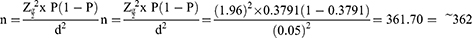Back to Journals » Journal of Blood Medicine » Volume 14
Anticoagulation Management in Patients Receiving Warfarin at Private Cardiac Centers in Addis Ababa, Ethiopia
Authors Getachew R, Tadesse TA , Shashu BA, Degu A , Alemkere G
Received 11 November 2022
Accepted for publication 1 February 2023
Published 10 February 2023 Volume 2023:14 Pages 107—117
DOI https://doi.org/10.2147/JBM.S397189
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Martin H Bluth
Roza Getachew,1 Tamrat Assefa Tadesse,1 Bekele Alemayehu Shashu,2 Amsalu Degu,3 Getachew Alemkere1
1Department of Pharmacology and Clinical Pharmacy School of Pharmacy, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 2Department of Internal Medicine School of Medicine, College of Health Sciences, Addis Ababa University, Addis Ababa, Ethiopia; 3Department of Pharmaceutics and Pharmacy Practice, School of Pharmacy & Health Sciences, United States International University Africa, Nairobi, Kenya
Correspondence: Tamrat Assefa Tadesse, Email [email protected]
Introduction: Anticoagulants are the cornerstone therapy for thromboembolism prevention and treatment. Warfarin is the frequently prescribed drug and remains the oral anticoagulant of choice in low- and middle-income countries, including Ethiopia. It is a narrow therapeutic index drug that needs high-quality anticoagulation monitoring with frequent international normalization ratio (INR) testing.
Objective: The study aimed to assess anticoagulation management with warfarin among adult outpatients at two selected private cardiac centers in Addis Ababa, Ethiopia.
Methods: A hospital-based retrospective study design that enrolled 374 patients receiving warfarin was employed at two private cardiac centres in Addis Ababa, Ethiopia. The time in the therapeutic range (TTR) was calculated using the Rosendaal method. The data were analyzed using Statistical Package for Social Science version 25.
Results: The mean age of the patients was 57 years, and 218 (58.3%) participants were females. Out of 3384 INR tests, 1562 (46.5%) were within the therapeutic range and the mean percentage of TTR was 47.24%. Only 25.67% of the patients spent their TTR ≥ 65%. The present study revealed that dose adjustments were required 1764 times. In non-therapeutic INR values of 1764 that required warfarin dose adjustment, 59.7% of the doses were adjusted. About 262 (70.1%) of co-prescribed medications had interaction with warfarin. Sixty-four patients (17.11%) experienced bleeding events.
Conclusion: Anticoagulation management with warfarin was suboptimal in private cardiac Addis Ababa, Ethiopia, private cardiac centers. Warfarin adjustment practice for nontherapeutic INR values was not minimal, and many patients encountered bleeding during their course of therapy.
Keywords: warfarin, anticoagulation, time in the therapeutic range, international normalization ratio, Ethiopia
Introduction
The safety and efficacy of warfarin therapy depend on careful monitoring and maintenance of the international normalized ratio (INR) within an optimal therapeutic range.1 Its narrow therapeutic range, frequent drug, herbal, and food interactions, and the effect of comorbidities place patients at risk of bleeding and thromboembolic complications if the recommended anticoagulation target ranges are not achieved.2–7 Furthermore, factors related to drugs, patients, and healthcare will compromise the overall quality of anticoagulation management with warfarin therapy which is reflected by actual time spent within the therapeutic INR, ie, time in the therapeutic range (TTR).3,8–10 The TTR estimates the percentage of time a patient’s INR is within the desired treatment range or goal and is widely used as an indicator of anticoagulation control.11
A minimum TTR of 65% is required for warfarin therapy to be regarded as effective,12,13 and below this value, warfarin is unlikely to prevent thromboembolic disease effectively. In another way, the risk of both thromboembolism and bleeding has also been shown to depend on the percentage of INR in the therapeutic range.14 However, in the real world, maintaining adequate anticoagulation with warfarin has proved to be challenging due to the complexity of warfarin therapy. This was demonstrated by studies conducted globally with low TTR (<65%).1,11,15–23 The problem seems the worst in Africa, as the lowest TTRs were reported in different regions of the continent.7,20,24–27 Similarly, in Ethiopia, only four studies were conducted in government health facilities and reported lower TTRs in the range of 29–38%.28–31 Despite the wide availability of warfarin as an oral anticoagulant, information on its level of anticoagulation control is scarce in Ethiopia, especially in private settings. Therefore, this study aimed to determine the proportion of time spent in the therapeutic INR range and investigate dose adjustment practice in patients taking warfarin in selected private health facilities in Addis Ababa, Ethiopia.
