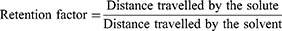Back to Journals » Journal of Experimental Pharmacology » Volume 14
Antidepressant-Like Activity of Solvent Fractions of the Root Bark of Carissa spinarum Linn. (Apocynaceae) in Rodents Involves Multiple Signaling Pathways
Authors Ali HS , Engidawork E
Received 20 September 2022
Accepted for publication 23 November 2022
Published 9 December 2022 Volume 2022:14 Pages 379—394
DOI https://doi.org/10.2147/JEP.S386015
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Roger Pinder
Hana Saif Ali, Ephrem Engidawork
Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia
Correspondence: Ephrem Engidawork, Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, P.O. Box 9086, Addis Ababa, Ethiopia, Email [email protected]
Background: The root bark of Carissa spinarum Linn. (Apocynaceae) is claimed to be used for the management of depression in Ethiopian folkloric medicine, and the crude extract has been reported to possess antidepressant-like activity in rodents.
Objective: This study aimed to evaluate the antidepressant-like effect of different fractions of the root bark in rodents and the possible underlying mechanisms in rats.
Methods: A 70% ethanol extract of the root bark was successively fractionated with n-butanol, ethyl acetate, and water. Animals of both sexes received 2% Tween 80, imipramine (30 mg/kg), or various doses (50, 100, 200 mg/kg) of the fractions. Duration of immobility was determined using the tail suspension test and the forced swim test. Locomotor activity was evaluated in the open field test. Serum corticosterone levels, total phenols, flavonoids, and alkaloids were determined. Preliminary mechanistic studies were also performed to explore possible mechanisms of action of the active fraction.
Results: All fractions but the aqueous fraction significantly (p< 0.001) decreased the duration of immobility in both tests, with the ethyl acetate fraction being the most active. The locomotor test revealed that the activity was not due to non-specific psycho-stimulant effects. Serum corticosterone levels were reduced by both fractions, with the ethyl acetate fraction again being the most effective. Mechanistic studies showed the involvement of multiple neurotransmission systems, including adrenergic, dopaminergic and cholinergic as well as L-Arginine-NO-cGMP pathway. Higher contents of phenols (42.42 vs 29.8 mgGAE/g), flavonoids (12.43 vs 2.07 mgQE/g), and alkaloids (0.17 vs 0.07 mgATE/g) were found in the ethyl acetate than in the n-butanol fraction.
Conclusion: The present findings collectively indicate that the ethyl acetate and n-butanol fractions are endowed with antidepressant-like activity due to the presence of phenols, flavonoids, and alkaloids, which are medium polar in nature.
Keywords: depression, Carissa spinarum Linn, antidepressant-like activity, solvent fractions, possible mechanisms
Introduction
Depression is a chronic and recurrent psychiatric disorder that affects mental and physical health, and has a significant impact on healthcare resources and costs.1 It is a heterogeneous mental disorder with psychological, behavioral, and physiological symptoms that affects one in five people worldwide. Moreover, it is a multifactorial disorder with different causes.2,3 It is one of the most burdensome diseases in the middle years of life in both developing and developed countries.4 The highest prevalence is estimated in sub-Saharan Africa, North Africa, and the Middle East.5
Despite the availability of several highly effective antidepressants that improve clinical and low work productivity, only 50–60% of patients receive adequate treatment, and those treated may develop resistance during treatment.4 Almost all of the antidepressants are associated with various side effects6–9 and are contraindicated in some diseases.6,10 They also showed potential interactions with food and other medications, and have been associated with liver dysfunction and increased risk of diabetes.11 To overcome these side effects, low efficacy, and inaccessibility,12 many scientists are seeking alternatives to conventional antidepressants from plant sources.
Plant-derived natural medicines have a crucial role in the development of natural drugs, which have antidepressant activity, as they are known to be affordable, safe and efficacious. Many plants that are used in the treatment of depression act by normalizing the disturbed neurotransmitters in the brain or related mechanisms, including the 5-HT system (hypericum species), the noradrenergic system (Valeriana wallichii), the dopamine system (Rosmarinus officinalis), the GABAergic system (Asparagus racemosus), the Glutamate system (Siphocampylus verticillatus), neurotrophins (Polygala tenuifolia), the HPA axis (Ptychopetalum olacoides) and the nitric oxide system (Aloysia gratissima).13,14
Carissa spinarum Linn. (Apocynaceae) (Supplementary Figure 1) is a medicinal plant known as a magic herb in most African countries and used in the treatment of various diseases, including depression.15 The plant harbors high content of bioactive secondary metabolites such as alkaloids, cardiac glycosides, saponins, flavonoids, tannins, anthraquinones, total phenols, and terpenoids, which are implicated in producing anti-depressant activity.16–18 C. spinarum has different vernacular names in Ethiopia, Hagmsa (in Affan Oromo) and Agam (in Amharic). The roots are extensively used in Ethiopian folk medicine to treat various ailments, either alone or in the form of mixtures.15,19–23
Moreover, pharmacological studies have confirmed most of the claimed uses, including diuretic,24,25 antinociceptive,26,27 anticonvulsant,28,29 anti-inflammatory and antioxidant,30 and antimicrobial31–33 activity. The crude root bark extract of C. spinarum is also reported to possess antidepressant activity.34 Therefore, the aim of this study was to evaluate the antidepressant-like activity of the solvent fractions using a series of paradigms and to explore the possible mechanisms.
Materials and Method
Drugs, Chemicals, and Reagents
Cyproheptadine HCl (Algorithm S.A.L - Lebanon); Diazepam BP (Gland Pharma Limited - Denmark); Ketamine Hydrochloride USP (Rotex Medica - Germany); L-Arginine HCl (Vita Pharmaceutical - Syria); Prazosin HCl (Mylan Inst., RKFD - USA); Standard Imipramine HCL BP (Remedica - Cyprus); Sulpiride (Sanofi-Aventis - France); Yohimbine (Sun Naturals - USA); Atropine (BDH Chemicals - England); Absolute Ethanol 99.9% (Iso Lab Chemicals - India); Dimethyl sulfoxide (DMSO) (Riedel.de Haen - Germany); Ethyl Acetate (Lobe Chemie - India); Methylene Blue (BDH Chemicals - England); N-Butanol (Loba Chemie - India); Normal saline Solution (Sansheng Pharmaceutics - Ethiopia); Petroleum Ether 40–60°C (Loba Chemie - India); Tween 80 (Uni-Chem - India), Aluminum Chloride Hexahydrate (Loba Chemie - India); BCG (Sisco research laboratories - India); Citric acid (Avonchem - UK); Disodium hydrogen orthophosphate Na2HPO4 (BDH Chemicals - England); Follin Ciocalteu; Gallic acid (Merch - Germany); Potassium Acetate (Blulux laboratories - India); Quercetin dihydrate (Sigma Aldrich - Germany); Sodium hydroxide pellets (Loba Chemie - India); and distilled water. All drugs were purchased from pharmacies in Ethiopia, Yemen, and the USA, while the chemicals were purchased from their respective vendors in Ethiopia. All were of analytical grades.
