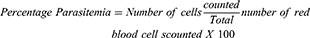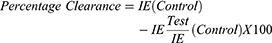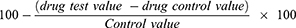Back to Journals » Infection and Drug Resistance » Volume 14
Antimalarial and Erythrocyte Membrane Stability Properties of Globimetula braunii (Engle Van Tiegh) Growing on Cocoa in Plasmodium berghei-Infected Mice
Authors Olanlokun JO , Ekundayo MT, Ebenezer O, Koorbanally NA , Olorunsogo OO
Received 6 May 2021
Accepted for publication 7 July 2021
Published 16 September 2021 Volume 2021:14 Pages 3795—3808
DOI https://doi.org/10.2147/IDR.S317732
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Héctor Mora-Montes
John Oludele Olanlokun,1 Mercy Toluwase Ekundayo,1 Oluwakemi Ebenezer,2 Neil Anthony Koorbanally,2 Olufunso Olabode Olorunsogo1
1Laboratories for Biomembrane Research and Biotechnology, Faculty of Basic Medical Sciences, College of Medicine, University of Ibadan, Ibadan, 4000, Nigeria; 2School of Chemistry and Physics, University of KwaZulu-Natal, Durban, 4006, South Africa
Correspondence: John Oludele Olanlokun
Department of Biochemistry, Faculty of Basic Medical Sciences, College of Medicine, University of Ibadan, Room NB 305, Ibadan, Nigeria
Email [email protected]
Introduction: Resistant malaria is a fatal disease. Globimetula braunii (African Mistletoe) is traditionally used for malarial treatment but this fact has not been scientifically reported.
Methods: Plasmodium berghei (NK65)-infected male Swiss mice (20± 2 g) were treated orally and once daily with 100, 200, and 400 mg/kg BW of methanol extract and its respective hexane, dichloromethane and ethyl acetate fractions for 9 days. P-alaxin was used as control drug P. berghei (ANKA)-infected mice were then treated with the most potent fraction for 5 days. Parasitemia and parasite clearance were determined by microscopy, while hematological parameters, heme, hemozoin, and mouse erythrocyte membrane stabilisation were assayed. The phytochemicals in the most potent fraction were identified using gas chromatography-mass spectrometry.
Results: Hexane fraction (HF)-treated mice (400 mg/kg BW) had the least mean parasite load (0.00 ± 0.00; 0.14 ± 0.05%) and highest clearance (100 ± 0.00; 75.50 ± 4.95%) compared with infected control (9.81 ± 0.09; 6.84 ± 0.09%) in susceptible and resistant models, respectively. Hexane fraction modulated hematological indices, minimised erythrocyte membrane damage in heat-induced (2.18 ± 0.94%) and hypotonic solution-induced (7.93 ± 0.93%) compared to artequin (5.05 ± 2.18; 6.38 ± 0.33%) and P-alaxin (67.45 ± 5.15; 56.78 ± 1.10%) in both models of membrane stabilisation, respectively. Hexane fraction (P< 0.01) increased heme and decreased hemozoin contents. Friedelan-3-one was identified as the most abundant triterpene.
Conclusion: The results indicated that G. braunii has anti-plasmodial properties and minimally dis-stabilised erythrocyte membrane. The major findings in this study are that n-hexane fraction of G. braunii possess excellent and moderate antiplasmodial activity against susceptible and resistant P. berghei, respectively. This was reflected via decreased parasite load, improved hematological parameters, increased heme and decreased hemozoin contents. Friedelan-3-one, a major constituent of the n-hexane fraction, may be responsible for this activity.
Keywords: Globimetula braunii, erythrocytes, heme, hemozoin, Plasmodium berghei, resistant malaria
Introduction
Malaria is a major infectious disease that has plagued many countries across the globe, claiming many lives. According to a World Malaria Report in 2019, over 90% of reported cases stem from Africa, with a significant percentage of these infections being fatal, especially among pregnant women and children.1 In 2019, there were 229 million estimated cases of malaria worldwide with about 409,000 fatalities while children whose ages are below 5 years accounted for 67% (274,000) casualties. The highest percentage of malaria cases and fatality (99.7%) is found in Africa.1 For example, in 2019 alone, the financial burden of malarial treatment and prevention rose to about US$ 3 billion.1 Due to this, several treatment regimens and preventive measures have been employed to combat Plasmodium infection and disease. Resistance to the available drugs and complications are the primary concern of physicians when treating malaria. Infection occurs after the injection of the parasite into the host in exchange for a blood meal by the female anopheles mosquito. Following the pre-erythrocytic stage in the liver, malaria parasites invade the red blood cells, after which the clinical symptoms such as fever and pain begin to manifest.2 Infection of red blood cells by the parasites causes a decrease in oxygen transport through anemia, ultimately leading to inadequate oxygen delivery to tissues, and tissue hypoxia.3
Once infected, malarial parasites proliferate and degrade the red blood cell components for their use, releasing hemozoin as a by-product of heme breakdown.4 This results in decreased oxygen transport and consumption causing more severe damage to tissues. Oxidative stress generated by invading parasites coupled with increased parasite load places stress on the red cell membrane, which becomes unstable and eventually undergo hemolysis.5 Interestingly, antimalarial drugs have varying mechanisms of action. One such example is chloroquine that interferes with hemozoin formation and this has been described as its mechanism of action.6 More potent and less toxic antimalarial drugs are currently in need due to drug resistance, and the high levels of cytotoxicity of current antimalarial drugs. Natural products with promising antiplasmodial properties that are less toxic to erythrocytes have been reported.7
Resistance to common orthodox drugs by malarial parasites has made the search for other drugs with wide therapeutic window quite inevitable. Therefore, there is an increase in research on medicinal plants with promising folkloric evidence of high antimalarial efficacy. Furthermore, large numbers of medicinal plants are frequently screened to identify, evaluate the pharmacological and medicinal properties of promising medicinal plants with good folkloric efficacy against some diseases especially malaria because of the resistant strains of the causative organisms. The folkloric antimalarial efficacy of medicinal herbs such as Trema orientalis, Alstonia boonei, and Khaya grandifoliola has been substantiated with scientific reports.8–10 In addition to this, African mistletoe is hemi-parasitic plants found on many tree crops of economic importance. They belong to the Loranthaceae family, and the medicinal importance of some genera have been identified. For example, Dendrophthoe pentandra is used in folkloric medicine to treat inflammation,11 and induces cell death in colon cancer11Englerina gabonensis and Sterculia tragacantha have antibacterial, antifungal and antioxidant properties,12 Tapinanthus sessilifolus and Agelanthus dodoneifloius have been reported to have antimalarial properties,13,14 while Phragmanthera incana has inhibitory effects against lipid peroxidation15,16Globimetula braunii, is a specie out of about five hundred species of this plant. Like other mistletoes, it is widely spread by seed dispersal via birds that feed on mistletoe fruits through fecal droppings or regurgitation Because of their diverse health benefits, mistletoes are generally believed to be “cure all” medicinal plants simply because of the phytochemicals present in the plants and their health benefits. G. braunii is African mistletoe commonly found on cocoa and other perennial plants. Its oxytoxic,17 laxative18 antioxidative,19 and high hydroxyl radical scavenging activities have been reported.20 It is locally used in South Western Nigeria as an antimicrobial and antimalarial effusion. This claim has not been scientifically substantiated. Although some phytochemical and pharmacological studies have been carried out on G. braunii,21 to the best of our knowledge, there is paucity of information on the antiplasmodial activity of the plant.
