Back to Journals » International Journal of Nanomedicine » Volume 19
Anticancer Mechanisms and Potential Anticancer Applications of Antimicrobial Peptides and Their Nano Agents
Authors Dong Z, Zhang X, Zhang Q, Tangthianchaichana J , Guo M, Du S, Lu Y
Received 18 October 2023
Accepted for publication 16 January 2024
Published 1 February 2024 Volume 2024:19 Pages 1017—1039
DOI https://doi.org/10.2147/IJN.S445333
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Ziyi Dong,1,2 Xinyu Zhang,1 Qing Zhang,1 Jakkree Tangthianchaichana,1,3 Mingxue Guo,1 Shouying Du,1 Yang Lu1
1Laboratory of Traditional Chinese Medicine, School of Chinese Materia Medica, Beijing University of Chinese Medicine, Beijing, People’s Republic of China; 2Research and Development Centre in Beijing, CSPC Pharmaceutical Group Limited, Beijing, People’s Republic of China; 3Chulabhorn International College of Medicine, Thammasat University, Pathum Thani, Thailand
Correspondence: Yang Lu; Shouying Du, Beijing University of Chinese Medicine, Number 11 East Section of the North Third Ring Road, Beijing, 100029, People’s Republic of China, Tel + 86-10-84738615, Email [email protected]; [email protected]
Abstract: Traditional chemotherapy is one of the main methods of cancer treatment, which is largely limited by severe side effects and frequent development of multi-drug resistance by cancer cells. Antimicrobial peptides (AMPs) with high efficiency and low toxicity, as one of the most promising new drugs to replace chemoradiotherapy, have become a current research hotspot, attracting the attention of worldwide researchers. AMPs are natural-source small peptides from the innate immune system, and certain AMPs can selectively kill a broad spectrum of cancer cells while exhibiting less damage to normal cells. Although it involves intracellular mechanisms, AMPs exert their anti-cancer effects mainly through membrane destruction effect; thus, AMPs also hold unique advantages in fighting drug-resistant cancer cells. However, the poor stability and hemolytic toxicity of peptides limit their clinical application. Fortunately, functionalized nanoparticles have many possibilities in overcoming the shortcomings of AMPs, which provides a huge prospect for better application of AMPs. In this paper, we briefly introduce the characteristics and different sources of AMPs, review and summarize the mechanisms of action and the research status of AMPs used as an anticancer therapy, and finally focus on the further use of AMPs nano agents in the anti-cancer direction.
Keywords: antimicrobial peptides, anticancer mechanisms, drug combination, anticancer application, peptide nano agents
Graphical Abstract:
Introduction
Cancer, as one of the most lethal diseases in the world, is still a serious threat to human survival.1 Chemotherapy alone or combined with surgery/radiotherapy plays an important role in improving the life expectancy of cancer patients. However, due to the lack of specificity of many chemotherapeutic drugs for cancer cells, they can also kill healthy cells that are in rapid proliferation phase while killing cancer cells, which may lead to serious side effects.2,3 Additionally, cancer cells are prone to develop resistance to chemotherapeutic drugs resulting from drug inactivation or efflux, target protein alteration, DNA damage repair, and signaling cascade alteration, which greatly limits the efficacy of such drugs.4,5 Therefore, it is urgent to find new potent drugs with high tumor selectivity.
In recent years, antimicrobial peptides (AMPs) have attracted the attention of researchers. AMPs are small-molecule peptides with positive charges and amphipathic properties.6 They are part of the innate immune defense system of organisms with multiple biological activities such as antibacterial activity, antifungal activity, anticancer activity, and immune system regulation.7,8 On the one hand, due to their positive charges and amphiphilic nature, AMPs can selectively bind to negatively charged cancer cell membranes through electrostatic attraction and form a transmembrane channel, destroying the integrity of the cell membrane, thereby killing cancer cells.9,10 Besides, AMPs can target various cellular processes within cancer cells, such as DNA and protein synthesis, protein folding, enzymatic activity, and cell wall synthesis.11,12 On the other hand, AMPs can be internalized into cancer cells via endocytosis mechanisms, leading to apoptosis and the inhibition of autophagy.13
At the same time, due to their unique membrane mechanism, AMPs do not easily induce drug resistance in tumor cells.14 Tumor multidrug resistance (MDR) is the main cause of tumor chemotherapy failure of tumor chemotherapy. The overexpression of P-glycoprotein (P-gp) in the tumor cell membrane is one of the main reasons for the formation of MDR.15 Fitly, P-gp participates in the formation of cell membranes and increases the negative charges on the surface of the tumor cell membrane, which is conducive to the combination of cationic AMPs and drug-resistant tumor cell membranes, thereby better exerting anti-cancer activity.16,17 Therefore, AMPs have great prospects for the treatment of drug-resistant tumors. In recent years, the use of AMPs alone or in combination with other drugs for cancer treatment has attracted extensive attention.18–20
Characteristics and Sources of Antimicrobial Peptides
Antimicrobial peptides (AMPs) are also called host defense peptides or antibiotic peptides. They are a type of biologically active peptides that exist widely in the biological world. AMPs are an important part of the innate immune defense system of almost every organism including bacteria, plants, insects, fish, amphibians, birds, and mammals.21,22 Representative AMP sequences from different sources are shown in Table 1.