Plant Material Collection and Preparation
The roots of C. spinarum were collected in August 2020 from Ashewa Meda, Burayu city administration (near Addis Ababa, the capital), Oromia region, Ethiopia. Identification and authentication of the plant sample was performed by a taxonomist, and a voucher specimen (HS001) was deposited at the National Herbarium, College of Natural and Computational Sciences, Addis Ababa University for future reference. The root was cleaned and carefully washed with tap water to remove dirt and soil debris. The root bark was then separated from the root wood, cut into small pieces and air dried in the shade. The dried root bark was pulverized using mortar and pestle.
Experimental Animals
Healthy Swiss albino mice (20–30 g, 6–8 weeks old) and Sprague-Dawley rats (200–250 g, 8–12 weeks old) of both sexes were bred and obtained from the animal house of the School of Pharmacy, Addis Ababa University, Addis Ababa, Ethiopia. All animals were housed in groups of 6 per cage and had access to standard laboratory pellets and water ad libitum under controlled conditions (12 h light and dark cycle and temperature of 23–25 °C). Before performing the pharmacological studies, the animals were acclimatized to laboratory conditions for 5 days. In addition, all animals used were handled and cared for according to internationally accepted guidelines for the care and use of laboratory animals.35 Ethical approval was obtained from an ethical review board of the School of Pharmacy, College of Health Sciences, Addis Ababa University (Reference number. ERB/SOP/176/12/2020).
Plant Extraction and Fractionation
Crude Extract
Five hundred and fifty grams of the ground root bark was defatted overnight with 1L of petroleum ether. It was then filtered and allowed to dry on a clean piece of aluminum. After complete evaporation of the petroleum ether, the plant material was macerated with 1.5 L of 70% ethanol for three days. The maceration process was repeated twice with the same volume. The extract was then filtered with a cotton gauze followed by Whatman filter paper No. 1, and filtration was accelerated with a vacuum pump. The filtrate was then concentrated under reduced pressure using a rotary evaporator at 40° C and stored in a refrigerator at 4° C. A sticky, dark brown colored crude extract was obtained with a percentage yield of 9.22% (w/w).
Solvent Fractionation
Thirty-three grams of the extract was mixed with acid-washed sand and placed in a small thimble in a Soxhlet apparatus for fractionation with various solvents. The sequence of solvents used for fractionation was based on their polarity index (n-butanol, ethyl acetate, and water).36 The n-butanol (CSB) and ethyl acetate (CSE) fractions were then collected sequentially and dried under reduced pressure in a rotary evaporator at 40° C. Finally, the marc left was macerated with 1 L of distilled water and freeze-dried using a lyophilizer (Labconco, Console Freeze Dry system, USA) to obtain the aqueous fraction (CSA). The final samples obtained were weighed and the percentage yields were 21.00% for the n-butanol, 11.55% for the ethyl acetate, and 61.7% for the aqueous fraction. The n-butanol and ethyl acetate fractions were sticky and light brown in color, while the aqueous fraction was black. The fractions were stored in tightly closed containers and kept in a refrigerator at −20° C until use.
Determination of Phytochemical Constituents and TLC Analysis
Determination of Total Phenolic Content
Total phenolic content (TPC) of the fractions was determined using the Follin-Ciocalteu method as described elsewhere37 with minor modifications. Follin-Ciocalteu reagent (2N) was diluted with distilled water (1:20). A series of concentrations (25, 12.5, 6.25, and 3.125 µg/mL) were then prepared in methanol using a standard solution of gallic acid (50 µg/mL). One mL of the standard solvent and each fraction (250 µg/mL) were mixed separately with 0.5 mL of Follin-Ciocalteu reagent in a test tube and allowed to stand at room temperature for 8 min. Sodium carbonate (2 mL of a 7.5% solution) was added and the mixture was allowed to stand at room temperature for 30 min. The absorbance was then measured at 765 nm using a UV- spectrophotometer. The same procedure was used to prepare a blank solution. The test was performed in triplicate and the average value was recorded. A linear calibration curve was plotted (Figure 1A)), with an equation (Y = 0.009296*X + 0.05072) and a correlation coefficient (R2) of 0.9997, and used to calculate the concentration of TPC, which was expressed as milligrams of gallic acid equivalent per gram (mgGAE/g).
 |
Figure 1 Calibration curve for Gallic acid (A), Quercetin (B) and Atropine (C) standard solutions. |
Determination of Total Flavonoid Content
Total flavonoid content (TFC) of the solvent fractions was determined by the colorimetric Aluminum chloride method as described elsewhere38 with minor modifications. A series of concentrations (25, 12.5, 6.25, 3.125, and 1.5625 µg/mL) were prepared in methanol using quercetin (1mg/mL) as a standard solution. Ten mg of each solvent fractions was dissolved in 10 mL of methanol to obtain 1 mg/mL of a stock solution. Half a milliliter of each solvent fraction and one milliliter of the standard solution were mixed separately in a test tube with 100 µL of 10% aluminum chloride, 100 µL of 1M potassium acetate, and 2.8 mL of distilled water. The mixture was incubated at room temperature for 30 min. The absorbance was measured at 415 nm using a UV- spectrophotometer. The same procedure was used to prepare a blank solution. The assay was performed in triplicate and the average value was noted. A linear calibration curve was plotted (Figure 1B)), with an equation of (Y = 0.02413*X - 0.01900) and a correlation coefficient (R2) of 0.9986, and used to determine TFC, which was expressed as milligrams of quercetin equivalent per gram (mgQE/g).
Determination of Total Alkaloid Content
Total alkaloid content (TAC) of the solvent fractions was determined using bromocresol green solution (BCG) and phosphate buffer solution prepared as described elsewhere.39 A series of concentrations (0.5, 0.25, 0.125, 0.0625, and 0.03125 mg/mL) were prepared using a standard pure atropine (1mg/mL) dissolved in methanol. Two mL of each solvent fraction (1 mg/mL) was dissolved in 2N HCl and then filtered using Whatman filter paper No.1. One mL of the filtered solution was then transferred to a separatory funnel and washed twice with 5 mL of chloroform. The pH was neutralized with 0.1 N NaOH. Five mL of the BCG solution and phosphate buffer were then added and shaken vigorously. Five mL of chloroform was added and the complex formed was transferred to a 10 mL volumetric flask and diluted to volume with chloroform. The absorbance of the complex formed was measured at 470 nm. A blank solution was prepared by the same procedure without the addition of atropine.40 The assay was performed in triplicate and the average value was noted. A linear calibration curve was plotted (Figure 1C)), with an equation of Y = 0.002184*X + 0.03608 and a correlation coefficient (R2) of 0.9935, and used to determine TAC, which was expressed as milligrams of Atropine equivalent per gram (mgATE/g).