In our continued interest in searching for new antimalarial medicinal plants and their bioactive constituents, we investigated the antiplasmodial activity of G. braunii. Various solvent extract and fractions of the leaves of this plant, using an established infection model, were explored. Due to malaria being noted for its resistance to conventional medicine, poor prognosis, and severe fatalities, the focus of the study was centered on both susceptible and resistant strains of malarial Plasmodium using animal models. In this regard, we substantiated the indigenous antimalarial claim of this plant using both chloroquine-sensitive (NK 65) and resistant (ANKA) models of P. berghei. We also identified the possible phytochemicals responsible for antimalarial activity in the plant’s most potent solvent fraction.
Materials and Methods
Collection of Plant Material, Extraction, and Partitioning in Vacuum Liquid Chromatography
The twigs with leaves of G. braunii were severed from the cocoa plant at Igbo Oloyin area in Ibadan and authenticated by Mr. Omotayo F.O. at the University Herbarium, Ekiti State University, Ado-Ekiti, Nigeria, and a voucher specimen (UHAE 2020064) deposited. The twigs were air-dried for two weeks at room temperature in the laboratory, and thereafter, the leaves were severed and pulverized using a mortar and pestle. The powdered sample was extracted using methanol at room temperature for 72 h. The methanol filtrate was concentrated using a rotary evaporator at 40°C to a dark green mass. The leaf methanol extract (ME) (50 g) of G. braunii was adsorbed on Thin Layer Chromatography (TLC) silica gel and air-dried. A column was packed with silica gel using n-hexane; the adsorbed sample was loaded and washed successively with four different solvents using vacuum liquid chromatography: n-hexane (HF), dichloromethane (DF), ethyl acetate (EF), and methanol (MF), respectively, to obtain their respective fractions (HF, DF, EF, and MF) and concentrated to dryness.
Phytochemical Screening
Determination of Total Phenolic Content
The total phenolic,22 tannins,23 alkaloids,24 flavonoids,25 saponins24 and terpenes26 contents of extract and fractions of G. braunii were quantitatively determined using the previously described methods.
Ethical Statement
All the experimental procedures involving laboratory animals were performed in compliance with the Principle of Laboratory Animal Care as specified in the Principle of Laboratory Animal Care (NIH publication No. 85–23) guidelines.27 The University of Ibadan Animal Care and Use Research Ethics Committee (UI-ACUREC) approved the study and approval number UI-ACUREC/17/0089 was assigned to the study.
Experimental Animals, Grouping, and Infection
The Ryley and Peter’s model of established infection was used in this study.28 Eight weeks old, all male Swiss mice (20 ± 2 g) were obtained from the Institute of Advanced Medical Research and Training, College of Medicine, University of Ibadan, Nigeria. The animals were kept in well-ventilated plastic cages and acclimatised for one week before the experiment. They were then intraperitoneally infected with the chloroquine-sensitive P. berghei-infected erythrocytes from a donor mouse (107 inoculums). Parasitemia was established after 72 hours, and the animals were sorted for grouping.28
Eighty-five infected mice (20±2 g) were randomly assigned to groups (n=5) and were treated orally and once daily as follows: the infected control was treated with the vehicle only (5% (v/v) dimethyl sulfoxide (DMSO)), the drug control group was treated with 10 mg/kg BW P-alaxin (Bliss GVS Pharma Limited, India), the test groups were treated with 50, 100 and 400 mg/kg BW each of ME, HF, DF, EF and MF of G. braunii. Treatment lasted for nine days. The most potent fraction was used for the chloroquine-resistant (ANKA strain) study. In this case, twenty-five male Swiss mice were infected with P. berghei ANKA strain, grouped (n=5) and were treated after the confirmation of parasitemia, orally and once daily for consecutive five days as follows: the infected control was treated with vehicle only (5% (v/v) dimethyl sulfoxide (DMSO)), the drug control mice were treated with 10 mg/kg BW artequin (a product of Oculus Pharmacare Limited, Nigeria), three doses; 50, 100 and 200 mg/kg BW of the most potent fraction were administered separately. Slides were collected daily for the assessment of percentage parasitemia and parasite clearance.
Termination of the Animals and Collection of Blood Samples
On the last day of each experiment, the animals were fasted overnight and terminated via cervical dislocation. The animals were quickly opened and the heart was located. Blood was obtained from the heart with the aid of 1 mL syringes and hypodermic needles. The needle, preloaded with 0.4 M preparation of EDTA to prevent blood clot, was inserted into the heart. Blood samples were collected from the heart via aspiration into EDTA bottles, swirled gently to ensure thorough mixing and kept.
Percentage Parasitemia and Percentage Clearance
Thin films of blood on slides were made from blood collected from the tail region of the animals, air-dried, and fixed in absolute methanol. These were air-dried again and stained with Giemsa stain, rinsed with buffered water, dried, and viewed under a microscope using the immersion oil objective (X100). Infected and uninfected erythrocytes were counted; percentage parasitemia and parasite clearance were calculated as follows:
Where IE= Infected Erythrocytes.
