Back to Journals » Neuropsychiatric Disease and Treatment » Volume 15
Antidepressant, anti-inflammatory, and antioxidant effects of electroacupuncture through sonic hedgehog–signaling pathway in a rat model of poststroke depression
Authors Cai W, Ma W, Wang GT, Li YJ, Shen WD
Received 12 February 2019
Accepted for publication 24 April 2019
Published 23 May 2019 Volume 2019:15 Pages 1403—1411
DOI https://doi.org/10.2147/NDT.S205033
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yuping Ning
Wa Cai,1,2 Wen Ma,1 Guan-Tao Wang,1 Yi-Jing Li,1 Wei-Dong Shen1,2
1Institute of Acupuncture and Anesthesia, Shanghai Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China; 2Department of Acupuncture, Shanghai Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, People’s Republic of China
Background: Poststroke depression (PSD) is the most frequent psychological sequela after stroke. Electroacupuncture (EA) treatment is effective for PSD. The study aimed at clarifying the mechanisms of EA’s antidepressant effects in a PSD rat model.
Methods: We used middle cerebral artery occlusion to establish the rat model of PSD. Tests of sucrose preference and locomotor activity were performed to examine depressive-like behaviors. We measured malondialdehyde, GSH, SOD, IL6, IL1β, TNFα, and 5HT with ELISA. The hippocampal Shh-signaling pathway was assessed by Western blot.
Results: EA significantly decreased sucrose preference and locomotor activities of PSD rats, reduced IL6, TNFα, increased GSH, and upregulated 5HT, and also slightly reduced IL1β and malondialdehyde, all of which were measured with ELISA. The Shh-signaling pathway assessed by Western blotting was activated by EA. Those changes were inhibited by the Shh-pathway inhibitor cyclopamine.
Conclusion: EA effectively alleviated depressive-like behaviors in PSD by suppressing inflammation and oxidative stress through activation of the Shh-signaling pathway.
Keywords: electroacupuncture, poststroke depression, sonic hedgehog–signaling pathway, oxidative stress, inflammation, 5HT
Introduction
Poststroke depression (PSD) is the most frequent psychiatric consequence of stroke, with a prevalence of a third of stroke survivors.1 PSD negatively affects stroke rehabilitation, cognitive function, and social activity, leading to increased mortality.2 Research has shown that PSD is associated with lower levels of 5HT, one of the monoamines that can be significantly upregulated by antidepressants.3 Both oxidative stress and inflammation have been found to play a crucial role in the pathogenesis of PSD.4
Shh is a glycoprotein that is prominent in neurogenesis of neural stem cells. It binds to its receptor Ptch1 and suppresses the transducing protein Smo, which subsequently triggers the transcription factor Gli1 to promote neurogenesis, including proliferation, migration, and differentiation of cells.5,6 Since hippocampal neurogenesis is involved in protecting against and promoting recovery from depression,7 the hippocampus is important in the development of depressive-like behaviors of PSD.
Numerous studies have confirmed that electroacupuncture (EA) is an effective treatment for PSD.8–11 A recent meta-analysis indicated that EA caused fewer adverse events than antidepressants in treating PSD.12 However, the mechanisms of EA treatment for PSD remain uncertain. The objective of this study was to find out whether the effectiveness of EA treatment for PSD is associated with the hippocampal Shh-signaling pathway.
Methods
Animals
The experimental protocol was approved by the Ethics Committee for Animal Experimentation of Shanghai University of Traditional Chinese Medicine and designed in accord with the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (publication 8023, revised 1978). Experiments were conducted according to the National Institute of Health’s Guide for the Care and Use of Laboratory Animals (publication 80–23), revised in 1996. The number of animals used and suffering caused was minimized in our experiments.
Male Sprague Dawley rats aged around 3 months were obtained from the Animal Center of Shanghai University of Traditional Chinese Medicine, Shanghai, China. Upon arrival, five rats were housed in each cage and acclimatized to a room with controlled temperature (24 °C±2 °C), humidity (60%±5%) and a natural 12 hr light/dark cycle. They were allowed to acclimatize to the environment for 7 days before experiments. All experimental rats (220–250 g) were allocated randomly into different treatment groups by an independent researcher. Rats in every group were mixed in behavioral tests during observation (9–11 am), and observers were blinded to group allocation. After experiments had finished, all rats were killed by overdoses of chemical anesthetics.