Methods
Study Setting
The study was conducted at two private cardiac centers in Addis Ababa, Ethiopia, namely Gesund Cardiac and Medical Center (GCMC) and Addis Cardiac Hospital (ACH). GCMC is a 31-bed cardiac center; annually, it serves around 41,500 patients and about 2000 of them receive warfarin. ACH is Ethiopia’s first specialized cardiovascular hospital with advanced investigations and treatment modalities for cardiac diseases, including heart surgery, implantable devices, and percutaneous coronary intervention. The hospital has 43 beds and serves 51,700 patients annually. Among these, 1683 patients were receiving warfarin therapy during our study period.
Study Design and Period
A retrospective study was carried out at ACH and GCMC from 1 February to 30 April 2021 on patients receiving warfarin.
Source and Study Population
All adult patients receiving warfarin for 3 months and above for various indications at study centers were the source population. All eligible patients on warfarin who had follow-ups at ACH and GCMC from 1 January 2019 to 31 December 2020 (2 years) were considered as the study population.
Sample Size Determination and Sampling Techniques
The sample size was determined using the single population proportion formula,32 ie,
where n = minimum sample size, Z = standard normal distribution (Zα/2=1.96) with 95% confidence interval, d = absolute precision or tolerable margin of error (d) = 5% (0.05) and P = prevalence is essential proportion). From a recent previous study in Northern Ethiopia, a proportion of 0.3791, ie, a mean TTR of 37.91%33 was used. With a 10% contingency to cater to incomplete medical records, the final sample size was 398. Finally, we reviewed 374 patients’ charts (187 from each site) that fulfilled the inclusion criteria during the study period. A 2-year list of hospital identification numbers of warfarin-treated patients was generated from the health record. The actual sampling fraction (kth) was calculated by dividing the total number of the study population attending the hospital during the study period (3671) by the estimated sample size of the study. Thus, approximately every 9 patients’ chart was reviewed.
Eligibility Criteria
Adult patients (≥18 years) who had been on warfarin for ≥3 months with two or more INR values were included in the study. Nonetheless, medical records of patients with incomplete information about the INR values and other pertinent findings were excluded from the study.
Data Collection Instruments and Techniques
The data abstraction instrument was prepared by reviewing different literature. The data were collected by three pharmacists and one nurse after providing relevant training. Then, all the required pieces of information such as demographic characteristics, indications of anticoagulation, comorbidities, drugs prescribed with warfarin, and corresponding INR results were recorded by reviewing the medical records of the patients. The data abstraction tool had been pretested on 5% of the study participants before actual data collection. All relevant amendments were made after pretesting the data abstraction tool. Percentages of TTR were calculated using the Rosendaal method,34 and the mean frequency of INR values and percentage of warfarin dose adjusted were determined.
Data Analysis and Interpretation
Data were analyzed using Statistical Package for Social Science (SPSS) version 25.0 software. Socio-demographic and clinical characteristics were summarized by percentages and frequencies. Micromedex Healthcare Series [online database] Version 5.135 was used to analyze drug interactions with warfarin.
Ethical Considerations
Ethical clearance was obtained from the Ethics Review Committee of the School of Pharmacy, College of Health Sciences of Addis Ababa University (ERB/SOP/244/13/2021). This study complies with the guidelines of the Declaration of Helsinki. Confidentiality of the information was maintained by avoiding the use of individual identifiers like names and the patients’ hospital identification numbers.
Results
The mean age of the study participants was 57± 18 years, with an age range of 18–92 years. The majority of the study participants were female (218, 58.3%). Heart failure (36.89%) and hypertension (17.2%) were the most common comorbidities in patients receiving warfarin in this study. Polypharmacy was seen in 51.34% of the patients (Table 1).
 |
Table 1 Socio-Demographic and Clinical Characteristics of Patients |
Indication of Warfarin
The most common indication of warfarin was for atrial fibrillation patients, followed by chronic rheumatic valvular heart disease (Figure 1).