Determination of Hematology Parameters
Hemoglobin Concentration
This was determined as previously described29 using the cyamet hemoglobin method. A blood sample (0.02 mL) was added to 4 mL of Drabkin’s diluent. The solution was permitted to remain for 10 min, and the resultant solution was read at 540 nm with the diluent as reference. The concentration of hemoglobin expressed in g/100 mL was calculated as shown below.
Dilution factor =201, Hemoglobin concentration of standard =0.0572g/mL.
Packed Cell Volume
Packed Cell Volume (PCV) was determined by the conventional method described by Schalm et al.29 This involves filing a capillary tube with blood; one end of the tube was sealed using plasticine. The tube centrifuge in the micro-hematocrit was centrifuged in a hematocrit centrifuge at 2000 rpm for 5 min. The PCV percentage was read directly from a graphic hematocrit reader.
Red Blood Cell Count
By means of rubber tubing attached to the end of the red cell pipette, blood was drawn to the 0.5 mark on the pipette. At a nearly horizontal level, the pipette was used to draw Haymen’s solution was drawn to the 101 mark. In order to ensure thorough mixing and dilution of the fluid in the bulb, the rubber tubing was removed, and the pipette rotated for 1 min. Diluted blood was then introduced carefully into the counting chamber with a clean coverslip in place. Meanwhile, the red cell counting was done using a microscope (X40 objective). Counting was carried out in 5 sets of small squares containing 80 small squares.
Total White Cell Count
The procedure followed was similar to that used for determining the red blood cell count of dilution. The dilution ratio was 1:20, and each of the 4 white cell counting areas was bounded by a single line. The multiplication factor was 50 when all the 4 counting areas were used.
Heme Content
Heme content was estimated from an equal volume of blood withdrawn from experimental animals. Briefly, 10 µL blood was mixed with 250 µL of 10% sodium dodecylsulfate (SDS) (w/v) and 250 µL of 1 M sodium hydroxide (NaOH), vortexed for 10 min. The tubes were incubated for 2 h at room temperature, and the total heme content was read at 404 nm. Supposing the molar absorption coefficient of heme to be 9.08104/M/cm, the concentration was articulated as micromole of heme/mL of blood.30
Hemozoin Content
Briefly, 10 µL blood was completely lysed by 0.08% saponin and centrifuged at 18,000 rpm in ultra-centrifuge. The supernatant was removed, and the pellet was washed (three times) with 250 µL of 25% SDS buffered with 25 mM Tris-HCl buffer (pH 7.4). The pellet was incubated at 37°C overnight in the buffer as mentioned above. After incubation, the tubes were centrifuged again at 18,000 rpm, and the pellet was hydrolysed to its monomers using 1M sodium hydroxide (NaOH). Absorbance was read at 404 nm. Hemozoin content was estimated and expressed as micromole heme/mL of blood.31
Red Cell Membrane Stabilising Activity
Two models were used for this study; hypotonic solution and heat-induced membrane dis-stabilisation.
Heat-Induced Membrane Dis-Stabilisation
Modified established procedure by Okoli et al32 was used to assess the erythrocyte membrane-stabilising effects of the extract and fractions of G. braunii. The assay mixture consisted of 0.5 mL hyposaline (0.42% (w/v) sodium chloride (NaCl)), 1.0mL of 0.15M sodium phosphate buffer (pH 7.4), the varying volume of iso-saline (0.85% (w/v) NaCl) and 0.5mL mice erythrocyte suspension. The drug control was pipette as above without 2% erythrocyte suspension, while the blood control contained all the reagents except the drug or the extract. The reaction mixture was mixed properly and incubated at 56°C for 30 min. The tubes were cooled under running water and then centrifuged at 3000 rpm for 5 min. The supernatant was collected, and the absorbance was read at 560 nm. The buffered sodium chloride solution served as blank. Artequin (Oculus Pharmacare Limited, Nigeria) and P-Alaxin (Bliss GVS Pharma Limited, Mumbai, India) were used as antimalarial drug controls.
The percentage membrane stability activities were estimated from the expression:
Hypotonic Solution-Induced Membrane Dis-Stabilisation
The membrane-stabilising effect of the extract and fractions of G. braunii using the hypotonic solution-induced mice erythrocyte hemolysis method was determined by using the modified method of Umapathy et al.33 To prepare the erythrocyte suspension, whole blood was obtained from mice via cardiac puncture using a syringe containing 0.4 M ethylenediamine tetraacetic acid (EDTA) at pH 7.4. The blood was centrifuged, and blood cells were washed three times with a solution of 154 mM NaCl in 10 mM sodium phosphate buffer (pH 7.4), centrifuged for 10 min at 5200 rpm. The test sample consisted of stock erythrocyte suspension (0.50 mL) mixed with 5 mL of hypotonic solution (50 mM NaCl) in 10 mM sodium phosphate-buffered saline (pH 7.4) containing either the extract/fraction of G. braunii (2.0 mg/mL) or acetylsalicylic acid (0.1 mg/mL). The control sample consisted of 0.5 mL of RBC mixed with hypotonic buffered saline alone. The mixture was incubated for 10 min at room temperature, centrifuged for 10 min at 5200 rpm, and the supernatant’s absorbance was measured at 540 nm.
Gas Chromatography-Mass Spectrometry (GC-MS) Analysis of the Most Potent Fraction
Gas chromatography-mass spectrometry (GC-MS) analysis of the most potent fraction was carried out using an Agilent 7890n gas chromatography coupled with mass detector triple Quad 7000A with an ion source temperature of 250°C and an Agilent ChemStation data system. The GC column was equipped with an HP-5MS column (30 m × 0.25 mm × 0.25 μm), an injector heated at 200°C, and a Flame Ionisation Detector (FID) at 230°C. The initial temperature of the oven was set at 40°C for 5 min, increased to 5°C/min to 180°C for 6 min, and then 10°C/min to 280°C for 12 min. Helium was used as a carrier gas at a 1 mL/min flow rate with a split ratio of 1:20. The GC-MS QP 2010 Plus was used to analyse the most potent fraction with ion source and interface temperature at 250°C; solvent cut time 2.5 min with relative detector gain mode and threshold 3000; scan MS ACQ mode; detector FTD; the mass range of m/z 40,400. Essential components were detected based on their retention indices and by comparison of their mass spectral fragmentation patterns by computer matching with in-built libraries.