Experimental protocol
In order to find the exact efficacy of EA and its associations with the Shh-signaling pathway, 48 rats were allocated randomly into six groups (n=8 for each group): sham, PSD, EA + PSD, EA + PSD + cyclopamine (intraperitoneal administrations of 0.2 mg/kg/day), EA + PSD + vehicle (intraperitoneal administrations of 10 μL saline/day), and fluoxetine + PSD (intragastric administrations of 14.4 mg/kg/day). The sham group was the comparison PSD-model group for depressive behaviors. Fluoxetine was compared to EA in treatment effectiveness. The use of cyclopamine aimed at clarifying the role of the Shh-signaling pathway. The EA + PSD + vehicle group was set as the control group of the EA + PSD + cyclopamine group to reduce minor subjective errors.
ELISA was used on antioxidants GSH, SOD, and oxidative MDA to evaluate levels of oxidative stress. Inflammatory cytokines IL1β, TNFα, and IL6 were measured with ELISA to assess inflammation. 5HT was also measured with ELISA to evaluate antidepressant effects. Shh, Smo, Ptch, and Gli1 were measured by Western blot to elucidate the role of the Shh-signaling pathway. Our study assessed depressive-like behavior through the sucrose-preference test (SPT) and locomotor activity (Figure 1).
PSD-model establishment
Transient focal cerebral ischemia was induced by 90 mins of left middle cerebral artery occlusion (MCAO), which was followed by reperfusion. The MCAO procedure was modified by a previous method.13 Rats were anesthetized with 5% chloral hydrate. The left common carotid artery, external carotid artery, and internal carotid artery were exposed and isolated after a ventral neck incision. A monofilament (Sunbio Biotech, Beijing, China) was then introduced into the internal carotid artery and gently advanced to block the blood flow of the left middle cerebral artery for 90 mins. The wound was sutured shortly after completion of ischemia procedures. The same surgical procedures were done in the sham group, except that the left middle cerebral artery was not occluded. Rats were allowed 3 days’ recovery before the start of any intervention.
Electroacupuncture treatment
The acupoints DU20 and DU24 were selected for EA treatment of PSD.14 Stainless-steel acupuncture needles (40×0.25 mm; Jiajian Medical Supplies Co Ltd, Wuxi, China) were inserted to a depth of about 3 mm using an electric current at a frequency of 2 Hz with an EA device (Hwato SDZ-II; Suzhou Medical Supplies, Suzhou, China) for about 30 mins per EA-treatment session.15 EA treatment lasted for a month (28 days), with 5 sessions a week.16
Behavior tests
The SPT and locomotor activity were assessed at baseline and on day 31 after treatment.
Sucrose-preference test
The SPT was conducted from 9 to 10 am to examine PSD-induced anhedonia. Rats were food- and water-deprived for 20 hrs prior to the test, followed by free access to one bottle with 2% sucrose solution (200 mL) and another bottle with water (200 mL) for 2 hrs. The sucrose-preference index was calculated:
sucrose preference = sucrose intake (g)/sucrose intake (g) + water intake (g).17
Locomotor activity
Locomotor activity was examined in a dark box (1×1 m chamber) with walls of 0.5 m height to assess rearing activity and general locomotor activity of rats. The floor of the chamber was divided into 25 equal square quadrants. Locomotor activity was measured when at least three paws were presented in a quadrant for 5 mins. Observer 2.0 software (Xinruan Information and Technology, Shanghai, China) was used to record the test.18
ELISA
Obtained from the abdominal aorta of anesthetized rats, blood was collected in anticoagulant-free tubes and quickly centrifuged to avoid glycolysis on day 31 after EA treatment. Serum samples were kept at –80 °C until assay. According to the manufacturer’s instructions, IL6, TNFα, and IL1β levels in serum were detected with ELISA kits (Xitang, Shanghai, China), and measurement of MDA (Beyotime, Haimen, China) and GSH and SOD (Qiaoyu, Shanghai, China) was performed. Serum levels of 5HT were measured with a sandwich-ELISA kit (R&D Systems).
