in an Italian survey. J Antimicrob Chemother. 1998;42(6):697–702. doi:10.1093/jac/42.6.697
23. Dallenne C, Da Costa A, Decré D, Favier C, Arlet G. Development of a set of multiplex PCR assays for the detection of genes encoding important beta-lactamases in Enterobacteriaceae. J Antimicrob Chemother. 2010;65(3):490–495. doi:10.1093/jac/dkp498
24. Ahmad SS, Ali FA. Detection of ESBL, AmpC and Metallo Beta-Lactamase mediated resistance in Gram-negative bacteria isolated from women with genital tract infection. Eur Sci J. 2014;10(9):193–209.
25. Dutta H, Nath R, Saikia L. Multi-drug resistance in clinical isolates of Gram-negative bacilli in a tertiary care hospital of Assam. Indian J Med Res. 2014;139(4):643.
26. Ilyas M, Khurram M, Ahmad S, Ahmad I. Frequency, susceptibility and co-existence of MBL, ESBL & AmpC positive Pseudomonas aeruginosa in Tertiary Care Hospitals of Peshawar, KPK, Pakistan. J Pure Appl Microbiol. 2015;9(2):981–988.
27. Mirsalehian A, Kalantar-Neyestanaki D, Taherikalani M, Jabalameli F, Emaneini M. Determination of carbapenem resistance mechanism in clinical isolates of Pseudomonas aeruginosa isolated from burn patients, in Tehran, Iran. J Epidemiol Glob Health. 2017;7(3):155–159. doi:10.1016/j.jegh.2017.04.002
28. Bashir D, Thokar MA, Fomda BA, et al. Detection of metallo-beta-lactamase (MBL) producing Pseudomonas aeruginosa at a tertiary care hospital in Kashmir. Afr J Microbiol Res. 2011;5(2):164–172.
29. Nithyalakshmi J, Vidhyarani R, Mohanakrishnan K, Sumathi G. ESBL producing Pseudomonas aeruginosa in clinical specimens: is it a scary nightmare or paper tiger? Indian J Microbiol Res. 2016;3(3):287–291. doi:10.5958/2394-5478.2016.00062.5
30. Zubair K, Iregbu K. Resistance pattern and detection of metallo-beta-lactamase genes in clinical isolates of Pseudomonas aeruginosa in a Central Nigeria Tertiary Hospital. Niger J Clin Pract. 2018;21(2):176–182. doi:10.4103/njcp.njcp_229_17
31. Rafiee R, Eftekhar F, Tabatabaei SA, Minaee Tehrani D. Prevalence of extended-spectrum and metallo β-lactamase production in AmpC β-lactamase producing Pseudomonas aeruginosa Isolates From Burns. Jundishapur J Microbiol. 2014;7(9):e16436. doi:10.5812/jjm
32. Laudy AE, Róg P, Smolińska-Król K, et al. Prevalence of ESBL-producing Pseudomonas aeruginosa isolates in Warsaw, Poland, detected by various phenotypic and genotypic methods. PLoS One. 2017;12(6):e0180121. doi:10.1371/journal.pone.0180121
33. Ahmed OB, Asghar AH, Bahwerth FS. Prevalence of ESBL genes of Pseudomonas aeruginosa strains isolated from Makkah Hospitals, Saudi Arabia. Euro J Biol Med Sci Res. 2015;3(6):12–18.
34. Abiri R, Mohammadi P, Shavani N, Rezaei M. Detection and genetic characterization of metallo-β-lactamase IMP-1 and VIM-2 in Pseudomonas aeruginosa strains from different hospitals in Kermanshah, Iran. Jundishapur J Microbiol. 2015;8(9):e22582. doi:10.5812/jjm
35. Chaudhary M, Payasi A. Rising antimicrobial resistance of Pseudomonas aeruginosa isolated from clinical specimens in India. J Proteomics Bioinform. 2013;6(1):005–009.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
