Back to Journals » Nanotechnology, Science and Applications » Volume 10
Antimicrobial characterization of silver nanoparticle-coated surfaces by “touch test” method
Authors Gunell M, Haapanen J, Brobbey KJ, Saarinen JJ, Toivakka M , Mäkelä JM, Huovinen P, Eerola E
Received 12 April 2017
Accepted for publication 21 August 2017
Published 14 November 2017 Volume 2017:10 Pages 137—145
DOI https://doi.org/10.2147/NSA.S139505
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Israel (Rudi) Rubinstein
Marianne Gunell,1,2 Janne Haapanen,3 Kofi J Brobbey,4 Jarkko J Saarinen,4 Martti Toivakka,4 Jyrki M Mäkelä,3 Pentti Huovinen,1 Erkki Eerola1,2
1Department of Medical Microbiology and Immunology, University of Turku, 2Department of Clinical Microbiology and Immunology, Microbiology and Genetics Service Area, Turku University Hospital, Turku, 3Aerosol Physics Laboratory, Department of Physics, Tampere University of Technology, Tampere, 4Laboratory of Paper Coating and Converting, Center for Functional Materials, Åbo Akademi University, Turku, Finland
Abstract: Bacterial infections, especially by antimicrobial resistant (AMR) bacteria, are an increasing problem worldwide. AMR is especially a problem with health care-associated infections due to bacteria in hospital environments being easily transferred from patient to patient and from patient to environment, and thus, solutions to prevent bacterial transmission are needed. Hand washing is an effective tool for preventing bacterial infections, but other approaches such as nanoparticle-coated surfaces are also needed. In the current study, direct and indirect liquid flame spray (LFS) method was used to produce silver nanoparticle-coated surfaces. The antimicrobial properties of these nanoparticle surfaces were evaluated with the “touch test” method against Escherichia coli and Staphylococcus aureus. It was shown in this study that in glass samples one silver nanoparticle-coating cycle can inhibit E. coli growth, whereas at least two coating cycles were needed to inhibit S. aureus growth. Silver nanoparticle-coated polyethylene (PE) and PE terephthalate samples did not inhibit bacterial growth as effectively as glass samples: three nanoparticle-coating cycles were needed to inhibit E. coli growth, and more than 30 coating cycles were needed until S. aureus growth was inhibited. To conclude, with the LFS method, it is possible to produce nanostructured large-area antibacterial surfaces which show antibacterial effect against clinically relevant pathogens. Results indicate that the use of silver nanoparticle surfaces in hospital environments could prevent health care-associated infections in vivo.
Keywords: silver, nanoparticle, E. coli, S. aureus, LFS, HAI
Introduction
Bacterial infections impose major consequences on global, national and individual levels, especially when antimicrobial resistant (AMR) bacteria are involved.1,2 AMR is a major threat globally, and it has been calculated that by the year 2050 there will be more than 10 million deaths worldwide linked to AMR bacteria.3 While people live longer, an increasing number of elderly people are staying in hospitals, geriatric institutions and long-care facilities, and thus they are more likely to get health care-associated infections (HAIs). In hospitals, microbes are easily transferred to environment and to other patients via surfaces such as bed linens, bed edges, trolleys, tables, water tap handles, toilet seats and door knobs. This bacterial transmission can lead to nosocomial infections which cause longer hospital stay, more severe infections and even death, especially when the transmission of AMR bacteria such as methicillin-resistant Staphylococcus aureus (MRSA) or carbapenemase-producing Enterobacteriaceae (CPE) are considered.4–6 Escherichia coli and S. aureus are reported of being the most common pathogens linked to HAIs and especially fluoroquinolone-resistant E. coli strains are causing infections in hospitals.7,8 Good hand hygiene and the use of alcohol-based hand rubs are the best way for preventing bacterial transmission and HAIs.9 Recent studies have nonetheless shown that case fatality rate in HAIs has increased,10 probably due to AMR and even multi-resistant bacteria. Despite the improved hand hygiene, efficient tools against infections and AMR bacteria are still needed.