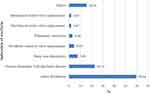 |
Figure 1 Indication of warfarin therapy. |
INR Distribution
During 2-year follow-up period, of 374 patients, 88.51% and 11.49% had target INR ranges of 2–3 and 2.5–3.5 with 1477 and 85 INR values within respective target ranges, respectively. The average number of INR tests per patient was 4. The detailed INR distribution is shown in Figure 2.
 |
Figure 2 INR values distributions within different intervals among patients taking warfarin for achieving target ranges (A :2.0−3.0, B: 2.5−3.5). |
Time in the Therapeutic Range
According to the Rosendaal method, the mean percentages of TTR were 47.24% (Figure 3), and only 25.67% of the patients spent their time above TTR of 65%.
 |
Figure 3 Time spent in different INR ranges in patients taking warfarin. |
Warfarin Dose Adjustment
This study revealed that dose adjustments were required 1764 times for nontherapeutic INR values. However, it was only adjusted 1053 times (873 for sub-therapeutic and 180 times for the supratherapeutic range). The details for warfarin dose adjustment for non-therapeutic INR values are given in Table 2.
 |
Table 2 Warfarin Dose Adjustment Practice for Non-Therapeutic INRs in Patients Receiving Warfarin |
Drug–Drug Interactions Among Patients Taking Warfarin
About 262 (70.0%) patients were prescribed medications with the potential of interacting with warfarin. Furosemide (89.84%), spironolactone (72.9%) and metoprolol succinate (63.63%) were the most repeatedly observed drugs that interact with warfarin in the present study. Only major and moderate interactions were reported based on the Micromedex interaction checker (Table 3).
 |
Table 3 Warfarin Drug Interactions Among Patients Taking Warfarin |
Bleeding Events
In this study, 64 (17.11%) patients encountered minor and major bleeding events. Of these, 42 patients experienced minor bleeding events (nasal and vaginal bleeding) with therapeutic INR value. Seven patients had INR values of 3.1–4.99, while 11 patients had INR values greater than 5, and 4 patients experienced intracranial hemorrhages with an INR value of >5.
Discussion
This study aimed to assess anticoagulation management with warfarin at private health facilities in Addis Ababa, Ethiopia. The majority of the study participants (58.3%) were females, and the mean age was 57 years. This female predominance was comparable to the study done in Kenya,36 and Ethiopia.29,37,38 In contrast, other studies conducted in Poland39 and Canada16 reported male predominance. Atrial fibrillation (43.85%) was the commonest indication for warfarin therapy in the current study.
The duration of time a patient is on oral anticoagulant influences the stability of INR. Moreover, dietary habits and the presence of drug interaction may alter INR levels associated with warfarin treatment.40 This study revealed that the mean frequency of INR monitoring per patient was every 47.85 days. The frequency of INR monitoring obtained in this study (every 47.85 days) was more infrequent when compared with the study done in western Ethiopia, where most patients (84%) had INR determined every month.37 In addition, a study from Kenya showed that the median frequency of INR monitoring was 18.5 days.36 Another study in Ethiopia reported the interval between two INR tests was 35 days.41 Comparatively, there was more frequent monitoring of INR in the study settings as compared to reports from a tertiary care teaching hospital in Ethiopia (64 days per patient).38 These disparities could probably be linked to the difference in the patient size population and specific trends in the management of patients receiving warfarin therapy in the study settings, which can affect their capacity to monitor INR frequently. Furthermore, it is also noticed that warfarin monitoring is patient-tailored. However, current guidelines suggest that at least a 4-week monitoring is essential. This time should be reduced to a few days on several occasions including in the case of frequent non-therapeutic INRs and the occurrence of bleeding and thrombotic events.
The TTR (47.24%) obtained in this study was higher than reports from Lithuania (40%),42 Uganda (41%),43 Spain (25.9%),44 Ethiopia (29%),38 Pakistan (34.9%),45 Botswana (30.8%)46 and Northern Ethiopia (33%).37 However, far away from recommended optimal TTR (≥70%), optimal anticoagulation in patients receiving warfarin47 and also low as compared to previous studies in New Zealand (63%)48 and Brazil (56.6%).49 This difference could probably be the higher level of health care in developing countries that had better anticoagulation monitoring services. In addition, this poor TTR in the study setting may be due to infrequent INR monitoring and a lack of separate anticoagulation clinics. The present study showed that 1764 INR tests were deemed to have warfarin dose adjustment. Nonetheless, doses were adjusted in only 1053 (59.69%) values. This can probably be linked to physicians’ adherence problems, lack of functional dose adjustment algorithm, warfarin drug–drug interaction, and drug–food interactions that affect warfarin serum levels. However, in the study settings, the management of nontherapeutic (sub-therapeutic) INR was better than in previous studies conducted in government hospitals in Ethiopia,29,38 although the management of nontherapeutic INR value was not satisfactory.