Western blot
The brains of deeply anesthetized rats were promptly removed on day 31 after EA treatment. Ischemic hippocampi were isolated and frozen in liquid nitrogen. Total protein measurement for Shh, Gli1, Ptch1, and Smo in ischemic hippocampi (n=8 in each group) was performed in accordance with the manufacturer’s instructions. With a BCA protein-assay reagent kit, concentrations of protein extracts were tested. Protein (50 μg) were separated by SDS/PAGE and transferred to polyvinylidene difluoride membranes. Membranes loaded with proteins of interest were incubated with 5% nonfat dried milk in PBS at room temperature for 1 hr and then probed with anti-Ptch1 polyclonal antibody (1:500; Abcam), anti-Gli1 polyclonal antibody (1:500; Sigma-Aldrich), anti-Shh polyclonal antibody (1:1,000; Cell Signaling Technology) and anti-Smo monoclonal antibody (1:500; Santa Cruz Biotechnology) overnight at 4 °C, washed with 0.1% Tris-buffered saline on the second day, and then incubated with fluorescence-labeled goat antirabbit secondary antibodies for 1 hr at room temperature. Imaging densitometry (Li-Cor Biosciences) was used to analyze the relative density of each band. Changes in protein activation are presented as ratios of β-actin.
Statistical analysis
SPSS 21.0 for Windows (IBM, Armonk, NY, USA) was used for statistical analyses. All data are presented as means ± SEM, and were analyzed with one-way ANOVA and paired t-tests. P<0.05 was considered statistically significant.
Results
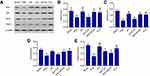
EA treatment improved the depressive-like behaviors of PSD
Figure 2A shows that PSD rats had less sucrose preference than rats in the sham group (P<0.01). Both EA and fluoxetine treatment significantly reversed low sucrose preference (P<0.01). PSD rats had significantly lower locomotor counts than sham-group rats (P<0.001, Figure 2B). Both EA and fluoxetine significantly increased locomotion counts (P<0.001). Sucrose preference and locomotor activity were assessed on day 31.
EA treatment alleviated oxidative stress and inflammation in PSD
Oxidative stress was measured through levels of GSH, SOD, and MDA on day 31 (Figure 3A–C). PSD rats had higher serum levels of MDA and lower levels of GSH than rats in the sham group. Both EA and fluoxetine treatment slightly reduced MDA. Moreover, serum levels of GSH were elevated significantly by EA (P<0.05, Figure 3B). Serum levels of IL6, TNFα, and IL1β increased significantly in PSD rats (P<0.01, Figure 3D–F). EA and fluoxetine alleviated inflammation in PSD via significantly decreasing serum levels of IL6 (P<0.05, Figure 3E) and TNFα (P<0.001, Figure 3F).
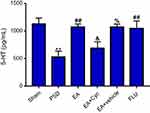
EA treatment regulated 5HT in PSD
As shown in Figure 4, serum levels of 5HT in PSD rats were significantly lower than sham rats (P<0.01, Figure 4). After treatment, 5HT levels in both the EA and fluoxetine groups were much higher than the PSD group (P<0.01, Figure 4).
EA treatment regulated Shh-signaling pathway in PSD
Expressions of Shh, Gli1, Smo, and Ptch1 proteins were measured through immunoblot analysis on day 31. Ratios of Shh, Gli1, Smo, and Ptch1 to β-actin in the PSD group were much lower than those in the sham group (P<0.05 [Figure 5B and D], P<0.01 [Figure 5C and E]). After EA and fluoxetine treatment, expression of all proteins had increased significantly (P<0.01 [Figure 5C], P<0.05 [Figure 5E]). The Gli1:β-actin ratio had also declined significantly after cyclopamine intervention (P<0.05, Figure 5C). Shh, Gli1, Smo, and Ptch1 upregulation by EA was found to be inhibited by cyclopamine, an antagonist of the Shh-signaling pathway (Figure 5). Meanwhile, cyclopamine slightly reversed the upregulation of GSH and downregulation of MDA and IL6, TNFα, and IL1β by EA (Figure 3). Compared to the EA group, 5HT in the EA + cyclopamine group was significantly decreased (Figure 4), with more serious depressive-like behaviors (P<0.01, Figure 2A and B).