Thin Layer Chromatography Analysis
Thin layer chromatography (TLC) was performed on precoated silica gel plates using the conventional one-dimensional ascending method. The plate was cut using a glass cutter and marked with a soft pencil. The active fraction was dissolved in methanol and spotted with a glass capillary at a distance of 1 cm. The plate was kept in a pre-saturated chamber with a solvent system containing a mixture of petroleum ether and ethyl acetate in a 1:2 ratio, covered and allowed to run 3/4th of the plate. The plate was removed and dried at room temperature for 30 min and observed under UV light (254 nm and 366 nm) (Figure 2).41,42 The movement of the active compound was calculated and expressed as retention factor (Rf) using the following equation:
 |
Figure 2 TLC profile of Ethyl acetate fraction under UV light at a wavelength of 254 nm (A) and 366 nm (B). |
Grouping and Dosing of Experimental Animals
For each model and solvent fraction, five groups of animals of both sexes were randomly assigned with 6 animals per group. Group I, served as a negative control and received vehicle (2% Tween 80) (TW80), group II served as a positive control and received standard drug, imipramine (30 mg/kg) (IP30), group III–V received increasing doses of the solvent fractions 50, 100, and 200 mg/kg, respectively. The solvent fractions and standard drug were dissolved in 2% Tween 80, prepared and administered orally one hour before the experimental sessions. The maximum volume administered was 10 mL/kg.43 Dose selection for the standard drug44 as well as the fractions34 was based on previous studies and a pilot experiment conducted before the main experiment.
Experimental Paradigms
Tail Suspension Test
The tail suspension test (TST) was performed as described elsewhere.45 Following 1 h after administration of vehicle/standard/fractions, each mouse was suspended by its tail upside-down, using an adhesive tape placed 1 cm from the tip of the tail, on a countertop at a height of 35–50 cm above the floor. The test was recorded with a digital video camera. The duration of immobility was measured in seconds using a stopwatch software, the XNote Timer, for the entire duration of the test. The duration of immobility is defined as the absence of any movement of the head and body.46,47
Forced Swim Test
The forced swim test (FST) or the Porsolt Despair test in rats was performed as described elsewhere48 with some modifications. The FST consists of two sessions. The initial 15-min pretest session followed by a 5-min test session 24 h later. Rats were forced to swim in an inescapable situation in a transparent glass cylinder (40 cm height; 18 cm diameter) filled with water 20 cm deep, with their paws not touching the bottom. The solvent fractions were administered 1, 4, and 24 h before the test. After swimming, the rat was taken out and dried with a clean towel, to maintain body temperature and prevent hypothermia, and returned to the cage. The water was also changed and replaced. Only the test sessions were recorded using a digital video camera, which was then used to evaluate the duration of immobility. The duration of immobility was measured using a stopwatch software, the XNote Timer. Floating and absence of any struggling behavior except those necessary to keep the head above the water for breathing were scored as immobile behavior, implying the development of an adaptive response to the stressful situation in the water.49
Open Field Test
The open field test (OFT) measures general locomotor activity and rule out any false-positive effect of the extract in mice. The open field apparatus is a rectangular inescapable box (68 cm x 68 cm x 45 cm) with the surface marked horizontally and vertically with lines forming a grid of 16 squares and a 60 W lamp placed above it. The solvent fractions were administered orally one hour before the test. The mouse was then placed in the center and crossing of the central and peripheral squares were tracked and counted for 5 min. The inner surface of the box was swapped and cleaned with alcohol and absorbent cotton to avoid any olfactory cues.50
Serum Corticosterone Assay
After completion of the FST and TST, each animal was immediately anesthetized with ketamine (80 mg/kg, i.p) and diazepam (10 mg/kg, i.p), and a cardiac puncture was performed. Blood was collected in an SST tube and kept at room temperature for 60 min. It was then centrifuged at 1000 g force for 10 min at 4°C and the separated serum was stored at −80°C until assayed. Corticosterone is a glucocorticoid hormone in rodents that is secreted by the adrenal cortex under various stressful conditions such as the forced swim test and tail suspension test. Therefore, it was analyzed and measured using an electrochemiluminescence Immunoassay (ECLIA) according to the manufacturer’s instructions (Cobas e 41-Roche Diagnostics GmbH, Mannheim, Germany).
Evaluation of Possible Mechanism(s) of Action
According to the pathophysiology of depression and the mechanism of action of conventional antidepressants, the possible mechanism (s) of action of the most active solvent fraction of the root bark of C. spinarum was investigated. This was performed by assessing the involvement of noradrenergic, serotonergic, dopaminergic, nitric oxide, and cholinergic-muscarinic system using different drugs at doses that do not modify the locomotor behaviors of rats in FST.51,52 Rats were randomly divided into nine different groups of 6 rats each. All drugs and the extract were dissolved in 0.5% v/v DMSO in normal saline. Prazosin (1 mg/kg, i.p., an α1-adrenoceptor antagonist) and yohimbine (1 mg/kg, i.p., an α2-adrenoceptor antagonist) were used for the noradrenergic system; cyproheptadine (3 mg/kg, i.p., a 5-HT2 receptor antagonist) for the serotonergic system; sulpiride (50 mg/kg, i.p., a dopamine D2 receptor antagonist) for the dopamine system; atropine (1 mg/kg, i.p., a muscarinic cholinergic receptor antagonist) for the cholinergic system; and L-Arginine (750 mg/kg, i.p. a precursor of NO) and methylene blue (10 mg/kg, i.p., an inhibitor of soluble guanylate cyclase (sGC)) used for the NO system. All drugs were administered 15 min before CSE (50 mg/kg, P.O.) and rats were subjected to FST 60 min after administration of the fraction.
Statistical Analysis
Data were expressed as mean ± standard error of the mean (SEM). Analyses were carried out using the Statistical Package for the Social Sciences (SPSS), version 25. One-way analysis of variance (ANOVA) followed by a Tukey’s post hoc test was used for testing and significance level was set at a P-value of less than 0.05. Curves for the quantification of the phytochemical constituents were generated using Graph Pad Prism software version 8.00 for Windows (Graph Pad Software Inc., San Diego, California, USA).
Results
Quantification of Secondary Metabolites and TLC Analysis
Phytochemical analysis of CSB and CSE, as these were found to be active in the experimental paradigms used, revealed that TPC was 29.8 mgGAE/g and 42.42 mgGAE/g, respectively. Likewise, TFC was 2.07 mgQE/g and 12.43 mgQE/g, and TAC was 0.07 mgATE/g and 0.17 mgATE/g, respectively, in CSB and CSE. TFC, TPC and TAC were higher in CSE than CSB. In addition, TAC was lower in both fractions compared to TFC and TPC, the order being TPC > TFC > TAC.