Statistical Analysis
Data were expressed as the mean ± SD of five determinations. Ungrouped data were analysed using a one-way while grouped data were analysed using two-way analysis of variance (ANOVA) followed by Tukey’s post hoc (one-way) and Dunnett’s multiple comparison test (two-way) using Graph Pad prism (6.0). The level of significance was set at P<0.05.
Results
Phytochemical Screening
Table 1 shows the quantitative phytochemical screening of extract and fraction of G. braunii. The total alkaloid content of HF at 50 mg/mL was significantly lower (P<0.05) than that of other solvent fractions whereas, DF and EF were significantly (P<0.01) higher in alkaloid content at the same concentration when compared with other solvent fractions. There was no significant difference in the alkaloid content of the extract and fractions of the plant at 100 and 200 mg/mL of the samples. At 50 mg/mL, phenolic content of ME, DF, EF and MF were significantly (P<0.05) higher than the HF while HF is significantly (P<0.05) lower in phenolic content when compared with other fractions. There was no significant difference in the phenolic content of the extract and fractions of G. braunii at 100 mg/mL. At 200 mg/mL, ME, EF and MF contained significantly (P<0.05) higher content of phenolics whereas the content of phenolic compounds were significantly (P<0.05) lower in HF. The tannin content of HF and EF at 50 mg/mL was significantly (P<0.05) higher than other solvent fractions while MF contained the least content of tannin (P<0.05). Similar results were obtained at 100 and 200 mg/mL concentration of the samples. The total flavonoid content was significantly (P<0.05) higher in ME and MF while HF contained the least (P<0.05) content of flavonoid at 50 mg/mL. Similar result was obtained at 100 mg/mL while there was significant (P<0.05) increase in flavonoid content in DF, EF and MF at 200 mg/mL. The saponin contents of ME, HF and DF were significantly (P<0.05) at 50 mg/mL. Similar results were obtained at 100 and 200 mg/mL. The terpene content of EF was significantly (P<0.05) higher while further increase (P<0.01) were observed in ME, HF and DF at 50 mg/mL. Similar result was obtained at 100 and 200 mg/mL (Table 1).
 |
Table 1 Quantitative Phytochemical Determination of Extract and Fractions of G. braunii |
Percentage Parasitemia and Parasite Clearance of NK65-Infected Mice Treated with Extract and Fractions of G. braunii
Measurement of the degree of parasitemia (%) and parasite clearance show that, indeed, G. braunii has antimalarial activity against chloroquine-susceptible (NK 65) strain of P. berghei. Interestingly, purification further enhances this activity. In brief, there was a decrease in percentage parasitemia and an increase in parasite clearance when infected mice were treated with ME. Hexane fraction (HF) had the least percentage parasitemia and the highest parasite clearance compared with other fractions. Although DF EF and MF also decreased parasite levels in the invaded erythrocytes, it was observed that their effects were not as high as in mice treated with HF. On day 9, the percentage parasitemia and parasite clearance of HF were not significantly (P<0.05) different from those of the animals treated with control drug. Although ME, DF, EF and MF also caused increase in parasite clearance, the effects were not significant. The order of percentage parasite clearance is control drug>HF>MC>DCM> MF>EF, while percentage parasitemia is of the reverse order (Figure 1A–E). Similarly, there was a corresponding increase in parasite clearance, the highest observed in HF and the least observed in EF (Figure 1F–J).
Hexane Fraction of G. braunii Modulates Blood Counts and Increases Heme Content
In Tables 2 and 3, the Packed Cell Volume (PCV) of mice infected with NK 65 strain of P. berghei increased albeit insignificantly and maximally at 200 mg/kg BW dose of n-hexane fraction while the least PCV value was obtained in mice infected with the parasites but that were not treated. Similar trend was obtained in the resistant model. The hemoglobin content of infected mice in the susceptible model treated with 200 mg/kg BW was significantly higher among other treated groups and was even higher than in mice treated with the standard drug. In the resistant model, all the doses of n-hexane fraction of G. braunii were significantly effective in increasing the hemoglobin content compared with the infected control. The red blood cell and platelet counts in the susceptible model at 200 mg/kg BW significantly (P<0.05) increased compared with the infected control where the least values were obtained. On the other hand, all the doses used for the treatment of infected mice in the resistant model significantly (P<0.05) increased RBC count than the infected control while there was no significant increase in the treated groups in platelet count when compared with the infected control. Total white blood cell count and lymphocyte differential significantly (P<0.05) increased in the 200 mg/kg BW treatment group in the susceptible model. However, the least values of these parameters were obtained in the infected control. Obviously, in the resistant model, total white blood cell and lymphocyte counts were significantly (P<0.05) higher than what was obtained in infected control mice. Neutrophil, monocyte and eosinophil counts did not vary linearly with the doses in the susceptible model and were not significantly higher when compared with the infected control. In the resistant model however, neutrophil count in the treated groups was significantly lower than what was observed in the infected control while there was no significant difference in monocyte and eosinophil counts between the infected control and the treated groups (Table 2). In Table 3, the heme contents of the infected control were the least in both models (8.7±0.3 and 14.8±0.6 µmole heme/mL of blood in susceptible and resistant models, respectively). At 200 and 400 mg/kg BW, the highest values for heme contents were obtained in mice infected with susceptible and resistant strains of P. berghei (11.5±0.0 and 17.6±0.4 µmole heme/mL of blood, respectively) although these values were not significantly higher than the infected control values.
 |
Table 2 Hematological Parameter of P. berghei-Infected Mice After Treatment with n-Hexane Fraction of G. braunii Leaf Extract |
 |
Table 3 Heme Contents of Mice Infected with Susceptible (NK 65) and Resistant (ANKA) Strains of P. berghei, Treated with n-Hexane Fraction of G. braunii |
Hexane Fraction of G. braunii Decreases Percentage Parasitemia and Hemozoin Content in Mice Infected with Resistant P. berghei
When the most potent fraction of G. braunii was tested on ANKA strain of P. berghei, there was a significant dose-dependent decrease in parasitemia and a corresponding increase in parasite clearance, respectively (Figure 2A and B). On day five of the experiment, the percentage parasitemia decreased to 0.14 at the highest dose (400 mg/kg BW) compared to infected control that stood at 6.89 (Figure 2A). In contrast, the percentage parasite clearance of HF at 400 mg/kg BW on day 5 was 75.50 (Figure 2B).