Discussion
PSD is the most frequent psychological sequela after stroke, and comes with neuronal injury, oxidative stress, and inflammation.18–20 The MCAO model is commonly used to induce ischemic brain injury in rats. Researchers have found that reduction in sucrose consumption and increase in immobility time in behavior tests are significant 2 weeks after MCAO surgery, indicating that the depressive-like behaviors are delayed effects of MCAO,21 which was also confirmed in our study. Although some results in our study, including behavior tests, Western blot, and ELISA, showed only a tendency toward alterations without statistical significance, which could be explained by some significant biochemical changes occurring prior to day 31, these tendencies were consistent with one another, as well as with findings of previous studies.
EA has been found to resist oxidation by reducing oxidants like reactive oxygen species (ROS), nitric oxide, inducible nitric oxide synthase, and COX2 in brain tissue after ischemic stroke22 and chronic unpredictable stress.23 Inflammatory cytokines, including IL1β, TNFα, and IL6 are reduced with EA in stroke survivors24 and depression patients.25 Significantly, our study had consistent findings: EA was found to resist oxidation effectively by reducing MDA and increasing GSH in brain tissue of PSD rats. IL1β, TNFα, and IL6 were found to be reduced by EA. Improvements in depressive-like behavior tests (SPT and locomotor activity) and upregulation of 5HT levels verified the antidepressant effect of EA, accompanied by the anti-inflammatory and antioxidant effects of EA in PSD.
Most strokes are reportedly ischemic and lead to brain injury via oxygen and glucose,26,27 resulting in a loss of antioxidant defense and overproduction of ROS.28 Depression is also related to increased oxidative stress and inhibition of antioxidant defense systems, causing neuronal damage.29 Recent research has indicated that the Shh-signaling pathway is involved in cerebral ischemia and the underlying mechanisms associated with counteracting excessive ROS production.30 It was found that Shh signaling reduced the detrimental effect of H2O2-like decreasing neuronal viability. Shh increased the activities of GSH-Px and attenuated the formation of MDA in H2O2-treated neurons. Shh plays a significant role in promoting neuritic outgrowth in H2O2-treated neurons in an in vitro model. Potential mechanisms of antioxidant effects by Shh may be associated with preventing mitochondrial dysfunction, inhibiting ROS release, and promoting the production of ATP.31 As the inhibitor of Smo in the Shh pathway, cyclopamine enhances the formation of MDA and reduces the activities of GSH-Px, Gli1, and Ptch1, which are critical effectors in the Shh pathway. A rat model of cerebral ischemia found that suppression of the Shh-signaling pathway aggravated ischemic injury.32
Many clinical studies have found that inflammation plays a crucial role in the pathogenesis of PSD.33–35 Notably, mutual interactions have been found between inflammation and oxidative stress. The Shh-signaling pathway can cope with inflammatory reactions,32,36,37 plays a part in ROS overproduction, and is regulated by brain injury–related inflammation.38 Cyclopamine decreases Gli expression and reduces proliferation of astrocytes in the injured cortex.38 Composed of astrocytes and capillary endothelial cells, blood–brain barrier (BBB) function is affected in stroke. Research has indicated that BBB dysfunction activates the Shh pathway during inflammation39–42 and that Shh released from astrocytes maintains the integrity of the BBB.43 IL1β was found to induce BBB disruption by downregulating Shh in astrocytes. Moreover, cross talk was found between the Wip1 and Shh pathways. Wip1 alleviated inflammation and BBB dysfunction via Shh signaling, which was increased by Wip1 overexpression and repressed by silencing of Wip1, indicating that Shh can interact with Wip1 to relieve inflammation and BBB disruption.40