Antimicrobial surfaces are one of the most promising innovations for preventing bacterial infections and bacterial transmission in hospital environments. Various heavy metal compounds, including TiO2, Cu2O, ZnO and Ag, are known to have antimicrobial properties since they disturb bacterial growth by inhibiting cell wall proton pumps, inhibiting DNA replication and transcription and causing damages to cell membrane.11–15 Silver vessels were already used in 1000 BC to prevent bacterial growth and make water potable.15 Nowadays, metal nanoparticles, especially silver nanoparticles have shown potential in various antimicrobial applications, including biomedical coatings and textiles.11,14,15 Silver nanoparticles are able to damage DNA or inactivate enzymes or they can induce cell death by increasing membrane permeability, and by changing the structure of membranes silver nanoparticles could also be used as an alternative for antimicrobial treatment.12,14,15 Advantage of using silver nanoparticles is based on their structure: nanoparticles (0.2–100 nm in size) have a high surface-to-volume ratio, thus small particles get good interaction with microbes and the structure enhances the antimicrobial activity.12,13 Nanoparticles and nanoparticle-coated surfaces can be produced with various methods. In the current study, a liquid flame spray (LFS)-coating technique was used. LFS is a thermal spray process, where liquid precursor solution is injected into turbulent H2/O2 flame. Due to high temperature of the flame, precursor solution evaporates and generates solid nanoparticles via various aerosol processes.16 The advantage of using LFS is that this method can be used for coating not only conventional materials, such as metal or glass, but also flexible and even heat-sensitive substrates, e.g., tissue paper and paperboards.17,18 In addition, LFS-coating method has applicability to roll-to-roll process, which is a beneficial feature when cost-effective antibacterial surfaces for various applications are developed and produced.19,20
In the current study, the LFS method was used for coating glass, polyethylene (PE) and PE terephthalate (PET) substrates with silver nanoparticles, and the “touch test” method was used for testing the antibacterial properties of produced surfaces. The thickness of silver nanoparticle layer and the direct vs. indirect LFS nanoparticle deposition method were evaluated to determine the antibacterial properties of the silver nanoparticle surfaces. The potential of LFS technique to produce large-scale antibacterial surfaces for hospital environments is also discussed.
Materials and methods
Substrates
Microscope cover glasses (borosilicate, thickness 0.16–0.19 mm; Thermo Fisher Scientific, Waltham, MA, USA), size 20 mm× 20mm, and PE- and PET-coated papers were used as substrates in nanoparticle coating. After LFS coating, A4 size paper was cut into 20 mm× 20 mm pieces to correspond the size of cover glass samples. Glass samples were cleaned with acetone, 2-propanol and deionized water prior to nanoparticle-coating process.
LFS coating
LFS is a versatile method for producing one- or multicomponent nanoparticles.18 In the LFS method, liquid precursor is injected into turbulent H2/O2/N2 flame. Liquid precursor was prepared by dissolving silver nitrate (AgNO3, 99.9%; Strem Chemicals, Newburyport, MA, USA) into deionized water with silver concentration of 250 mg/mL. Precursor feed rate was fixed at 2.0 mL/min, gaining silver nanoparticle production rate of 500 mg/min. Gas flow rates for H2, O2 and N2 were fixed at 20, 10 and 5 L/min, respectively. Details of LFS process are described in previous publications.17,18,20 In the direct deposition method, a carousel-type coating device was used where samples passed through the flame one or several times.17 In the carousel, the speed of the sample through the flame was fixed at 50 m/min, and the distance between the burner nozzle and substrate was fixed at 20 cm. One sweep through the flame is defined in this study as one coating cycle. One coating cycle with the chosen parameters produces sub-monolayer of silver nanoparticles, and by increasing the number of coating cycles, surface becomes more covered with nanoparticles. In the indirect deposition method, flame is introduced into a specially designed flow tube (tube diameter ca. 20 cm), where the flow of nanoparticle cools down and becomes more homogeneous. Deposition of nanoparticles occurs at the other end of the flow tube. LFS parameters are the same in the indirect as in the direct deposition, but coating time at the end of the tube is varied.