The most commonly prescribed warfarin-interacting drugs were furosemide, followed by spironolactone and metoprolol succinate in the study settings. The prevalence of drug–drug interactions with warfarin was found to be 70%. This result was comparable with a study conducted in Thailand that identified 71% warfarin drug interaction,1 but it is better than that of Ayder Referral Hospital, Ethiopia (99.2%).50 Other studies at Tertiary Care Hospitals in Ethiopia identified a lower percentage of warfarin drug interaction.41,51 This high percentage of drug–drug interaction with warfarin may be justified by the high prevalence of co-prescribed interacting medications.
The most common complication of warfarin therapy was bleeding. This study reveals 64 (17.11%) minor and major (intracranial hemorrhage) bleeding events, which were lower in incidence as compared with studies from Ethiopia (25%) and Thailand (32.56%).52,53 However, a retrospective study in Brazil reported comparable bleeding events (4.2% major and 10.3% minor bleeding episodes) with the present study.49
Limitations of the Study
Due to the retrospective nature of the study, the data will be affected by the completeness and accuracy of the information in the study settings. Therefore, it is difficult to clarify some problems in definite detail, such as the cause of non-adherence or the type of herbs that interact with warfarin, factors that may affect anticoagulation like adherence, diet, physical activity, and genetic variability. There was a risk of information bias as this retrospective study relied on information from patient files. It is also prone to missing patient information if they had sought other health facilities that were not documented.
Conclusion
Anticoagulation management with warfarin was suboptimal in the study settings, which can significantly affect the desired treatment outcomes of the patients. In addition, most of the co-prescribed medications had undesired drug–drug interactions with warfarin and need attention. Therefore, optimal interventional strategies are required to improve the treatment outcomes of patients receiving warfarin therapy.
Abbreviations
ACH, Addis Cardiac Hospital; AF, atrial fibrillation; DVT, deep vein thrombosis; INR, GCMC, Gesund Cardiac and Medical Center; INR, international normalization ratio; SPSS, Statistical Package for Social Science; TTR, time in therapeutic range; VTE, venous thromboembolism.
Data Sharing Statement
The datasets used in this study are available from the corresponding author upon request.
Declarations Ethics Approval and Consent to Participate
This study was approved by the Ethical Review committee of the School of Pharmacy, College of Health Sciences Addis Ababa University, Addis Ababa, Ethiopia (ERB/SOP/244/13/2021). Then, a support letter was written by the Department of Pharmacology and Clinical Pharmacy, School of Pharmacy to GCMC and ACH, and permission was obtained from the study settings to conduct the study. Information obtained from data collected during the study was only handled by the research team. Personal identifiers were not used, and data were analyzed in aggregate. The Ethics Committee waived the need for informed consent since this was an observational study using existing data.
Acknowledgments
The authors would like to acknowledge the staff members of ACH and GCMC healthcare facilities for their unreserved support during the entire data collection process for this project.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis, and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no competing interests.