Researchers have reported that the antidepressant effects of fluoxetine were mediated by increased neurogenesis of the dentate gyrus (DG) of the adult hippocampus.44 Impairment of DG neurogenesis might be one of the etiological factors of PSD. Cerebral ischemia triggered neurons generated to migrate from the subgranular zone into the granule-cell layer of the DG.45,46 Wang et al47 found that ischemia hippocampal neurogenesis was inhibited in PSD rats, suggesting an interaction between depressive-like behaviors and ischemia-stimulated neurogenesis. Shh signaling can mediate the differentiation and proliferation of adult hippocampal neural progenitor cells (NPCs). If hippocampal NPCs are isolated from the hippocampus, Shh stimulates their proliferation,48 whereas cyclopamine inhibits it. Harbored by adult hippocampus in the subgranular zone of the DG, NPCs can generate neurons that integrate into the DG and receive synaptic inputs from other hippocampal neurons.49 Since deletion of Shh or Smo in nestin-expressing NPCs leads to reduced neurogenesis, Shh signaling is indispensable in hippocampal neurogenesis in adult rats.50 Furthermore, due to the high prevalence of cognitive impairments in late-life depression, these were included in the diagnostic criteria of depressive-like disorders in the current Diagnostic and Statistical Manual of Mental Disorders 5 classification.51 Studies have found that changes in the hippocampus were closely associated with cognitive impairments,52,53 which led to delayed neurological recovery time and negatively affected other depressive-like symptoms of stroke survivors, lowering the life quality of PSD patients.54 Future research should be done to find associations between the hippocampal Shh-signaling pathway and cognitive impairments in PSD.
In our study, the significant upregulation of Shh, Gli1, Smo, and Ptch1 in rat hippocampi in the EA group and fluoxetine group suggested that EA and fluoxetine activated the Shh-signaling pathway, while cyclopamine counteracted it. Additionally, anti-inflammatory and antioxidant effects of EA were inhibited by cyclopamine, consequently reversing the upregulation of 5HT by EA and aggravating depressive-like behaviors of PSD. Interestingly, cyclopamine significantly inhibited EA-mediated increases in sucrose preference, but no significant change was found in locomotor activity, which indicated that inhibiting the Shh-signaling pathway might have more association with anhedonia than with poor motivation. Our study found that the underlying mechanisms of EA’s antidepressant, anti-inflammatory, and antioxidant effects on PSD were closely associated with activation of the Shh-signaling pathway.
Conclusion
This study aimed at clarifying potential mechanisms of EA treatment for PSD. We found that EA can effectively relieve depressive-like behaviors of PSD by suppressing inflammation and oxidative stress via activation of the hippocampal Shh-signaling pathway, suggesting that EA can be an effective treatment for PSD. Further research is needed to explore whether EA is associated with hippocampal neurogenesis mediated by Shh signaling.
Abbreviation list
EA, electroacupuncture; GSH, glutathione; LA, locomotor activity; MCAO, middle cerebral artery occlusion; MDA, malondialdehyde; NPCs, neural progenitor cells; PSD, poststroke depression; SPT, sucrose-preference test; WB, Western blot.
Ethics approval and consent to participate
All experimental procedures were approved by the Ethics Committee for Animal Experimentation of Shanghai University of Traditional Chinese Medicine and performed according to the National Institutes of Health’s Guide for the Care and Use of Laboratory Animals (publication 8023, revised 1978).
Acknowledgments
WC was supported by the Graduate Innovation Training Program, Shanghai University of Traditional Chinese Medicine (grant Y201805). WDS was supported by the Shanghai Committee of Science and Technology, China (grant 16401970402/18401970601), the Three-Year Action Plan for the Development of Traditional Chinese Medicine, Shanghai, China (grant ZYSNXD-CC-HPGC-JD-014), and the Shanghai Municipal Commission of Health and Family Planning, China (grant ZYKC201701001).