Hemozoin contents of mice infected with susceptible and resistant strains of P. berghei but treated with graded doses of HF of G.braunii are presented in Figure 2C and D. In both cases, the hemozoin content of the infected control increased significantly (P<0.0001 and P<0.01 in the susceptible and resistant models, respectively) compared with the drug control. Although the standard drug used in the susceptible model significantly (P<0.05) decreased hemozoin content when compared with the 100 mg/kg BW of hexane fraction, there was no significant difference between the effect of the standard drug, 200 and 400 mg/kg BW of hexane fraction (Figure 2C). In the resistant model, 400 mg/kg BW of HF caused a significant (P<0.01) decrease in hemozoin content when compared with the drug control and the infected control. Also, the 400 mg/kg BW dose significantly (P<0.05) decreased hemozoin content when compared with either 100 or 200 mg/kg BW (Figure 2D).
Hexane Fraction of G. braunii Contains Friedelan-3-one, and It Stabilises Red Cell Membrane in Mice
The GC-MS fingerprint presented in Figure 3A shows that the Friedelan-3-one and its derivatives are the main constituents present in the HF of G. braunii. There are 80 peaks in all. The peaks, retention time, and relative abundance of the first five most abundant compounds are presented in Table 4. The peak of the most abundant compound is 76, and its retention time is 32.267, the relative abundance is 27.68%, and the intensity is 8,500,000. The compound with the highest relative abundance is friedelan-3-one. Although friedelan-3-one has been purified and identified in some medicinal plants, its presence and identification in G. braunii are reported here for the first time. The effect of G. braunii on the maintenance of erythrocyte membrane integrity was determined using heat-induced and hypotonic solution-induced models. Results showed that HF of G. braunii stabilises the erythrocyte membrane (Figure 3B and C). Results further showed that, indeed, some antimalarial drugs could destabilise the erythrocyte membrane. In both heat-induced and hypotonic solution models, the artequin and P-alaxin caused erythrocyte membrane destabilisation. However, P-alaxin destabilises the erythrocyte membrane more than artequin.
 |
Table 4 The Most Abundant Phytochemical Constituents of n-Hexane Fraction of G. braunii |
Discussion
Scientific evidence have shown that natural products are excellent sources of therapeutic and prophylactic interventions for managing, treating, and preventing diseases. This study shows that G. braunii is rich in important phytochemicals, which may be responsible for its medicinal properties. The methanol extract is rich in nearly all the phytochemicals identified with the quantity varying in the solvent fractions. This is due to the solubility of the phytochemicals in the solvents used. In this way, the quantity per unit volume of the phytochemicals increase in the solvent fraction in which they are more soluble. Although all the phytochemicals are present in the solvent fractions, the phenolic content of HF was the least. HF also has the least content of alkaloids and flavonoids. It is interesting to note, however, that HF is rich in terpenes. It is well established that the antimalarial properties of phenolic derivatives including flavonoids as well as xanthones, stilbenes, coumarins, lignans, tannins, and quinones; and terpenoid compounds sesquiterpenes, diterpenes, triterpenes, steroids, and alkaloids have been reported.34
A good curative antimalarial drug or drug candidate is expected to have an excellent potency to clear malaria parasites from its host. Interestingly, it could be reasoned that, indeed, HF of G. braunii has substantial antiplasmodial property against the chloroquine-sensitive strain of P. berghei. Besides, it also has moderate antimalarial effect against the chloroquine-resistant strain. This is because it has the least percentage parasitemia and the highest parasite clearance. Antimicrobial and antioxidant properties of different solvent extract and fractions of G. braunii have been reported.35,36 Apart from the antimicrobial properties of medicinal plants, their antioxidant potential is a significant feature that can be screened for antiplasmodial potential.37,38 This antioxidant potential may be an indicator of the therapeutic mechanism of antimalarial herbs.38 It is interesting also to note that, unlike some antimalarial herbs that are only effective against a susceptible strain of Plasmodium species, hexane fraction of G. braunii has moderate antiplasmodial activity against chloroquine-resistant (ANKA) strain of P. berghei. This feature may be the reason why this plant is often locally used for malarial therapy.
In addition to several symptoms, the Plasmodium infection has a deleterious effect on blood cells. This includes anemia,39 leucopenia,40 and thrombocytopenia.41 Malaria affects erythrocytes via hemolysis of infected and uninfected erythrocytes and bone marrow dyserythropoiesis.39 The obligatory destruction of erythrocytes at schizont rupture is the major cause of hemolytic anemia in malaria.42 In this study, HF of G. braunii improves PCV, red blood cell counts, and oxygen transport molecule, hemoglobin, in both models in a dose-dependent manner. Leucopenia and thrombocytopenia are commonly reported in Plasmodium infections and constitute the major fatal effects of malarial pathogenesis. The data in this study reported an increase in white blood cell and platelet counts, and these are the first lines of defense against malaria. Leucopenia, in some cases, maybe the least prevalent cytopenia in Plasmodium infection; its chronic incidence may denote the density and severity of infection.43 Although platelets regulate blood homeostasis, it is an essential component of the body’s defense system against invading pathogenic organisms.44 In malarial disease, platelet binds to Plasmodium-infected erythrocytes and kills the parasite within the cell.45
There are different reports on neutropenia, lymphopenia, and monocytosis in malarial disease. Some studies have observed neutropenia,46 lymphopenia,47 and monocytosis in acute malaria.48 It was reported in these studies that different stages of malaria infection, ranging from acute to severe, may determine the levels of white blood cells and their differentials in Plasmodium infection. In this study, the neutropenia and lymphopenia were observed in the infected controls while these blood parameters increased dose-dependently in the susceptible and resistant models of P. berghei infection. There was no significant difference between monocyte counts in the infected and treated controls.
Decreased hemoglobin content, increased oxidative stress, and hemozoin content are parts of the significant outcomes of hemolytic anemia in malaria.49 Degradation of RBC caused a decline in heme content in untreated malaria. However, in this study, heme content improved, as parasite load decreased. Hemozoin is a mechanism by the parasite to evade the oxidative effect of high heme iron content, and this by-product of heme degradation increased in untreated malarial disease.50 Inhibition of the polymerisation of heme to hemozoin has been the proposed target of some antimalarial drugs.6 In this study, HF of G. braunii decreased hemozoin and increased heme contents of blood cells in both models of malarial study. This implies that it decreased hemozoin content probably as a result of a decrease in the parasite load that could have polymerised heme to hemozoin. Furthermore, its effect on heme may be as a result of increasing heme biosynthesis and the prevention of its breakdown.