References
1. Albabtain MA, Alharthi MM, Dagriri K., et al. Assessment of the quality of anticoagulation management with warfarin in a tertiary care center. Saudi Med J. 2020;41(11):1245–1251. doi:10.15537/smj.2020.11.25456
2. Laäs DJ, Naidoo M. An evaluation of warfarin use at an urban district-level hospital in KwaZulu-Natal Province, South Africa. South Af Med J. 2018;108(12):1046–1050. doi:10.7196/SAMJ.2018.v108i12.13256
3. Witt DM, Nieuwlaat R, Clark NP, et al. American Society of Hematology 2018 guidelines for the management of venous thromboembolism: optimal management of anticoagulation therapy. Blood Adv. 2018;2(22):3257–3291. doi:10.1182/bloodadvances.2018024893
4. Zeitler EP, Eapen ZJ, Clinical D, Nc D. Anticoagulation in heart failure: a review. J Atrial Fibrillation. 2015;8(1). doi:10.4022/jafib.1250
5. Di MA, Frigerio B, Spadarella G, et al. Old and new oral anticoagulants: food, herbal medicines and drug interactions. Blood Rev. 2017;31(4):193. doi:10.1016/j.blre.2017.02.001
6. Fibrillation A, Nelson WW, Choi JC, et al. Impact of co-morbidities and patient characteristics on international normalized ratio control over time in patients with nonvalvular. Am J Cardiol. 2013;112(4):509–512. doi:10.1016/j.amjcard.2013.04.013
7. Karuri S, Nyamu D, Opanga S, Menge T. Factors associated with time in therapeutic range among patients on oral anticoagulation therapy in a tertiary teaching and referral hospital in Kenya. East Cent Af J Pharm Sci. 2019;22(3):85–95.
8. Alghadeeer S, Alzahrani AA, Alalayet WY, Alkharashi AA, Alarifi MN. Anticoagulation control of warfarin in pharmacist-led clinics versus physician-led clinics: a prospective observational study. Risk Manag Healthc Policy. 2020;13:1175–1179. doi:10.2147/RMHP.S248222
9. Mwita JC, Francis JM, Oyekunle AA, Gaenamong M, Goepamang M, Magafu MGMD. Quality of anticoagulation with warfarin at a tertiary hospital in Botswana. Clin Appl Thrombosis Hemostasis. 2018;24(4):596–601. doi:10.1177/1076029617747413
10. Gabriel P, De BM, Sznejder H, et al. Anticoagulation therapy in patients with non-valvular atrial fibrillation in a private setting in Brazil: a real-world study. Arq Bras Cardiol. 2020;114:457.
11. Farsad B, Abbasinazari M, Dabagh A, Bakshandeh H. Evaluation of time in therapeutic range (TTR) in patients with non-valvular atrial fibrillation receiving treatment with warfarin in Tehran, Iran: a cross-sectional study. J Clin Diagnostic. 2016;20(9):20–22. doi:10.7860/JCDR/2016/21955.8457
12. Prinsloo DN, Gould TJ, Viljoen CA, Basera W, Ntsekhe M. International normalised ratio control in a non-metropolitan setting in Western Cape Province, South Africa. South African Med J. 2021;111(4):355–360. doi:10.7196/SAMJ.2021.v111i4.15171
13. Pastori D, Farcomeni A, Saliola M, Del F, Pignatelli P. Temporal trends of time in therapeutic range and incidence of cardiovascular events in patients with non-valvular atrial fibrillation. Eur J Intern Med. 2018;1. doi:10.1016/j.ejim.2018.04.007
14. Ntlokotsi S, Moshesh MF, Mntla P, Towobola OA, Mogale MA. Optimum INR intensity and therapeutic INR control in patients with mechanical heart valve prosthesis on warfarin oral anticoagulation at Dr George Mukhari academic hospital: a three-year retrospective study. South African Family. 2018;60(6):192–196.
15. Caldeira D, Cruz I, Morgado G, et al. Evaluation of time in therapeutic range in anticoagulated patients: a single-center, retrospective, observational study. Revista Portuguesa de Cardiologia. 2014;34(1):1–5. doi:10.1016/j.repc.2014.08.005
16. Gateman D, Trojnar ME, Agarwal G, Gateman D, Trojnar ME, Agarwal G. Time in therapeutic range Un RIN dans la fourchette thérapeutique. Canadian Family Phys. 2017;63:425–431.