For glass samples, two different approaches were used when the antimicrobial properties of silver nanoparticle coatings were tested. In the first approach, tested cover glasses were coated with direct and indirect LFS nanoparticle deposition, using 30 coating cycles and 30 s coating time, respectively, and the results were compared with each other and to control samples. In the second approach, tested cover glasses were coated with direct deposition method using 1, 2, 4, 8, 16 and 32 coating cycles to find out the optimal threshold level when the nanoparticle surface became antibacterial, i.e., when there were enough nanoparticles in a given surface area to prevent bacterial growth. Similar microscope cover glasses without nanoparticle coatings were used as references in both approaches.
For PE- and PET-coated paper samples, only the threshold- level testing was used, since the direct deposition method showed better results with glass samples. For PE samples and PET samples, direct deposition with 1, 5, 10 and 30 coating cycles and direct deposition with 1, 3, 5, 10 and 30 coating cycles, respectively, were used to test the optimal threshold level for nanoparticle coatings. PE- and PET-coated papers without nanoparticle coating were used as reference samples.
Silver nanoparticle and surface characterization
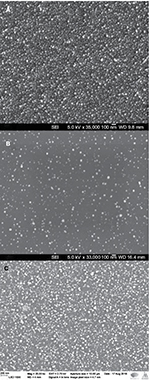
Scanning electron microscopy (SEM; Jeol JSM-6335F; JEOL [Nordic], Sollentuna, Sweden) was used to image the sample surfaces before and after coating with silver nanoparticles. SEM images were obtained by sputtering the samples with a thin carbon coating and using an accelerating voltage of 2.7 kV with about 5–6 mm working distance. The method of determining the morphology of silver nanoparticles and measuring particle distribution on surfaces is described in more detail in the previous publication.21 X-ray photoelectron spectroscopy (XPS) was used to chemically quantify the nanoparticles on the surface in atomic percentage. XPS spectra were obtained using PHI Quantum 2000 (Physical Electronics, Chanhassen, MN, USA) as described in the previous publication.21
Antimicrobial testing
The antimicrobial properties of the produced nanoparticle surfaces were tested against Gram-negative rod-shaped E. coli American Type Culture Collection (ATCC) 25922 and Gram-positive coccus, S. aureus ATCC 29213 (methicillin-susceptible strain). E. coli and S. aureus were chosen since they are the most common cause for HAIs.2 Evaluation of direct vs. indirect nanoparticle deposition was also performed with other clinically relevant bacteria: Acinetobacter sp., Enterococcus sp., Pseudomonas aeruginosa, Streptococcus pyogenes, Streptococcus pneumoniae and MRSA.
The antimicrobial testing of nanoparticle-coated samples was performed with modified replica-plating method.22 In touch test method, overnight grown bacteria were diluted in 0.9% NaCl to obtain bacterial suspension equal to 0.5 McFarland standard (approximately 1.5 × 108 colony-forming unit [CFU]/mL). Of this bacterial suspension, 50 µL was pipetted on top of the nanoparticle-coated glass and paper samples. Samples were incubated at room temperature (RT) or +37°C for 6, 24 and 48 h on an empty petri plate. After the incubation, viable bacteria from the nanoparticle-coated samples were replicated by stamping the sample on top of the blood agar plate (tryptic soy agar W/5% SB (II); BD, Franklin Lakes, NJ, USA), nanoparticle surface against agar, for 30 s and then removed (touch test). Blood agar plates were then incubated at +37°C o/n and the number of CFUs, if applicable, was determined on the following day (Figure 1). Bacterial growth was scaled as follows: 0 – no growth, 1 – 0–50 (weak growth), 2 – 50–100 (moderate growth), 3 – >100 colonies (good growth), i.e., the culture method was semiquantitative. When the direct and indirect LFS deposition method was evaluated, assay was performed 10 times for E. coli and S. aureus and three times for other pathogens. In the threshold screening, cover glass, PE and PET samples were tested three times for E. coli and S. aureus. Mean and SD were calculated for each trial, and they are presented in Figures 2–4 and Table 1. A one-tailed Student’s t-test was used when comparing antimicrobial activity between uncoated and silver nanoparticle-coated glass and PE and PET samples.