The maintenance of erythrocyte membrane stability in the course of malarial treatment is a subject for debate in the malaria research study. Certain drugs such as artesunate, mefloquine, and quinine are believed to exert their antimalarial effects by destabilising the erythrocyte membrane, thus causing eryptosis.51 Unfortunately, the observation that red cell death inducers as antimalarial treatments do not specifically target infected erythrocytes but would instead cause membrane destabilisation of uninfected red cells. This puts uninfected red cells at high risk of hemolytic anemia.52 However, in this study, we observed significant maintenance of red cell stabilisation by n-hexane fraction of G. braunii, preventing red cell membrane lysis. This effect, as observed in this study, shows that the antimalarial mechanism of action of compounds in the n-hexane fraction of G. braunii may differ from those of the orthodox drugs that elicit their antiplasmodial effects via eryptosis. The unselective hemolytic effects of orthodox antimalarial drugs on un-infected erythrocytes may constitute adverse effects of such drugs.
The GC-MS analysis of hexane fraction of G. braunii gave friedelan-3-one and its derivatives as its principal constituent. Precisely, friedenlan-3-one constitutes 27.68% which is the highest percentage of all phytochemicals present. Furthermore, other significant constituents are either friedelan-3-one derivatives or their structural variants. This is the first time that this triterpene would be identified in G. braunii. It is interesting to note that a previous study has identified friedelan-3-one in Harungana madagascariensis LAM. (Hypericaceae), tested against chloroquine-resistant (W2) strain of P. falciparum and showed high antiplasmodial activity53 at 7.70µM. Furthermore, friedelan-3-one purified from Pterocarpus santalinoides L’Herit ex Dc showed antimicrobial (bactericidal and fungicidal) effects against pathogenic organisms.54 This may be one of the reasons why this plant is used extensively as an antimicrobial agent. It shows the medicinal properties of phytochemicals present in plants as drug leads and promising drug candidates in treating diseases, particularly malaria.55,56 Finally, it is interesting to note here that the n-hexane fraction of G. braunii was the most potent fraction and it contains the active principle, friedelan-3-one, a terpene. Artemisinin, the current antimalarial compound purified from Artemisia annua was purified using toluene/ethanol and n-hexane/ethyl acetate solvents.57,58 Like sesquiterpene artemisinin, this compound or another from this n-hexane fraction may offer hope for the purification of another antimalarial drug in the nearest future.
In conclusion, we have shown that a specie of African mistletoe, G. braunii, has antiplasmodial properties against chloroquine-sensitive (NK 565) and resistant (ANKA) strains of P. berghei in mice. The reduction in the parasite load may be attributed to the active constituent. Also, it may be likely that the conglomeration of phytochemicals present in the plant acted synergistically for a high reduction in parasite burden as observed in this the study. Furthermore, the effect of the plant extract and fractions on the improvement of the hematological indices would ensure a balance in this vital body tissue, especially in infectious disease as malaria where blood homeostasis is critical to survival and recovery from the disease. This way, the chronic effect of infection such as thrombocytopenia and anemia would be prevented. Furthermore, its antimicrobial activity would prevent secondary infection in malarial treatment. Notably, the primary active constituents present in the most potent fraction, friedelan-3-one, did not show toxicity to erythrocytes. The concentrations tested were greater than 20 mM, about three-fold more significant than 7.7 µM, the concentration with antimalarial activity.37 This shows that membranes of erythrocytes would be stable, hemolysis of uninfected erythrocytes is prevented and thus, the extract and fractions of G. braunii is user-friendly. Further, it shows that hemolysis of the erythrocytes is prevented when G. braunii is employed for malarial treatment, further confirming our report on erythrocyte membrane stabilisation. The positive effect of the plant extract on chloroquine-resistant (ANKA) strain of P. berghei shows that one of the serious fatal infection by Plasmodium parasite can be effectively cured and thus reducing the fatalities and the adverse socio-economic effects of malaria.
Acknowledgments
The authors appreciate Professor O.G. Ademowo, the Director, Malarial Research Section, Institute of Advanced Medical Research and Training, who freely supplied chloroquine-sensitive (NK 65) and resistant (ANKA) strains of P. berghei used in this study. The authors also appreciate Mr. F.O. Omotayo, Director, Plant Herbarium Section, Department of Plant Science, Ekiti State University, Ado-Ekiti, Nigeria, for plant identification.
Author Contributions
JOO conceived the research idea, infected the animals, performed microscopy, took part in other assays, and interpreted the data. He wrote the draft manuscript and is the corresponding author. MTE took care of the animals prepared reagents and took part in research assays, OE performed the GC-MS analysis, manuscript editing and data analysis, NAK supplied equipment for GC-MS, interpreted the spectra, read and corrected the manuscript, and OOO supplied equipment, read the draft manuscript and took part in manuscript editing. All authors contributed to data analysis, drafting or revising the article, have agreed on the journal to which the article will be submitted, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Disclosure
The authors declare no potential conflict of interests with respect to this work or the research, authorship or publication of this article.
References
1. World Health Organization. World malaria report; 2019.
2. Marrelli MT, Brotto M. The effect of malaria and anti-malarial drugs on skeletal and cardiac muscles. Malar J. 2016;15(1):1–6. doi:10.1186/s12936-016-1577-y
3. Yeo TW, Lampah DA, Kenangalem E, Tjitra E, Price RN, Anstey NM. Impaired skeletal muscle microvascular function and increased skeletal muscle oxygen consumption in severe falciparum malaria. J Infect Dis. 2013;207(3):528–536. doi:10.1093/infdis/jis692
4. Ihekwereme CP, Esimone CO, Nwanegbo EC. Hemozoin inhibition and control of clinical malaria. Adv Pharmacol Sci. 2014;2014:984150. doi:10.1155/2014/984150
5. Percário S, Moreira DR, Gomes BA, et al. Oxidative stress in malaria. Int J Mol Sci. 2012;13(12):16346–16372. doi:10.3390/ijms131216346
6. Fong KY, Wright DW. Hemozoin and antimalarial drug discovery. Future Med Chem. 2013;5(12):1437–1450. doi:10.4155/fmc.13.113
7. Noungoue DT, Chaabi M, Ngouela S, et al. Antimalarial compounds from the stem bark of Vismia laurentii. Z Naturforsch C. 2009;64(3–4):210–214. doi:10.1515/znc-2009-3-410
8. Olanlokun JO, David OM, Afolayan AJ. In vitro antiplasmodial activity and prophylactic potentials of extract and fractions of Trema orientalis (Linn.) stem bark. BMC Complement Altern Med. 2017;17(1):1–11. doi:10.1186/s12906-017-1914-x
9. Olanlokun JO, Okoro PO, Lawal OS, et al. Betulinic acid purified from Alstonia boonei inhibits folate biosynthesis in malarial Plasmodium, enhances mitochondrial pore opening and F1F0 ATPase in mice. J Mol Stru. 2021;1239:130454. doi:10.1016/j.molstruc.2021.130454.