Author contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hackett ML, Pickles K. Part I: frequency of depression after stroke: an updated systematic review and meta-analysis of observational studies. Int J Stroke. 2014;9(8):1017–1025. doi:10.1111/ijs.12357
2. Ellis C, Zhao Y, Egede LE. Depression and increased risk of death in adults with stroke. J Psychosom Res. 2010;68(6):545–551. doi:10.1016/j.jpsychores.2009.11.006
3. Paolucci S. Advances in antidepressants for treating post-stroke depression. Expert Opin Pharmacother. 2017;18(10):1011–1017. doi:10.1080/14656566.2017.1334765
4. Nabavi SF, Dean OM, Turner A, Sureda A, Daglia M, Nabavi SM. Oxidative stress and post-stroke depression: possible therapeutic role of polyphenols? Curr Med Chem. 2015;22(3):343–351. doi:10.2174/0929867321666141106122319
5. Bambakidis NC, Petrullis M, Kui X, et al. Improvement of neurological recovery and stimulation of neural progenitor cell proliferation by intrathecal administration of sonic hedgehog. J Neurosurg. 2012;116(5):1114–1120. doi:10.3171/2012.1.JNS111285
6. Ihrie RA, Shah JK, Harwell CC, et al. Persistent sonic hedgehog signaling in adult brain determines neural stem cell positional identity. Neuron. 2011;71(2):250–262. doi:10.1016/j.neuron.2011.05.018
7. Deng W, Aimone JB, Gage FH. New neurons and new memories: how does adult hippocampal neurogenesis affect learning and memory? Nat Rev Neurosci. 2010;11(5):339–350. doi:10.1038/nrn2822
8. Chen SH. Clinical observation of electroacupuncture in the treatment of post-stroke depression. Chin J Integr Med. 2012;19(20):3094–3095.
9. Dong JP, Sun WY, Wang S. Clinical observation on head point-through-point electroacupuncture for treatment of poststroke depression. Chin Acupunct Moxibustion. 2007;27(4):241–244.
10. Guo S, Li A, Chen X. Effects of electric-acupuncture and fluoxetine on depression and neurological function of post-stroke depression patients. Shandong Med J. 2011;51(25):9–11.
11. Kw G, Yb G. Electro-acupuncture therapy for the post-stroke depression. J Clini Acupunct Moxibustion. 2014;30(11):35–37.
12. Li XB, Wang J, Xu AD, et al. Clinical effects and safety of electroacupuncture for the treatment of post-stroke depression: a systematic review and meta-analysis of randomised controlled trials. Acupunct Med. 2018;36(5):284–293. doi:10.1136/acupmed-2016-011300
13. Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20(1):84–91. doi:10.1161/01.STR.20.1.84
14. Chen SX, Liu FF. [Effect of Tiaoshen Kaiyu acupuncture (Regulating vitality and dredging stasis) combined with psychological intervention on patients of mild depression after stroke]. Zhen Ci Yan Jiu = Acupunct Res. 2018;43(1):39–43. doi:10.13702/j.1000-0607.170049
15. Wang YW, Wang CM, Sun YT. Therapeutic observation of electroacupuncture at different frequencies for post-stroke depression. Shanghai J Acupunct Moxibustion. 2015;34(9):822–824.
16. Chen D, Sun YZ. Clinical observation on treating post-stroke depression by electropuncture plus auricular pressure. Clin J Chin Med. 2016;8(2):35–36.
17. Yu Y, Wang R, Chen C, et al. Antidepressant-like effect of trans-resveratrol in chronic stress model: behavioral and neurochemical evidences. J Psychiatr Res. 2013;47(3):315–322. doi:10.1016/j.jpsychires.2012.10.018
18. Li W, Ling S, Yang Y, Hu Z, Davies H, Fang M. Systematic hypothesis for post-stroke depression caused inflammation and neurotransmission and resultant on possible treatments. Neuro Endocrinol Lett. 2014;35(2):104–109.