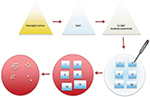  | Figure 1 Schematic picture of “touch test” method. |
Results
Direct vs. indirect LFS deposition
Results showed that the direct silver nanoparticle deposition, with 30 coating cycles, had clear antibacterial effect against both S. aureus ATCC 29213 (p < 0.005) and E. coli ATCC 25922 (p < 0.05). There were no E. coli CFUs detected in any of the samples when incubated for 3, 6, 24 or 48 h at +37°C; however, a few separate S. aureus colonies were detected after 3 and 24 h incubation (Figure 2). The indirect silver nanoparticle deposition was also effective against E. coli (p < 0.05), when less than five colonies were detected in samples after 3 h incubation and no bacterial colonies were detected in any samples in latter time-point samples. However, the indirect silver nanoparticle coating did not have influence on S. aureus, and moderate bacterial growth was detected in all time points (Figure 2). Bacteria could also be cultured from all reference samples in every time point: E. coli growth decreased evenly and rapidly, whereas S. aureus stayed viable throughout the whole incubation period (Figure 2).
When nanoparticle coatings were tested against other clinically relevant bacteria, similar results as with S. aureus and E. coli were obtained. Nanoparticle coatings made with direct nanoparticle deposition showed better antibacterial properties compared with indirect deposition. Only MRSA and Enterococcus faecalis were viable in direct LFS samples which were incubated 6 h in RT (Table 1). In all the other time points, there were no bacterial colonies detected in direct LFS samples. The indirect LFS coating had almost no effect on Enterococci, whereas clear inhibition was seen in all the other tested pathogens when samples were incubated over 6 h or at +37°C. In addition, MRSA was clearly inhibited (p < 0.005) with indirect coating when samples were incubated at +37°C (Table 1). Since better results were achieved by direct silver nanoparticle deposition, only the direct deposition method was used for further testing.
Threshold screening
Results from coating cycle threshold screening showed that already one LFS-coating cycle was enough to make a glass surface antibacterial against E. coli (p < 0.001). E. coli colonies were not detected in any of the nanoparticle-coated samples in any time points. However, E. coli was able to grow on reference glasses after 6 and 24 h incubation; thus, growth was slightly inhibited when samples were incubated at +37°C (Figure 3A). More coating cycles were needed to inhibit S. aureus growth. After 6 h RT incubation, S. aureus was able to grow on all coated samples although the number of CFUs decreased along with the increasing number of coating cycles (Figure 3B). After 24 h incubation at RT, S. aureus was able to grow only in samples with one coating cycle, same as in samples incubated for 6 h at +37°C. After 24 h incubation at +37°C, S. aureus growth was inhibited in all silver nanoparticle-coated samples (p < 0.01); however, S. aureus was growing well on reference glasses when incubated at RT or +37°C (Figure 3B).