10. Agbedahunsi JM, Elujoba AA, Makinde JM, et al. Antimalarial activity of Khaya gradifoliola stem bark. Pharm Biol. 1998;36(1):8-12. doi:10.1076/phbi.36.1.8.4613
11. Endharti AT, Wulandari A, Listyana A, Norahmawati E, Permana S. Dendrophthoe pentandra (L.) Miq extract effectively inhibits inflammation, proliferation and induces p53 expression on colitis-associated colon cancer. BMC Compl Alt Med. 2016;16(1):374. doi:10.1186/s12906-016-1345-0
12. Sima-Obiang C, Rln MM, Ndong-Atome GR, Ondo JP, Obame-Engonga LC, Nsi-Emvo E. Chemical composition, antioxidant and antimicrobial activities of stem barks of Englerina gabonensis Engler and Sterculia tragacantha Lindl from Gabon. Int J Phytomed. 2017;9(3):501–510. doi:10.5138/09750185.2124
13. Okpako L, Ajaiyeoba E. In vitro and in vivo antimalarial studies of Striga hermonthica and Tapinanthus sessilifolius extracts. Afr J Med Med Sci. 2004;33:73–75.
14. Builders M, Uguru M, Aguiyi C. Antiplasmodial potential of the African mistletoe: agelanthus dodoneifolius Polh and Wiens. Indian J Pharm Sci. 2012;74(3):223. doi:10.4103/0250-474X.106064
15. Ogunmefun OT, Fasola TR, Saba AB, Akinyemi AJ. Inhibitory effect of Phragmanthera Incana (Schum.) harvested from cocoa (Theobroma Cacao) and kolanut (Cola Nitida) trees on Fe2+ induced lipid oxidative stress in some rat tissues - in vitro. Int J Biomed Sci. 2015;11(1):16–22.
16. Karunaichamy K, Arp K, Paliwal A. Biomass and nutrient dynamics of mistletoe (Dendrophtoe falcata) and neem (Azadirachta indica) seedlings. Curr Sci. 1999;76(6):840–843.
17. Oboh I, Nworgu Z. Oxytocic properties of the aqueous extract of Globimetula braunii (loranthaceae). Pak J Pharm Sci. 2008;21(4):356-360.
18. Adediwura FJ, Temitope O, Oluwakemi A. Phytochemical and laxative studies of Globimetula braunii (Engle) van tiegh growing on Cola acuminata (Schott & Endl). Afr J Tradit Complement Altern Med. 2008;5:419.
19. Okpuzor J, Ogbunugafor H, Kareem G. Antioxidative properties of ethyl acetate fraction of Globimetula braunii in normal albino rats. J Biol Sci. 2009;9(5):470–475. doi:10.3923/jbs.2009.470.475
20. Akinmoladun AC, Obuotor EM, Farombi EO. Evaluation of antioxidant and free radical scavenging capacities of some Nigerian indigenous medicinal plants. J Med Food. 2010;13(2):444–451. doi:10.1089/jmf.2008.0292
21. Okpanachi GO, Oyl AR, Musa H, Abdulsamad A, Sani MB, Ya’u J. Phtytochemical screening and hypoglycemic property of Globimetula braunii (Loranthaceae) leaf extracts. J Pharm Res. 2018;22(1):1–11.
22. Singleton VL, Orthofer R, Lamuela-Raventós RM. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999;299:152–178.
23. Van Buren JP, Robinson WB. Formation of complexes between protein and tannic acid. J Agric Food Chem. 1969;17(4):772–777. doi:10.1021/jf60164a003
24. Williams S. Official Methods of Analysis. Association of Official Analytical Chemists; 1984.
25. Park Y-S, Jung S-T, Kang S-G, et al. Antioxidants and proteins in ethylene-treated kiwifruits. Food Chem. 2008;107(2):640–648. doi:10.1016/j.foodchem.2007.08.070
26. Indumathi C, Durgadevi G, Nithyavani S, Gayathri P. Estimation of terpenoid content and its antimicrobial property in Enicostemma litorrale. Int J ChemTech Res. 2014;6:4264–4267.
27. Public Health Service (PHS). Public Health Service Policy on Humane Care and the Use of Laboratory Animals. Washington, DC: Human Health Research Act, US Department of Health and Humane Services; 1996:99–158.
28. Ryley J, Peters W. The antimalarial activity of some quinolone esters. Ann Trop Med Parasitol. 1970;64(2):209–222. doi:10.1080/00034983.1970.11686683
29. Schalm OW, Jain NC, Carroll EJ. Veterinary Hematology. Lea & Febiger; 1975.
30. Asakura T, Minakata K, Adachi K, Russell M, Schwartz E. Denatured hemoglobin in sickle erythrocytes. J Clin Invest. 1977;59(4):633–640. doi:10.1172/JCI108681
31. Orjih AU, Fitch CD. Hemozoin production by Plasmodium falciparum: variation with strain and exposure to chloroquine. Biochim Biophys Acta Gen Subj. 1993;1157(2):270–274. doi:10.1016/0304-4165(93)90109-L
32. Okoli CO, Akah PA, Onuoha NJ, Okoye TC, Nwoye AC, Nworu CS. Acanthus montanus: an experimental evaluation of the antimicrobial, anti-inflammatory and immunological properties of a traditional remedy for furuncles. BMC Complement Altern Med. 2008;8(1):27. doi:10.1186/1472-6882-8-27
33. Umapathy E, Ndebia EJ, Meeme A, et al. An experimental evaluation of Albuca setosa aqueous extract on membrane stabilization, protein denaturation and white blood cell migration during acute inflammation. J Med Plants Res. 2010;4:789–795.