19. Ng F, Berk M, Dean O, Bush AI. Oxidative stress in psychiatric disorders: evidence base and therapeutic implications. Int J Neuropsychopharmacol. 2008;11(6):851–876. doi:10.1017/S1461145707008401
20. Yang L, Zhang Z, Sun D, et al. Low serum BDNF may indicate the development of PSD in patients with acute ischemic stroke. Int J Geriatr Psychiatry. 2011;26(5):495–502. doi:10.1002/gps.2552
21. Pang C, Cao L, Wu F, et al. The effect of trans-resveratrol on post-stroke depression via regulation of hypothalamus-pituitary-adrenal axis. Neuropharmacology. 2015;97:447–456. doi:10.1016/j.neuropharm.2015.04.017
22. Jung YS, Lee SW, Park JH, Seo HB, Choi BT, Shin HK. Electroacupuncture preconditioning reduces ROS generation with NOX4 down-regulation and ameliorates blood-brain barrier disruption after ischemic stroke. J Biomed Sci. 2016;23:32. doi:10.1186/s12929-016-0249-0
23. Lu J, Shao RH, Jin SY, Hu L, Tu Y, Guo JY. Acupuncture ameliorates inflammatory response in a chronic unpredictable stress rat model of depression. Brain Res Bull. 2017;128:106–112. doi:10.1016/j.brainresbull.2016.11.010
24. Liu W, Wang X, Zheng Y, et al. Electroacupuncture inhibits inflammatory injury by targeting the miR-9-mediated NF-kappaB signaling pathway following ischemic stroke. Mol Med Rep. 2016;13(2):1618–1626. doi:10.3892/mmr.2015.4745
25. Lu J, Shao RH, Hu L, Tu Y, Guo JY. Potential antiinflammatory effects of acupuncture in a chronic stress model of depression in rats. Neurosci Lett. 2016;618:31–38. doi:10.1016/j.neulet.2016.02.040
26. Le DA, Wu Y, Huang Z, et al. Caspase activation and neuroprotection in caspase-3- deficient mice after in vivo cerebral ischemia and in vitro oxygen glucose deprivation. Proc Natl Acad Sci U S A. 2002;99(23):15188–15193. doi:10.1073/pnas.232473399
27. Lloyd-Jones D, Adams R, Carnethon M, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–e181. doi:10.1161/CIRCULATIONAHA.108.191261
28. Niizuma K, Yoshioka H, Chen H, et al. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim Biophys Acta. 2010;1802(1):92–99. doi:10.1016/j.bbadis.2009.09.002
29. Maes M, Galecki P, Chang YS, Berk M. A review on the oxidative and nitrosative stress (O&NS) pathways in major depression and their possible contribution to the (neuro)degenerative processes in that illness. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):676–692. doi:10.1016/j.pnpbp.2010.05.004
30. Yao PJ, Petralia RS, Mattson MP. Sonic hedgehog signaling and hippocampal neuroplasticity. Trends Neurosci. 2016;39(12):840–850. doi:10.1016/j.tins.2016.10.001
31. He W, Cui L, Zhang C, et al. Sonic hedgehog promotes neurite outgrowth of cortical neurons under oxidative stress: involving of mitochondria and energy metabolism. Exp Cell Res. 2017;350(1):83–90. doi:10.1016/j.yexcr.2016.11.008
32. Ji H, Miao J, Zhang X, et al. Inhibition of sonic hedgehog signaling aggravates brain damage associated with the down-regulation of Gli1, Ptch1 and SOD1 expression in acute ischemic stroke. Neurosci Lett. 2012;506(1):1–6. doi:10.1016/j.neulet.2011.11.027
33. McKechnie F, Lewis S, Mead G. A pilot observational study of the association between fatigue after stroke and C-reactive protein. J R Coll Physicians Edinb. 2010;40(1):9–12. doi:10.4997/JRCPE.2010.103
34. Rothenburg LS, Herrmann N, Swardfager W, et al. The relationship between inflammatory markers and post stroke cognitive impairment. J Geriatr Psychiatry Neurol. 2010;23(3):199–205. doi:10.1177/0891988710373598
35. Yang L, Zhang Z, Sun D, Xu Z, Zhang X, Li L. The serum interleukin-18 is a potential marker for development of post-stroke depression. Neurol Res. 2010;32(4):340–346. doi:10.1179/016164110X12656393665080