Silver nanoparticle-coated PE surface inhibited bacterial growth better than PET surface. Already one LFS coating cycle on PE surface was enough to inhibit E. coli bacterial growth (p < 0.05), E. coli colonies were detected only in PE samples with one LFS coating cycle when incubated for 6 h either at RT or +37°C. When more coating cycles and longer incubation times were used, no E. coli colonies were detected (p < 0.005; Figure 4A). When PET samples were incubated for 6h in RT, 10 LFS coating cycles were needed to inhibit E. coli growth in samples incubated at RT for 6 h. At least three coating cycles were needed to inhibit E. coli growth when samples were incubated a longer time or at +37°C (Figure 4C). When reference samples were incubated at RT, E. coli growth was only slightly inhibited whereas incubation at +37°C decreased bacterial growth linearly (Figure 4A and C). Silver nanoparticle-coated PE and PET surfaces had clearly less effect on S. aureus compared with E. coli, although PE surface inhibited S. aureus growth better. When PE samples were incubated at RT, surfaces with 10 coating cycles decreased S. aureus growth linearly and 30 coating cycles inhibited bacterial growth totally in all the other samples except in those incubated for 6 h (Figure 4B). When PE samples were incubated at +37°C, S. aureus growth was decreased also on surfaces with five coating cycles and the growth was clearly inhibited (p < 0.005) in samples with 10 coating cycles (Figure 4B). More than five coating cycles were needed to decrease S. aureus growth in PET samples and with 30 coating cycles bacterial growth was evidently inhibited (p < 0.001), especially when incubated at +37°C (Figure 4D). In reference PE and PET samples, there was no decrease in S. aureus bacterial growth during the 48 h incubation.
Nanoparticle surface characterization
As shown also in our previous publication,21 SEM imaging suggested that synthesized silver nanoparticles are spherical with primary particle size of 20–50 nm, nanoparticles are homogeneously distributed and they form a monolayer on the surface (Figure 5). SEM imaging also showed that the surface with direct 30 coating cycles has clearly more silver nanoparticles than surface with indirect 30 s deposition (Figure 5A–C). With higher amount of coating cycles, silver nanoparticles may form bigger clusters when several primary nanoparticles attach to each other.
Discussion
In the current study, silver nanostructures were produced with the LFS method, which enables easy and cost-effective way of coating different kinds of materials, even paper board, and the method is applicable in large-scale production processes.19 The glass, PE and PET samples were coated with silver nanoparticles, and the results showed that with the direct LFS method it was possible to produce surfaces which were antimicrobial against all of the tested bacteria. The antimicrobial properties of silver nanoparticle surfaces were tested against E. coli and S. aureus, because not only they are commonly used reference bacteria in laboratory settings but also they are an example of the most important threat of AMR in hospital settings: fluoroquinolone-resistant E. coli and MRSA. In addition, other seven clinically relevant pathogens whose impact on HAIs and AMR is enormous were tested.2,7,8 The touch test method was used since it was the most convenient and easiest way of testing the antimicrobial properties of nanoparticle surfaces. In addition, being in vitro model, the touch test method was comparable to situation in hospitals where people are touching different surfaces and thus transfer bacteria to other surfaces.23–25 In the touch test method, tested bacteria were in close contact with silver nanostructures, allowing silver nanoparticles to interact with the bacterial cell membrane and thus cause cell death.26
Tested surfaces inhibited E. coli and S. aureus growth almost totally during the 48 h incubation time. SEM imaging has shown that surface with direct 30 coating cycles has clearly more silver nanoparticles than surface with indirect 30 s deposition. We have previously shown that the particle spacing depends also on the number of coating cycles, i.e., the surface area covered by nanoparticles increases along with the number of sweeps.21 This explains the better performance of the direct deposition samples. Silver nanoparticles are more widely dispersed on surface when more coating cycles are used, and since we wanted to maximize the silver nanoparticle amount on the surface, silver concentration of 250 mg/mL was chosen. Based on the previous knowledge from silver nanoparticle synthesis by LFS, it is known that with higher silver concentration there is a possibility of formation of undesired bigger residual particles and the presence of AgNO3 droplets on the surface.17,18 However, the amount of silver nanoparticles is decreased when lower concentration is used.