17. Chan P. Time in therapeutic range and percentage of inrs in therapeutic range as measure of quality of anticoagulation control in atrial fibrillation patients. Can J Cardiol. 2015;32(10):1247.e23–1247.e28. doi:10.1016/j.cjca.2015.10.029
18. Han SY, Palmeri ST, Broderick SH, et al. Quality of anticoagulation with warfarin in patients with nonvalvular atrial fibrillation in the community setting. J Electrocardiol. 2013;46(1):45–50. doi:10.1016/j.jelectrocard.2012.08.011
19. Botsile E, Mwita JC. Incidence and risk factors for thromboembolism and major bleeding in patients with mechanical heart valves: a tertiary hospital-based study in Botswana. Cardiovasc J Afr. 2020;31(4):185–189. doi:10.5830/CVJA-2020-006
20. Jonkman LJ, Gwanyanya MP, Kakololo MN, Verbeeck RK, Singu BS. Assessment of anticoagulation management in outpatients attending a warfarin clinic in Windhoek, Namibia. Drugs Ther Perspect. 2019;35(7):341–346. doi:10.1007/s40267-019-00630-y
21. Ugur A, Turk O, Tuncer E, Alioglu E, Yuksel K. Evaluation of the impact of warfarin s time-in-therapeutic range on outcomes of patients with atrial fibrillation in Turkey: perspectives from the Observational, Prospective WATER Registry. Cardiology J. 2015;1:548. doi:10.5603/CJ.a2015.0035
22. Quinn LM, Richardson R, Cameron KJ, Battistella M. Evaluating time in therapeutic range for hemodialysis patients taking warfarin. Clin Nephrol. 2015;83(2):80–85. doi:10.5414/CN108400
23. de Castro KP. A Patient decision aid for anticoagulation therapy in patients with nonvalvular atrial fibrillation: development and pilot study. JMIR Cardio. 2021;5(2):e23464. doi:10.2196/23464
24. Sonuga BO, Hellenberg DA, Cupido CS, Jaeger C. Profile and anticoagulation outcomes of patients on warfarin therapy in an urban hospital in Cape Town, South Africa. Af J Prim Heal Care Fam Med. 2016;8(1):e1–e8. doi:10.4102/phcfm.v8i1.1032
25. Ouali S, Ben Halima A, Chabrak S, et al. Epidemiological characteristics, management, and outcomes of atrial fibrillation in Tunisia: results from the National Tunisian Registry of Atrial Fibrillation (NATURE-AF). Clin Cardiol. 2021;44(4):501–510. doi:10.1002/clc.23558
26. Mariita K, Nyamu DG, Maina CK, Karimi PN. Patient factors impacting on oral anticoagulation therapy among adult outpatients in a Kenyan referral hospital. Af J Pharmacol Therapeutics. 2016;1:548.
27. Sadhabariss D, Brown SL. Warfarin: time in therapeutic range, a single centre study on patients using warfarin for stroke prevention in non-valvular atrial fibrillation and prosthetic heart valves. SA Hear. 2021;18(1):28–38. doi:10.24170/18-1-4771
28. Fenta TG, Assefa T, Alemayehu B. Quality of anticoagulation management with warfarin among outpatients in a tertiary hospital in Addis Ababa, Ethiopia: a retrospective cross-sectional study. Af J Pharmacol. 2017:1–7. DOI:10.1186/s12913-017-2330-0
29. Yimer NS, Abiye AA, Hussen SU, Tadesse TA. Anticoagulation control, outcomes, and associated factors in patients with atrial fibrillation receiving warfarin at tertiary care hospital in Ethiopia. Clin Appl Thromb. 2021;27:107602962110497. doi:10.1177/10760296211049786
30. Liyew Z, Tadesse A, Bekele N, Tsegaye T. Evaluation of anticoagulation outcome among patients taking warfarin: a single-center experience, Northwest Ethiopia. Res Sq. 2017;20–25.
31. Masresha N, Muche EA, Atnafu A, Abdela O. Evaluation of warfarin anticoagulation at University of Gondar comprehensive specialized. J Blood Med. 2021;12:189–195. doi:10.2147/JBM.S282948
32. Pourhoseingholi MA, Vahedi M, Rahimzadeh M. Sample size calculation in medical studies. Gastroenterol Hepatol Bed Bench. 2013;6(1):14–17.
33. Masresha N, Muche EA, Atnafu A, Abdela O. Evaluation of warfarin anticoagulation at University of Gondar comprehensive specialized hospital, north-west Ethiopia. J Blood Med. 2021;12:189–195. doi:10.2147/JBM.S282948
34. Rosendaal FR, Cannegieter SC, Van der Meer FJM, Briet E. A method to determine the optimal intensity of oral anticoagulant therapy. Thromb Haemost. 1993;69(3):236–239. doi:10.1055/s-0038-1651587
35. Thomson Micromedex. Micromedex Healthcare Series [Intranet Database]. Version 5.1. Greenwood Village, Colo: Thomson Micromedex; 2005.
36. Karuri WS. Quality of Oral Anticoagulation Management Among Patients on Follow Up At Kenyatta National Hospital. University of Nairobi; 2016.