36. Dai RL, Zhu SY, Xia YP, et al. Sonic hedgehog protects cortical neurons against oxidative stress. Neurochem Res. 2011;36(1):67–75. doi:10.1007/s11064-010-0264-6
37. Hu Q, Li T, Wang L, et al. Neuroprotective effects of a smoothened receptor agonist against early brain injury after experimental subarachnoid hemorrhage in rats. Front Cell Neurosci. 2016;10:306. doi:10.3389/fncel.2016.00018
38. Amankulor NM, Hambardzumyan D, Pyonteck SM, Becher OJ, Joyce JA, Holland EC. Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J Neurosci. 2009;29(33):10299–10308. doi:10.1523/JNEUROSCI.2500-09.2009
39. Brilha S, Ong CWM, Weksler B, Romero N, Couraud PO, Friedland JS. Matrix metalloproteinase-9 activity and a downregulated hedgehog pathway impair blood-brain barrier function in an in vitro model of CNS tuberculosis. Sci Rep. 2017;7(1):16031. doi:10.1038/s41598-017-16250-3
40. Zhen H, Zhao L, Ling Z, Kuo L, Xue X, Feng J. Wip1 regulates blood-brain barrier function and neuro-inflammation induced by lipopolysaccharide via the sonic hedgehog signaling signaling pathway. Mol Immunol. 2018;93:31–37. doi:10.1016/j.molimm.2017.09.020
41. Singh VB, Singh MV, Piekna-Przybylska D, Gorantla S, Poluektova LY, Maggirwar SB. Sonic hedgehog mimetic prevents leukocyte infiltration into the CNS during acute HIV infection. Sci Rep. 2017;7(1):9578. doi:10.1038/s41598-017-10241-0
42. Singh VB, Singh MV, Gorantla S, Poluektova LY, Maggirwar SB. Smoothened agonist reduces human immunodeficiency virus type-1-induced blood-brain barrier breakdown in humanized mice. Sci Rep. 2016;6:26876. doi:10.1038/srep19082
43. Alvarez JI, Dodelet-Devillers A, Kebir H, et al. The Hedgehog pathway promotes blood-brain barrier integrity and CNS immune quiescence. Science. 2011;334(6063):1727–1731. doi:10.1126/science.1206936
44. Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301(5634):805–809. doi:10.1126/science.1083328
45. Jin K, Minami M, Lan JQ, et al. Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci U S A. 2001;98(8):4710–4715. doi:10.1073/pnas.081011098
46. Zhu DY, Liu SH, Sun HS, Lu YM. Expression of inducible nitric oxide synthase after focal cerebral ischemia stimulates neurogenesis in the adult rodent dentate gyrus. J Neurosci. 2003;23(1):223–229. doi:10.1523/JNEUROSCI.23-01-00223.2003
47. Wang SH, Zhang ZJ, Guo YJ, Teng GJ, Chen BA. Hippocampal neurogenesis and behavioural studies on adult ischemic rat response to chronic mild stress. Behav Brain Res. 2008;189(1):9–16. doi:10.1016/j.bbr.2007.11.028
48. Lai K, Kaspar BK, Gage FH, Schaffer DV. Sonic hedgehog regulates adult neural progenitor proliferation in vitro and in vivo. Nat Neurosci. 2003;6(1):21–27. doi:10.1038/nn983
49. Vivar C, Potter MC, Choi J, et al. Monosynaptic inputs to new neurons in the dentate gyrus. Nat Commun. 2012;3:1107. doi:10.1038/ncomms2101
50. Machold R, Hayashi S, Rutlin M, et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron. 2003;39(6):937–950. doi:10.1016/S0896-6273(03)00561-0
51. Roca M, Vives M, Lopez-Navarro E, Garcia-Campayo J, Gili M. Cognitive impairments and depression: a critical review. Actas Esp Psiquiatr. 2015;43(5):187–193.
52. Lee SH, Choi BY, Kim JH, et al. Late treatment with choline alfoscerate (l-alpha glycerylphosphorylcholine, alpha-GPC) increases hippocampal neurogenesis and provides protection against seizure-induced neuronal death and cognitive impairment. Brain Res. 2017;1654(Pt A):66–76. doi:10.1016/j.brainres.2016.10.011
53. Sun JH, Tan L, Yu JT. Post-stroke cognitive impairment: epidemiology, mechanisms and management. Ann Transl Med. 2014;2(8):80.
54. Shprakh VV, Suvorova IA. [Post-stroke vascular dementia: risk factors and clinical neuro-imaging features]. Adv Gerontol. 2010;23(2):293–300.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.