34. Jaa’afar MK, Jamil S, Basar N. Antioxidant activity of leaf extracts of Globimetula braunii (Engler) van Tiegh parasitizing on Piliostigma Thonningii and Parkia biglobosa. UTM Jl Teki. 2017;79(5):43–47.
35. Oriola AO, Aladesanmi AJ, Akinkunmi EO, Olawuni IJ. Antioxidant and antimicrobial studies of some hemi-parasitic West African Plants. Eur J Med Plants. 2020;31(3):17–26.
36. Bero J, Frédérich M, Quetin‐Leclercq J. Antimalarial compounds isolated from plants used in traditional medicine. J Pharm Pharmacol. 2009;61(11):1401–1433. doi:10.1211/jpp.61.11.0001
37. Arrey Tarkang P, Nwachiban Atchan AP, Kuiate J-R, Okalebo FA, An G, Agbor GA. Antioxidant potential of a polyherbal antimalarial as an indicator of its therapeutic value. Adv Pharmacol Sci. 2013;2013:678458. doi:10.1155/2013/678458
38. Dadzie I, Avorgbedo SA, Appiah-Opong R, Cudjoe O. Cytotoxic and antioxidant effects of antimalarial herbal mixtures. Int J Microbiol. 2020;2020:1–5. doi:10.1155/2020/8645691
39. White NJ. Anaemia and malaria. Malar J. 2018;17(1):1–17. doi:10.1186/s12936-018-2509-9
40. Anabire NG, Aryee PA, Helegbe GK. Hematological abnormalities in patients with malaria and typhoid in Tamale metropolis of Ghana. BMC Res Notes. 2018;11(1):353. doi:10.1186/s13104-018-3456-9
41. Awoke N, Arota A. Profiles of hematological parameters in Plasmodium falciparum and Plasmodium vivax malaria patients attending Tercha General Hospital, Dawuro Zone, South Ethiopia. Infect Drug Resist. 2019;12:521.
42. Taylor T, Agbenyega T. Malaria. In: Hunter’s Tropical Medicine and Emerging Infectious Disease.
43. Lampah DA, Yeo TW, Malloy M, et al. Severe malarial thrombocytopenia: risk factor for mortality in Papua, Indonesia. J Infect Dis. 2015;211(4):623–634. doi:10.1093/infdis/jiu487
44. Clemetson KJ. The role of platelets in defence against pathogens. Homostaseologie. 2011;31(4):264–268. doi:10.5482/ha-1152
45. McMorran BJ, Burgio G, Foote SJ. New insights into the protective power of platelets in malaria infection. Commun Integr Biol. 2013;6(3):e23653. doi:10.4161/cib.23653
46. Dale DC, Wolff SM. Studies of the neutropenia of acute malaria. Blood. 1973;41(2):197–206. doi:10.1182/blood.V41.2.197.197
47. Hviid L, Kemp K. What is the cause of lymphopenia in malaria? Infect Immun. 2000;68(10):6087–6089. doi:10.1128/IAI.68.10.6087-6089.2000
48. Kassa D, Petros B, Messele T, Admassu A, Adugna F, Wolday D. Parasito-haematological features of acute Plasmodium falciparum and P. vivax malaria patients with and without HIV co-infection at Wonji Sugar Estate, Ethiopia. Ethiop J Health Dev. 2005;19(2):132–139. doi:10.4314/ejhd.v19i2.9982
49. Pham TT, Lamb TJ, Deroost K, Opdenakker G, Van den Steen PE. Hemozoin in malarial complications: more questions than answers. Trends Parasitol. 2021;37(3):223–226. doi:10.1016/j.pt.2020.09.016
50. Singh SV, Manhas A, Kumar Y, et al. Antimalarial activity and safety assessment of Flueggea virosa leaves and its major constituent with special emphasis on their mode of action. Biomed Pharmacother. 2017;89:761–771. doi:10.1016/j.biopha.2017.02.056
51. Bissinger R, Barking S, Alzoubi K, Liu G, Liu G, Lang F. Stimulation of suicidal erythrocyte death by the antimalarial drug mefloquine. Cell Physiol Biochem. 2015;36(4):1395–1405. doi:10.1159/000430305
52. Totino PR, Magalhães AD, Silva LA, Banic DM, Daniel-Ribeiro CT, de Fátima Ferreira-da-cruz M. Apoptosis of non-parasitized red blood cells in malaria: a putative mechanism involved in the pathogenesis of anaemia. Malar J. 2010;9(1):1–5. doi:10.1186/1475-2875-9-350
53. Lenta BN, Ngouela S, Boyom FF, et al. Anti-plasmodial activity of some constituents of the root bark of Harungana madagascariensis LAM. (Hypericaceae). Chem Pharm Bull. 2007;55(3):464–467. doi:10.1248/cpb.55.464
54. Odeh IC, Tor-Anyiin TA, Igoli JO, Anyam JV. In vitro antimicrobial properties of friedelan-3-one from Pterocarpus santalinoides L’Herit, ex Dc. Afr J Biotechnol. 2016;15(14):531–538. doi:10.5897/AJB2015.15091
55. Olanlokun JO, Bodede O, Prinsloo G, Olorunsogo OO. Comparative antimalarial, toxicity and mito-protective effects of Diospyros mespiliformis Hochst. ex A. DC. and Mondia whitei (Hook. f.) Skeels on Plasmodium berghei infection in mice. J Ethnopharmacol. 2021;268:113585. doi:10.1016/j.jep.2020.113585
56. David OM, Olanlokun JO, Owoniyi BE, Ayeni M, Ebenezer O, Koorbanally NA. Studies on the mitochondrial, immunological and inflammatory effects of solvent fractions of Diospyros mespiliformis Hochst in Plasmodium berghei-infected mice. Sci Rep. 2021;11(1):1–15. doi:10.1038/s41598-021-85790-6
57. Horosanskaia E, Triemer S, Seidel-Morgenstem A, Lorenz H. Purification of artemisinin from the product solution of a semisynthetic reaction within a single crystallization step. Org Process Res Dev. 2019;23(9):2074–2079. doi:10.1021/acs.oprd.9b00175
58. Malwade C, Buchholz H, Rong BG, et al. Crystallization of artemisinin from chromatography fractions of Artemisia annua extractor. Process Res Dev. 2016;20(3):646–652. doi:10.1021/acs.oprd.5b00399
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.