TLC study of CSE (as it is the most active fraction) showed the presence of three different spots, one major spot with an Rf value 0.43 and two minor spots with Rf values of 0.69 and 0.77 respectively (Figure 2).
Tail Suspension Test
Data obtained for the three fractions in the TST model are shown in Tables 1 and 2. In mice treated with the three doses of CSE, there was a significant reduction (p< 0.001) in the duration of immobility compared to TW80. The duration of immobility appeared to decrease with dose, with the middle (100 mg/kg, CSE100) and higher (200 mg/kg, CSE200) doses producing a significantly greater reduction (p < 0.05) than the lower dose (50 mg/kg, CSE50). CSB also produced the same pattern as CSE, except that CSB200 had a comparable effect to IP30. Indeed, the reduction in the duration of immobility produced by IP30 was significantly greater (p<0.05) than all doses of CSE as well as CSB50 and CSB100. By contrast, CSA did not significantly reduce the duration of immobility at the doses used in the present study. A comparison between CSE and CSB revealed no apparent difference between the different doses of the two fractions, except that CSB200 (p<0.001) produced a significantly greater reduction than CSE50.
 |
Table 1 Antidepressant-Like Activity of Ethyl Acetate and n-Butanol Fractions of the Root Bark of C. spinarum in Mice Using Tail Suspension Test |
 |
Table 2 Antidepressant-Like Activity of an Aqueous Fraction of the Root Bark of C. spinarum in Mice Using Tail Suspension Test |
Forced Swim Test
Since the CSA had no effect in the TST, the FST was performed for the CSE and CSB fractions. Table 3 summarizes the data obtained from the FST using the two fractions at different doses used in the study. Accordingly, both fractions significantly reduced (p<0.001) the duration of immobility in a dose-dependent manner compared with TW80. In addition, the lower doses of both fractions had a significantly smaller reduction (p<0.01) than the middle and higher doses, although no detectable difference was observed between the latter two doses. CSE50 and CSB50 produced a significantly smaller reduction (p<0.001) in the duration of immobility than IP30. Although CSB100 and CSB200 also produced a significantly lower (p<0.001) duration of immobility than IP30, no apparent difference was observed between IP30 and the other two CSE doses (CSE100 & CSE200). In contrast to TST, the effect of CSE on FST was consistently higher than that of the corresponding doses of CSB for each dose used. For example, CSE200 showed a significantly greater reduction in immobility time than CSB100 (p<0.01) and CSB200 (p<0.05).
 |
Table 3 Antidepressant-Like Activity of Ethyl Acetate and n-Butanol Fractions of the Root Bark of C. spinarum in Rats Using Forced Swim Test |
Open Field Test
Table 4 shows a summary of data obtained from the OFT. Accordingly, neither the standard nor the fractions resulted in a significantly different number of crosses compared to TW80. Similarly, no apparent difference was found between the different doses of the fractions and the standard, ruling out the possibility that the antidepressant-like activity was due to nonspecific psychostimulation.
 |
Table 4 The Effect of Ethyl Acetate and n-Butanol Fractions of the Root Bark of C. spinarum on Locomotion in Mice Using Open Field Test |
Serum Corticosterone Assay
The effect of CSE and CSB on serum corticosterone levels in TST is shown in Table 5. CSE significantly (p<0.01) decreased serum corticosterone levels at all doses compared with TW80 in a dose-dependent manner. Although IP30 significantly decreased the level compared to CSE50 (p<0.05), no obvious difference was observed between IP30 and the other two doses. All CSB doses had no significant effect on corticosterone levels, as no detectable changes were observed compared to TW80. In addition, with the exception of CSB50 (p<0.05), no significant difference was observed for the other doses compared to IP30.
 |
Table 5 The Effect of Ethyl Acetate and n-Butanol Fractions of the Root Bark of C. spinarum on Serum Corticosterone Level in Mice Using Tail Suspension Test |
Serum corticosterone levels were also measured in rats subjected to FST. As shown in Table 6, CSE50, CSB50, and CSB100 had no effect on corticosterone levels. In contrast, CSE100 (p < 0.05) and CSE200 (p<0.05) significantly reduced levels compared to TW80. On the other hand, a significantly greater reduction was seen with IP30 compared to all doses of CSE (p<0.05). However, larger individual differences precluded statistically significance between IP30 and CSB.
 |
Table 6 The Effect of Ethyl Acetate and n-Butanol Fractions of the Root Bark of C. spinarum on Serum Corticosterone Level in Rat Using Forced Swim Test |
Evaluation of the Possible Mechanism(s) of Action
According to the results of the antidepressant-like activity in the current study, CSE proved to be the most active fraction. Therefore, this fraction was used to investigate the possible mechanism(s) of action involved using different pharmacological agents.
The effect of different pharmacological agents (drugs) on the antidepressant-like activity of CSE using the FST model is shown in Figure 3. Pretreatment with yohimbine, sulpiride, atropine, and L-arginine significantly reversed the antidepressant-like activity of CSE50 (p < 0.05). In contrast, pretreatment with prazosin, cyproheptadine, and methylene blue had no effect on the antidepressant-like activity of CSE50. Among the agents that reversed the activity, only sulpiride and L-arginine were able to return the duration of immobility to the level of TW80.
Discussion
In the present study, an attempt was made to evaluate the antidepressant-like activity and explore the possible mechanism (s) of action of solvent fractions of a root bark of a plant used in Ethiopia to treat depression.
Several experimental models are essentially used to assess and evaluate the efficacy and potency of agents with antidepressant-like activity. In this study, the FST (Despair Behavior test) and TST were used due to their ease of use, high reliability, and specificity, as well as for their sensitivity to all classes of antidepressants.53
Oral administration of the solvent fractions of the root bark of C. spinarum at different doses indicated that CSE and CSB exhibited antidepressant-like activity in the rodent models of depression used in the present study. CSA on the other hand, did not show significant antidepressant-like activity, as reported elsewhere.34 Moreover, a nonsignificant increase in the total number of squares crossed in OFT with CSE could be an indicator of some anxiolytic effect. However, there was no apparent effect of CSE and CSB on the locomotor activity, indicating that the effect was not due to a nonspecific psychostimulant effect of the fractions.
Depression as a byproduct of stress or as a stress-related disorder triggers a neuronal, endocrine, and behavioral response. In FST and TST, rodents are exposed to an inescapable situation that leads to endocrine disruption and a change in corticosterone levels as a result of activating the major neuroendocrine stress system, the HPA axis.54,55 Therefore, corticosterone level can be used as a possible marker of depression. CSE, especially at the middle and higher doses, consistently decreased corticosterone levels in both TST and FST, although such a consistent decrease was not observed in CSB. This effect may be used as additional evidence for the antidepressant-like activity of the fractions, with CSE showing better activity than CSB.