37. Liyew Z. Evaluation of Anticoagulation Outcome among Patients Taking Warfarin: a Single-Center Experience, Northwest Ethiopia. Adv Hematol. 2021;2021:5489.
38. Fenta TG, Assefa T, Alemayehu B. Quality of anticoagulation management with warfarin among outpatients in a tertiary hospital in Addis Ababa, Ethiopia: a retrospective cross-sectional study. BMC Health Serv Res. 2017;17(1):1–7. doi:10.1186/s12913-017-2330-0
39. Ciurus T, Cichocka-Radwan A, Lelonek M. Factors affecting the quality of anticoagulation with warfarin: experience of one cardiac centre. Kardiochirurgia I Torakochirurgia Pol. 2015;12(4):334–340. doi:10.5114/kitp.2015.56784
40. McAlister FA, Wiebe N, Hemmelgarn BR. Time in therapeutic range and stability over time for warfarin users in clinical practice: a retrospective cohort study using linked routinely collected health data in Alberta, Canada. BMJ Open. 2018;8(1):1–9. doi:10.1136/bmjopen-2017-016980
41. Yimer NS, Abiye AA, Hussen SU, Tadesse TA. Anticoagulation control, outcomes, and associated factors in patients with atrial fibrillation receiving warfarin at tertiary care hospital in Ethiopia. Clin Appl Thromb. 2021;2:27. doi:10.1177/10760296211049786
42. Health P, Setting C. The quality of anticoagulation therapy among warfarin-treated patients with atrial fibrillation in a primary health care setting. Medicina. 2019;55:1–11. doi:10.3390/medicina55010015
43. Lertsanguansinchai P, Huntrakul A, Rungpradubvong V. Factors predicting poor anticoagulant control on warfarin in a Thai population with non ‑ valvular atrial fibrillation (NVAF): the ACAChE score. Int J Arrhythmia. 2021;22(1). doi:10.1186/s42444-021-00038-8
44. Sa MA. Quality of vitamin K antagonist anticoagulation in spain: prevalence of poor control and associated factors. Revista Española de Cardiología. 2015;68(9):761–768.
45. Hakeem H. Evaluation of quality of warfarin therapy by assessing patient s time in therapeutic range at a tertiary care hospital in Pakistan. JPMA. 2018;68(9):1339–1344.
46. Mwita JC, Francis JM, Oyekunle AA, Gaenamong M, Goepamang M, Magafu MGMD. Quality of anticoagulation with warfarin at a tertiary hospital in Botswana. Clin Applied Thromb. 2017;1:596. doi:10.1177/1076029617747413
47. Task A, Members F, Hindricks G, et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS) The Task Force for the diagnosis and management of atrial fibrillation of the Europe. Clin Applied Thromb. 2020;373–498.
48. McAuliffe GN, De Silva F, Upton A, Chan G. International normalised ratio monitoring in the community populations of the Auckland and Northland regions of New Zealand: time in therapeutic range and frequency of testing. Int Med J. 2018;48(12):1487–1491. doi:10.1111/imj.14032
49. de Barros e Silva PGM, Sznejder H, Vasconcellos R, et al. Anticoagulation therapy in patients with non-valvular atrial fibrillation in a private setting in Brazil: a real-world study. Arq Bras Cardiol. 2020;114(3):457–466. doi:10.36660/abc.20180076
50. Teklay G, Shiferaw N, Legesse B, Bekele ML. Drug-drug interactions and risk of bleeding among inpatients on warfarin therapy: a prospective observational study. Thrombosis J. 2014;12(1):1–8. doi:10.1186/1477-9560-12-20
51. Tadesse TA, Woldu MA. Prevalence of warfarin drug interaction and warfarin education practice in outpatient setups of university teaching hospital. J Basic Clin Pharm. 2018;262–266.
52. Liyew Z, Tadesse A, Bekele N, Tsegaye T. Evaluation of anticoagulation control among patients taking warfarin in University of Gondar Hospital, Northwest Ethiopia. Adv Hematol. 2021;2021:1–8. doi:10.1155/2021/7530997
53. Feungfu L. Evaluation of time in therapeutic range among patients receiving warfarin therapy: a retrospective cohort study at one private hospital in Thailand. Int J Med. 2021;48(2):107–114.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.