Incubation temperature also had an influence on bacterial growth inhibition, and when samples were incubated at RT less inhibition was detected. This could be explained by the fact that when samples were incubated at +37°C, bacterial suspension was dried up during the first 30 min of incubation, and thus drying was the main cause of bacterial growth inhibition. Although +37°C is the optimal temperature for bacterial growth, the aim was to develop a nanoparticle surfaces which could be used in a normal ambient temperature, i.e., hospitals and long-term care facilities; thus, results from the RT testing are more important. Surface material also has an effect on bacterial survival. It has been reported that MRSA survives the longest on plastic surfaces and bacterial transmission happens the best from smooth surfaces.27 Results showed that both S. aureus and E. coli cells were more viable on nanoparticle-coated PE and PET surfaces compared with glass samples. When using the LFS method, silver nanoparticles are bound to substrate via van der Waals forces, and therefore, nanoparticle coating is by no means stable. Since bacteria are able to survive on a dry surface over days and weeks,24,27 there is a need to use techniques which could enhance nanoparticle surface stability, for example, coatings or priming nanoparticles on surfaces. This is especially important for further development if these surfaces are going to be used in hospital environments where surfaces are predisposed to high wearing.
Conclusion
This study was performed to evaluate the potential of the LFS method by producing silver nanoparticle surfaces which could prevent bacterial transmission even in hospital environments. It has been shown in this study that with the direct LFS method we could produce surfaces which showed antibacterial effect not only against E. coli but also against S. aureus and other clinically relevant pathogens. The touch test method was used and it was an easy, repeatable and reliable way of testing antibacterial properties of silver nanoparticle-coated glass, PE and PET samples. It was shown in this study that at least 30 nanoparticle-coating cycles were needed to inhibit S. aureus growth in PE and PET samples, whereas three nanoparticle-coating cycles were enough to kill E. coli cells. With glass samples, two nanoparticle layers were needed to show antibacterial effect. To conclude, LFS is a cost-effective method for producing silver nanoparticle large-scale area antibacterial surfaces. Results indicate that the use of silver nanoparticle-coated surfaces and paper products in hospital environments could prevent bacterial transmission and HAIs also in vivo.
Acknowledgments
This work was supported by the Academy of Finland under the project “Nanostructured large-area antibacterial surfaces (nLABS, grant no 275 475).” JJS wishes to thank the Academy of Finland (grants nos: 250 122, 256 263 and 283 054) for the financial support. Heidi Isokääntä and Minna Lamppu are thanked for their excellent technical assistance with antibacterial testing.
Disclosure
The authors report no conflicts of interest in this work.
References
Levy SB, Marshall B. Antibacterial resistance worldwide: causes, challenges and responses. Nat Med. 2004;10(12 suppl):S122–S129. | ||
World Health Organization. The Evolving Threat of Antimicrobial Resistance: Options for Action. France: GPS Publishing; 2012. | ||
O’Neill J. Review on Antimicrobial Resistance. Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations. Wellcome Trust and the UK Government; 2014. | ||
Ray MJ, Lin MY, Weinstein RA, Trick WE. Spread of carbapenem-resistant enterobacteriaceae among Illinois healthcare facilities: the role of patient sharing. Clin Infect Dis. 2016;63(7):889–893. | ||
Visalachy S, Palraj KK, Kopula SS, Sekar U. Carriage of multidrug resistant bacteria on frequently contacted surfaces and hands of health care workers. J Clin Diagn Res. 2016;10(5):DC18–DC20. | ||