The antidepressant-like activity observed in the present study could be attributed to the presence of phenols, flavonoids, and alkaloids in the fractions. Many scientific studies have reported that plants with antidepressant-like activity are rich in these secondary metabolites52,56 and produce effect at doses used in the present study.13,57 Quantitative analysis showed that CSE contained more secondary metabolites than CSB, which, at least, in part explains the difference in activity between the two fractions. The difference in polarity of the solvents used for fractionation could be the basis for the selective distribution of secondary metabolites in CSE compared to CSB. These secondary metabolites are thought to exert their antidepressant-like effect individually or in synergy by influencing the various neurotransmitters involved in the neurobiology of depression. Phenols, flavonoids (polyphenol) and alkaloids, which were shown to be constituents of C. spinarum, are among the best studied secondary metabolites whose mechanisms may involve stimulation of 5-HT, noradrenaline, dopamine, and GABAergic neurotransmission systems.56,58,59 Indeed, alkaloids have been found to decrease immobility behavior in FST and several preclinical studies have demonstrated their antidepressant-like activity,60 although their concentration in C. spinarum was very low. TLC study of CSE indicates the presence of different spots with different Rf values, which reinforces the notion that the root bark is endowed with different secondary metabolites, which contribute to its antidepressant-like activity.61
Since CSE was found to be the most active fraction in the variety of depression paradigms used in the present study, further investigation of the possible mechanism(s) using this fraction was conducted based on several hypotheses proposed to explain the pathophysiology of depression.
α2-Adrenoceptors are inhibitory autoreceptors that autoregulate the noradrenergic system. The affinity and density of these receptors are greatly increased in depressed patients,62 so that activation of the receptors has a crucial influence on behavioral activation and target neuron function.63 Therefore, dysregulation of α2-adrenoceptors is associated with depression.64 The fact that yohimbine, but not prazosin, was able to reverse the antidepressant-like activity of CSE suggests that the fraction mediates its effect via α2- adrenoceptors but not α1-adrenoceptors. This finding is consistent with reports indicating yohimbine51,65 but not prazosin66,67 that reversed the antidepressant-like activity of plant extracts. Collectively, these studies suggest that plant extracts preferentially interact with α2-adrenoceptors to produce antidepressant-like effects. Indeed, studies suggest that some flavonoids interact with α2-adrenoceptors to produce depression-like symptoms in animals,68 while some flavonoids exhibit antidepressant-like effects.69
Pretreatment with cyproheptadine did not reverse the antidepressant-like activity of CSE, suggesting that the effect was not mediated via 5-HT2 receptor neurotransmission. Similar results have been reported elsewhere.70 The dopaminergic system is an extremely important target involved in the regulation of depression through its important role in reward stimulus processing, cognition, mood, attention, and learning.71,72 Pretreatment with sulpiride significantly reversed the antidepressant-like activity of CSE, suggesting that the effect may also be related to the dopaminergic system. Indeed, several studies have suggested the role of sulpiride in altering the antidepressant-like activity of various plant extracts in rodents66,73 as well as the influence of some flavonoids on the dopaminergic system.74,75
The cholinergic system plays an important role in the regulation of central functions such as arousal, attention, cognition, and memory. The cholinergic neurons are connected to the hippocampus and the VTA, and are involved in function of the reward system and mood regulation. Impairment of this system is responsible for development of the cognitive symptoms observed in patients with depression. Pretreatment with atropine significantly reversed the antidepressant-like activity of CSE, suggesting that the muscarinic cholinergic system is involved in the antidepressant-like activity of the fraction. Similar observations have been made in other studies.73,76 Moreover, reports are available showing that the antidepressant-like activity of some isolated flavonoids is abolished by atropine, suggesting the involvement of the cholinergic system in their action.77
The involvement of nitric oxide in physiological neuronal functions such as synaptic plasticity, including depression and neurological disorders is well recognized.78,79 NO is formed from L-arginine by the enzyme NO synthase and inhibited by L-arginine analogs. Pretreatment with L-arginine reversed the antidepressant-like activity of CSE, indicating the involvement of NO in the activity of the fraction. NO probably plays a dual role in the modulation of depression in TST and FST, as either increased or decreased synthesis can produce an antidepressant-like effect.80,81 Although it is generally believed that L-arginine enhances the antidepressant-like activity of various agents, there are cases in which other effects have been reported. For example, moderate doses of L-arginine have antidepressant-like activity, whereas higher doses have no effect.80 In addition, co-administration of L-arginine with an effective dose of modafinil has been shown to attenuate the antidepressant-like activity of modafinil.82 Thus, attenuation of the activity of CSE by L-arginine could be related to either the use of high dose (750 mg/kg) or co-administration with the effective dose of CSE. In a parallel experiment, pretreatment with methylene blue (an inhibitor of nitric oxide synthase and an inhibitor of soluble guanylate cyclase (sGC)) did not reverse the antidepressant-like activity of CSE. By contrast, reports in the literature show the antidepressant-like activity of methylene blue in FST,83,84 which could not be replicated in the present study. Such discrepancies may be related to the multifaceted role of NO in depression and the achievement of an optimal concentration of nitrites in the brain during treatment with agents acting through this pathway to produce an antidepressant-like effect.81
Although this study found that adrenergic, dopaminergic, cholinergic and NO may be involved in the mechanism of action of the antidepressant-activity of CSE, only L-arginine and sulpiride were able to return the duration of immobility to control levels, suggesting that dopaminergic and NO are the most likely mechanisms through which CSE mediates its effect.
Individual variation and sample size could be factors that contribute to variations observed in the measurement of corticosterone level. Measuring brain corticosterone than serum level could have given a better picture. Nevertheless, the use of several models for the study could offset these limitations.
Conclusions
The results of this study shows that CSE and CSB from the root bark of C. spinarum have antidepressant-like activities in TST and FST, the former appearing to be more active than the latter. This was further enhanced by a decrease in serum corticosterone levels. The study suggests that the antidepressant-like activity of CSE might be mediated via α2-adrenergic, D2-dopaminergic receptors, the muscarinic cholinergic system, and the L-arginine-NO pathway. The quantification study suggests that flavonoids, phenols, and alkaloids present in the solvent fractions could be responsible for the observed activity. Further studies are needed to isolate compounds from the CSE fraction that could be used to develop new compounds with anti-depressant activity.
Abbreviations
ECLIA, Electro-chemiluminescence Immunoassay; FST, Forced swim test; OFT, Open field test; TLC, Thin layer Chromatography; TST, Tail suspension test; TW80, Tween 80.
Authors’ Details
Department of Pharmacology and Clinical Pharmacy, School of Pharmacy, College of Health Sciences, Addis Ababa University, P.O. Box 9086, Addis Ababa, Ethiopia.
Data Sharing Statement
The data sets used and/or analyzed during the current work are available and included in this article.
Ethics Approval
The protocol was approved by institutional review board of the School of Pharmacy with Reference no. (ERB/SOP/176/12/2020).
Acknowledgments
The authors are grateful to the Ethiopian Public Health Institute (EPHI) for sponsoring the biochemical analysis (Serum corticosterone). HS would like to acknowledge Addis Ababa University for providing laboratory facilities.
Author Contributions
Both authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received.
Disclosure
The authors declare that they have no conflicts of interest in relation to this work nor regarding the publication of this paper. This paper was uploaded to the Addis Ababa University repository as a thesis in March 2022 (http://etd.aau.edu.et/handle/123456789/31685).85
References
1. Arnaud A, Suthoff E, Tavares RM, Zhang X, Ravindranath AJ. The increasing economic burden with additional steps of pharmacotherapy in major depressive disorder. Pharmacoeconomics. 2021;39(6):691–706. doi:10.1007/s40273-021-01021-w
2. Filatova EV, Shadrina MI, Slominsky PA. Major depression: one brain, one disease, one set of intertwined processes. Cells. 2021;10(6):1283. doi:10.3390/cells10061283
3. Surana AR, Wagh RD. Phytochemical analysis and antidepressant activity of ixora coccinea extracts in experimental models of depression in mice. Turk J Pharm Sci. 2018;15:130–135. doi:10.4274/tjps.14622
4. Wang PS, Simon G, Kessler RC. The economic burden of depression and the cost-effectiveness of treatment. Int J Methods Psychiatr Res. 2003;12(1):22–33. doi:10.1002/mpr.139
5. Santomauro DF, Mantilla Herrera AM, Shadid J, et al. Global prevalence and burden of depressive and anxiety disorders in 204 countries and territories in 2020 due to the COVID-19 pandemic. Lancet. 2021;398:1700–1712. doi:10.1016/S0140-6736(21)02143-7
6. Friedman ES, Anderson IM. Handbook of Depression. Springer Healthcare Limited.
7. Haller E, Geier M, Finley P. Antidepressants, pharmacology. In: Encyclopedia of the Neurological Sciences. Elsevier. 2014:219–223.
8. Laban TS, Saadabadi A. Monoamine Oxidase Inhibitors (MAOI). Treasure Island (FL): StatPearls; 2021.
9. Yildiz A, Gönül AS, Tamam L. Mechanism of actions of antidepressants: beyond the receptors; 2002.
10. Petersen TJ, Hopkins G, Kunwar A, Schwartz TL. Drug development, psychotherapy development, and clinical use. In: Depression: Treatment Strategies and Management. Philadelphia, PA, US: Taylor & Francis; 2006:41–83.
11. Ashraf H, Salehi A, Sousani M, Sharifi MH. Use of complementary alternative medicine and the associated factors among patients with depression. Evid Based Complement Altern Med. 2021;27:2021.
12. Lee G, Bae H. Therapeutic effects of phytochemicals and medicinal herbs on depression. Biomed Res Int. 2017;2017. doi:10.1155/2017/6596241
13. Farahani MS, Bahramsoltani R, Farzaei MH, Abdollahi M, Rahimi R. Plant-derived natural medicines for the management of depression: an overview of mechanisms of action. Rev Neurosci. 2015;26(3):305–321. doi:10.1515/revneuro-2014-0058
14. Zeni AL, Zomkowski AD, Dal-Cim T, Maraschin M, Rodrigues AL, Tasca CI. Antidepressant-like and neuroprotective effects of Aloysia gratissima: investigation of involvement of L-arginine-nitric oxide-cyclic guanosine monophosphate pathway. J Ethnopharmacol. 2011;137(1):864–874. doi:10.1016/j.jep.2011.07.009
15. Moravec I, Fernandez E, Vlkova M, Milella L. Ethnobotany of medicinal plants of northern Ethiopia. Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas. 2014;13(2):126–134.
16. Ansari I, Patil DT, Brief A. Review on phytochemical and pharmacological profile of Carissa spinarum L. Asian J Pharm Clin Res. 2018;11(9):12–18. doi:10.22159/ajpcr.2018.v11i9.26316
17. Nantongo JS, Odoi JB, Abigaba G, Gwali S. Variability of phenolic and alkaloid content in different plant parts of Carissa edulis Vahl and Zanthoxylum chalybeum Engl. BMC Res Notes. 2018;11(1):1–5. doi:10.1186/s13104-018-3238-4
18. Teke GN, Kuete V. Acute and subacute toxicities of african medicinal plants. In: Toxicological Survey of African Medicinal Plants. Elsevier; 2014:63–98.
19. Esubalew ST, Belete A, Lulekal E, Gabriel T, Engidawor E, Asres K. Review of ethnobotanical and ethnopharmacological evidences of some Ethiopian medicinal plants traditionally used for the treatment of cancer. Ethiop J Health Dev. 2017;31:161.
20. Gidey M, Asfaw Z. Review on ethnobotanical studies on traditional medicinal plants used to treat livestock and human ailments in Tigray Region, Ethiopia. Adv J Biol Sci Res. 2015;3:8–36.
21. Moges A, Moges Y. Ethiopian common medicinal plants: their parts and uses in traditional medicine - ecology and quality control. In: Plant Science - Structure, Anatomy and Physiology in Plants Cultured in vivo and in Vitro. IntechOpen; 2020:89.
22. Tefera BN, Kim Y-D. Ethnobotanical study of medicinal plants in the Hawassa Zuria District, Sidama Zone, Southern Ethiopia. J Ethnobiol Ethnomed. 2019;15(1):25. doi:10.1186/s13002-019-0302-7
23. Teklehaymanot T. Ethnobotanical study of knowledge and medicinal plants use by the people in Dek Island in Ethiopia. J Ethnopharmacol. 2009;124(1):69–78. doi:10.1016/j.jep.2009.04.005
24. Kebamo S, Makonnen E, Debela A, Geleta B. Evaluation of diuretic activity of different solvent fractions of methanol extract of Carissa edulis root bark in rats. Med Chem (Los Angeles). 2015;5:472–478. doi:10.4172/2161-0444.1000304
25. Nedi T, Mekonnen N, Urga K. Diuretic effect of the crude extracts of Carissa edulis in rats. J Ethnopharmacol. 2004;95(1):57–61. doi:10.1016/j.jep.2004.06.017
26. Maina GS, Kelvin JK, Maina MB, et al. Antinociceptive properties of dichloromethane: methanolic leaf and root bark extracts of Carissa edulis in rats. J Phytopharmacol. 2015;4(2):106–112.
27. Ngulde SI, Sandabe UK, Barkindo AA, Tijjani MB, Hussaini IM. Antinociceptive and Anti-inflammatory activities of the ethanol extract of Carissa edulis vahl. root bark in rats and mice. Int J Modern Biol Med. 2013;4(2):85–95.