Shams AM, Rose LJ, Edwards JR, et al. Assessment of the overall and multidrug-resistant organism bioburden on environmental surfaces in healthcare facilities. Infect Control Hosp Epidemiol. 2016;37(12):1426–1432. | ||
Peleg AY, Hooper DC. Hospital-acquired infections due to gram-negative bacteria. N Engl J Med. 2010;362(19):1804–1813. | ||
Weiner LM, Webb AK, Limbago B, et al. Antimicrobial-resistant pathogens associated with healthcare-associated infections: summary of data reported to the national healthcare safety network at the centers for disease control and prevention, 2011–2014. Infect Control Hosp Epidemiol. 2016;37(11):1288–1301. | ||
Allegranzi B, Bagheri Nejad S, Combescure C, et al. Burden of endemic health-care-associated infection in developing countries: systematic review and meta-analysis. Lancet. 2011;377(9761):228–241. | ||
Wang RF, Shen SH, Yen AM, et al. Risk factors for incidence and case-fatality rates of healthcare-associated infections: a 20-year follow-up of a hospital-based cohort. Epidemiol Infect. 2016;144(1):198–206. | ||
Vardanyan Z, Gevorkyan V, Ananyan M, Vardapetyan H, Trchounian A. Effects of various heavy metal nanoparticles on Enterococcus hirae and Escherichia coli growth and proton-coupled membrane transport. J Nanobiotechnology. 2015;13:69. | ||
Rudramurthy GR, Swamy MK, Sinniah UR, Ghasemzadeh A. Nanoparticles: alternatives against drug-resistant pathogenic microbes. Molecules. 2016;21(7):E836. | ||
Hajipour MJ, Fromm KM, Akbar Ashkarran A, et al. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012;30(10):499–511. | ||
Jung WK, Koo HC, Kim KW, Shin S, Kim SH, Park YH. Antibacterial activity and mechanism of action of the silver ion in Staphylococcus aureus and Escherichia coli. Appl Environ Microbiol. 2008;74(7):2171–2178. | ||
Rai M, Yadav A, Gade A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 2009;27(1):76–83. | ||
Tikkanen J, Gross KA, Berndt CC, et al. Characteristics of the liquid flame spray process. Surf Coat Technol. 1997;90(3):210–216. | ||
Aromaa M, Arffman A, Suhonen H, et al. Atmospheric synthesis of superhydrophobic TiO2 nanoparticle deposits in a single step using liquid flame spray. J Aerosol Sci. 2012;52:57–68. | ||
Haapanen J, Aromaa M, Teisala H, et al. Binary TiO2/SiO2 nanoparticle coating for controlling the wetting properties of paperboard. Mater Chem Phys. 2015;14(9–150):230–237. | ||
Mäkelä JM, Haapanen J, Aromaa M, et al. Roll-to-roll coating by liquid flame spray nanoparticle deposition. MRS Online Proc Libr (OPL) Arch. 2015;1747:37-42. | ||
Mäkelä JM, Aromaa M, Teisala H, et al. Nanoparticle deposition from liquid flame spray onto moving roll-to-roll paperboard material. Aerosol Sci Technol. 2011;45(7):827–837. | ||
Brobbey KJ, Haapanen J, Gunell M, et al. One-step flame synthesis of silver nanoparticles for roll-to-roll production of antibacterial paper. Appl Surf Sci. 2017;420:558–565. | ||
Paavilainen T, Osterblad M, Leistevuo T, Huovinen P, Kotilainen P. Screening for antimicrobial resistance in normal bacterial flora of the skin using the replica plating method. Eur J Clin Microbiol Infect Dis. 2000;19(12):956–959. | ||
Huang SS, Datta R, Platt R. Risk of acquiring antibiotic-resistant bacteria from prior room occupants. Arch Intern Med. 2006;166(18):1945–1951. | ||
Neely AN, Maley MP. Survival of enterococci and staphylococci on hospital fabrics and plastic. J Clin Microbiol. 2000;38(2):724–726. | ||
Zarpellon MN, Gales AC, Sasaki AL, et al. Survival of vancomycin-intermediate Staphylococcus aureus on hospital surfaces. J Hosp Infect. 2015;90(4):347–350. | ||
Soman S, Ray JG. Silver nanoparticles synthesized using aqueous leaf extract of Ziziphus oenoplia (L.) mill: characterization and assessment of antibacterial activity. J Photochem Photobiol B. 2016;163:391–402. | ||
Peng Y, Song C, Yang C, Guo Q, Yao M. Low molecular weight chitosan-coated silver nanoparticles are effective for the treatment of MRSA-infected wounds. Int J Nanomedicine. 2017;12:295–304. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.