28. Ya’u J, Yaro AH, Malami S, et al. Anticonvulsant activity of aqueous fraction of Carissa edulis root bark. Pharm Biol. 2015;53:1329–1338. doi:10.3109/13880209.2014.981280
29. Ya’u J, Yaro AH, Abubakar MS, Anuka JA, Hussaini IM. Anticonvulsant activity of Carissa edulis (Vahl) (Apocynaceae) root bark extract. J Ethnopharmacol. 2008;120(2):255–258. doi:10.1016/j.jep.2008.08.029
30. Woode E, Ansah C, Ainooson GK, Abotsi WM, Mensah AY, Duweijua M. Anti-inflammatory and antioxidant properties of the root extract of Carissa edulis (forsk.) Vahl (Apocynaceae). J Sci Tech. 2007;27:5–15.
31. Berhanu G, Atalel D, Kandi V. A review of the medicinal and antimicrobial properties of Carissa spinarum L. Am J Biomed Res. 2020;8:54–58.
32. Jenson BN Pharmacognostic and antimicrobial studies on the leaf extracts of Carissa Edulis Vahl. (Apocynaceae) and Senna Alata L. (Fabaceae) used in the management of skin infections; 2018:165.
33. Ngulde SI, Sandabe UK, Tijjani MB, Barkindo AA, Hussaini IM. Phytochemical constituents, antimicrobial screening and acute toxicity studies of the ethanol extract of Carissa edulis Vahl. root bark in rats and mice. Am J Res Commun. 2013;1(9):99–110.
34. Ya U, Malami S, Ngura HE, Ngura H. Antidepressant activity of ethanol extract and residual aqueous fraction of Carissa edulis (Apocynaceae) root bark in mice. Trop J Nat Prod Res. 2017;1(3):129–132. doi:10.26538/tjnpr/v1i3.9
35. National Research Council. Guide for the Care and Use of Laboratory Animals.
36. Sarker SD, Latif Z, Gray AI. Natural product isolation. In: Sarker SD, Latif Z, Gray AI, editors. Natural Products Isolation. Totowa, NJ: Humana Press; 2006:1–25.
37. Maria R, Shirley M, Xavier C, et al. Preliminary phytochemical screening, total phenolic content and antibacterial activity of thirteen native species from Guayas province Ecuador. J King Saud Univ Sci. 2018;30(4):500–505. doi:10.1016/j.jksus.2017.03.009
38. Gandagule U, Duraiswamy B, Bhurat M, Nagdev S, Zalke A, Gupta L. Estimation of total phenolic content and total flavonol content in leaves and stem extracts of ventilago maderspatana Gaertn. and leaves, stem and stem bark extracts of ZiZiphus xylopyrus (Retz) Willd. Inventi J. 2018;2019:5.
39. Van Tan P. The determination of total alkaloid, polyphenol, flavonoid and saponin contents of Pogang gan (Curcuma sp.). Int J Biol. 2018;10:4.
40. Tabasum S, Khare S, Jain K. Spectrophotometric quantification of total phenolic, flavonoid, and alkaloid contents of abrus precatorius L. Seeds. Asian J Pharm Clin Res. 2016;9(2):371–374.
41. Dawa I, Mamza U, Sodipo O, Abdulrahman FI, Balami V, Yakubu J. Phytochemical evaluation and gas chromatography-mass spectrometric analysis of column fractions of Carissa edulis leaf extract. Chem Sci. 2021;10:59–68.
42. Oduor LP. Investigation of in vitro antiplasmodial activities of Carissa edulis, Azadirachta indica, Cassia siamea and Harrisonia abyssinica on Plasmodium falciparum Doctoral dissertation. Egerton University; 2016:98.
43. Diehl K-H, Hull R, Morton D, et al. A good practice guide to the administration of substances and removal of blood, including routes and volumes. J Appli Toxicol. 2001;21(1):15–23. doi:10.1002/jat.727
44. Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi:10.1038/266730a0
45. Steru L, Chermat R, Thierry B, Simon P. The tail suspension test: a new method for screening antidepressants in mice. Psychopharmacology. 1985;85(3):367–370. doi:10.1007/BF00428203
46. Can A, Dao DT, Terrillion CE, Piantadosi SC, Bhat S, Gould TD. The tail suspension test. J Vis Exp. 2012;3769. doi:10.3791/3769
47. Costa-Nunes JP, Cline BH, Araújo-Correia M, et al. Animal models of depression and drug delivery with food as an effective dosing method: evidences from studies with celecoxib and dicholine succinate. Biomed Res Int. 2015;2015. doi:10.1155/2015/596126
48. Porsolt RD, Anton G, Blavet N, Jalfre M. Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol. 1978;47:379–391. doi:10.1016/0014-2999(78)90118-8
49. Krishnan V, Nestler EJ. Animal models of depression: molecular perspectives. Curr Top Behav Neurosci. 2011;7:121–147.
50. Demissie S, Shibeshi W, Engidawork E. Evaluation of the antidepressant-like activity of solvent fractions of the aerial parts of Hypericum revolutum (Hypericaceae) in rodent models. Ethiop Pharm J. 2017;33(2):95–106. doi:10.4314/epj.v33i2.3
51. Abiola AL, Ayofe AM, Adedoyin A. Neurobehavioural mechanism of antidepressant effect of methanol stem bark extract of Adansonia digitata (Linn) in tail suspension test in mice. Adv Pharmacol Pharm. 2019;7(1):5–13. doi:10.13189/app.2019.070102
52. Akinpelu L, Adegbuyi A, Agboola S, et al. Antidepressant activity and mechanism of aqueous extract of Vigna Unguiculata ssp. Dekindtiana (L.) walp dried aerial part in mice. Int J Neurosci Behavioral Sci. 2017;5(1):7–18. doi:10.13189/ijnbs.2017.050102
53. Cryan JF, Markou A, Lucki I. Assessing antidepressant activity in rodents: recent developments and future needs. Trends Pharmacol Sci. 2002;23(5):238–245. doi:10.1016/S0165-6147(02)02017-5
54. Bangasser DA, Valentino RJ. Sex differences in stress-related psychiatric disorders: neurobiological perspectives. Front Neuroendocrinol. 2014;35:303–319.
55. Brigitta B. Pathophysiology of depression and mechanisms of treatment. Dialogues Clin Neurosci. 2002;4(1):7–20. doi:10.31887/DCNS.2002.4.1/bbondy
56. Malik H, Javaid S, Fawad Rasool M, et al. Amelioration of scopolamine-induced amnesic, anxiolytic and antidepressant effects of ficus benghalensis in behavioral experimental models. Medicina. 2020;56(3):144. doi:10.3390/medicina56030144
57. Kumar A, Lakshman K, Velmurugan C, Sridhar SM, Saran G. Antidepressant activity of methanolic extract of amaranthus spinosus. Basic Clin Neurosci. 2014;5(1):11–17.
58. García-Ríos RI, Mora-Pérez A, Ramos-Molina AR, Soria-Fregozo C. Neuropharmacology of secondary metabolites from plants with anxiolytic and antidepressant properties. In: In Behavioral Pharmacology - from Basic to Clinical Research. IntechOpen; 2020.
59. Kennedy DO, Wightman EL. Herbal extracts and phytochemicals: plant secondary metabolites and the enhancement of human brain function. Adv Nutr. 2011;2(1):32–50. doi:10.3945/an.110.000117
60. Al-Youssef HM, Hassan WHB. Phytochemical and pharmacological aspects of Carissa edulis vahl: a review. Int J Curr Res Chem Pharm Sci. 2014;1(9):12–24.
61. Umar UA, Hassan LG, Maradun KL. TLC analysis and antioxidant activity of garden egg leaves. Bayero J Pure Appl Sci. 2019;12:443–448. doi:10.4314/bajopas.v12i1.67S
62. Maletic V, Eramo A, Gwin K, Offord SJ, Duffy RA. The role of norepinephrine and its α-adrenergic receptors in the pathophysiology and treatment of major depressive disorder and schizophrenia: a systematic review. Front Psychiatry. 2017;8:42. doi:10.3389/fpsyt.2017.00042
63. Stone EA, Lin Y, Rosengarten H, Kramer HK, Quartermain D. Emerging evidence for a central epinephrine-innervated α1-adrenergic system that regulates behavioral activation and is impaired in depression. Neuropsychopharmacol. 2003;28(8):1387–1399. doi:10.1038/sj.npp.1300222
64. Cottingham C, Wang Q. α2 adrenergic receptor dysregulation in depressive disorders: implications for the neurobiology of depression and antidepressant therapy. Neurosci Biobehav Rev. 2012;36(10):2214–2225. doi:10.1016/j.neubiorev.2012.07.011
65. Ishola I, Akinyede A, Sholarin A. Antidepressant and anxiolytic properties of the methanolic extract of momordica charantia Linn (Cucurbitaceae) and its mechanism of action. Drug Res. 2013;64(7):368–376. doi:10.1055/s-0033-1358712
66. Bettio LEB, Machado DG, Cunha MP, et al. Antidepressant-like effect of extract from Polygala paniculata: involvement of the monoaminergic systems. Pharm Biol. 2011;49(12):1277–1285. doi:10.3109/13880209.2011.621958
67. Mensah JA Antidepressant effect and the possible mechanism (S) of action of secondary metabolites from trichilia monadelpha in murine models Doctoral dissertation. University of Ghana; 2016.
68. Kaur R, Chopra K, Singh D. Role of α2 receptors in quercetin-induced behavioral despair in mice. J Med Food. 2007;10(1):165–168. doi:10.1089/jmf.2005.063
69. Butterweck V, Jürgenliemk G, Nahrstedt A, Winterhoff H. Flavonoids from hypericum perforatum show antidepressant activity in the forced swimming test. Planta Med. 2000;66(1):3–6. doi:10.1055/s-2000-11119
70. Ishola IO, Chatterjee M, Tota S, et al. Antidepressant and anxiolytic effects of amentoflavone isolated from Cnestis ferruginea in mice. Pharmacol Biochem Behav. 2012;103(2):322–331. doi:10.1016/j.pbb.2012.08.017
71. Dunlop BW, Nemeroff CB. The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry. 2007;64:327. doi:10.1001/archpsyc.64.3.327
72. Ploski JE, Vaidya VA. The neurocircuitry of PTSD and major depression: insights into overlapping and distinct circuit dysfunction - a tribute to Ron Duman. Biol Psychiatry. 2021;90:109–117. doi:10.1016/j.biopsych.2021.04.009
73. Ishola IO, Olayemi SO, Yemitan OK, Umeh EA. Antidepressant and anxiolytic effects of the methanol root extract of Capparis thonningii: involvement of monoaminergic, cholinergic and GABAergic systems. Drug Res. 2015;65(4):205–213. doi:10.1055/s-0034-1376963
74. Pannu A, Sharma PC, Thakur VK, Goyal RK. Emerging role of flavonoids as the treatment of depression. Biomolecules. 2021;11(12):1825. doi:10.3390/biom11121825
75. Wang X, Zhang L, Hua L, Xing D, Du L. Effect of flavonoids in scutellariae radix on depression-like behavior and Brain Rewards: possible in dopamine system. Tinshhua Sci Technol. 2010;15:460–466. doi:10.1016/S1007-0214(10)70088-2
76. Owope T, Ishola I, Akinleye M, Oyebade R, Adeyemi O. Antidepressant effect of cnestis ferruginea Vahl ex DC (Connaraceae): involvement of cholinergic, monoaminergic and L-arginine-nitric oxide pathways. Drug Res. 2016;66:235–245. doi:10.1055/s-0035-1565174
77. Onasanwo A, Ilenre K, Faborode O. The impact of kolaviron (A bioflavonoid of garcinia kola seed) on depression status in laboratory rodents: roles of monoaminergic systems. Annals Depress Anxiety. 2015;2(1):1042.
78. Dhir A, Kulkarni SK. Nitric oxide and major depression. Nitric Oxide. 2011;24:125–131. doi:10.1016/j.niox.2011.02.002
79. Zhou Q-G, Zhu X-H, Nemes AD, Zhu D-Y. Neuronal nitric oxide synthase and affective disorders. IBRO Report. 2018;5:116–132. doi:10.1016/j.ibror.2018.11.004
80. da Silva G, Matteussi AS, Dos Santos ARS, Calixto JB, Rodrigues ALS. Evidence for dual effects of nitric oxide in the forced swimming test and in the tail suspension test in mice. NeuroReport. 2000;11(17):3699–3702. doi:10.1097/00001756-200011270-00022
81. Inan SY, Yalcin I, Aksu F. Dual effects of nitric oxide in the mouse forced swimming test: possible contribution of nitric oxide-mediated serotonin release and potassium channel modulation. Pharmacol Biochem Behav. 2004;77(3):457–464. doi:10.1016/j.pbb.2003.12.024
82. Omidi-Ardali H, Badi AG, Saghaei E, Amini-Khoei H. Nitric oxide mediates the antidepressant-like effect of modafinil in mouse forced swimming and tail suspension tests. J Basic Clin Physiol Pharmacol. 2021;32(2):25–31. doi:10.1515/jbcpp-2020-0021
83. Delport A, Harvey BH, Petzer A, Petzer JP. Methylene blue and its analogues as antidepressant compounds. Metab Brain Dis. 2017;32(5):1357–1382. doi:10.1007/s11011-017-0081-6
84. Heiberg IL, Wegener G, Rosenberg R. Reduction of cGMP and nitric oxide has antidepressant-like effects in the forced swimming test in rats. Behav Brain Res. 2002;134:479–484. doi:10.1016/S0166-4328(02)00084-0
85. Saif H. Antidepressant-like activity of solvent fractions of the root bark of Carissa spinarum Linn. (Apocynaceae) in rodents. Thesis. Addis Ababa University; 2022.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.