 |
Table 1 AMP Sequences from Different Sources |
AMPs are small peptides that are usually composed of 10–100 amino acid residues, most of which are cationic.49,50 Therefore, AMPs are often referred to as cationic antimicrobial peptides (CAPs). The primary structure of most AMPs shares the following characteristics: N-terminal is rich in lysine, arginine, and other cationic amino acids; C-terminal is rich in alanine, valine, glycine, and other hydrophobic amino acids, taking up to 50% of the entire amino acid sequence.51 In addition, AMPs mainly have the following four secondary structures52,53 (as shown in Figure 1): 1. α-helical structure, such as as Melittin,54 Magainin;55 and Cecropins;56 3. Extended helices structure, which usually occurs in a linear peptide that is rich in proline and arginine or tryptophan residues, such as bovine-derived AMP (indolicidin)28 and Drosophila-derived AMP (drosocin);57 4. Loop structure, with which AMPs often contain an intramolecular disulfide bond at the C-terminus to form a loop, while the N-terminus is a linear structure, forming a cyclic chain structure, such as bovine neutrophils-derived AMP (bactenecin)58 and frog skin-derived AMP (brevinins).59 Among them, α-helical AMPs are the most studied type of AMPs. The α-helical structure is a nearly perfect hydrolipid amphiphilic structure, that is, one side of the longitudinal axis of the helix is a positively charged hydrophilic region, and the opposite side is a hydrophobic region. This amphiphilic structure is the key to the activity of AMPs.60,61
 |
Figure 1 Four secondary structures of antimicrobial peptides. |
Although AMPs have the above-mentioned common features, their sequences and activities are different. These peptides are produced in large quantities at the infection and/or inflammation sites of the organism, and can rapidly and broadly kill Gram-positive and Gram-negative bacteria, fungi, parasites, and certain viruses.62,63 In addition, AMPs also play an important role in the inflammatory response, immune activation, and wound healing.64,65 It is worth noting that increased studies have shown that many AMPs have significant selective anti-cancer activity66–68 and such AMPs can also be called anti-cancer peptides (ACPs). Table 269 gives examples of AMPs with different activities.
 |
Table 2 Representative Antimicrobial Peptides with Different Activities |
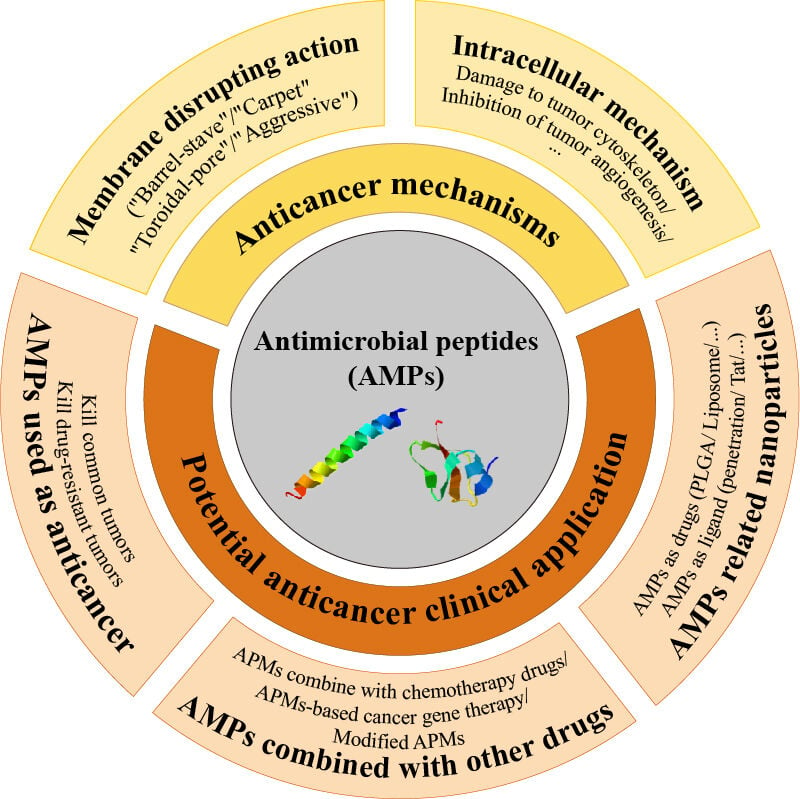
Anticancer Mechanisms of AMPs
The anti-cancer mechanism of AMPs can be divided into two types:84 selective membrane disruption action and non-membranolytic actions.85,86 The selective membrane disruption action is similar to its bactericidal action: the cationic AMPs directly interact with the cancer cell membrane through electrostatic attraction to form transient pores and destroy the integrity of the cell membrane and finally cause cell death.87 Besides, non-membranolytic actions of AMPs include the destruction of the cytoskeleton in cancer cells, inhibition of DNA and protein synthesis, inhibition of tumor angiogenesis, regulation of immunity, and induction of tumor cell apoptosis or necrosis. Each mechanism is not entirely separate, AMPs may show a complex anti-tumor mechanism. AMPs may first interact with the tumor cell membrane, enter into the cells and then act on the intracellular targets, or induce cell apoptosis or necrosis.88,89
Basis for Anticancer Action of AMPs: The Particularities of Cancer Cell Membranes
Similar to its antibacterial mechanism, almost all the AMPs can initially interact with the negatively charged cancer cell membranes, and the disruption of cancer cell membranes is considered to be the primary mechanism for the anticancer effect of AMPs, which determines the broad-spectrum anticancer effect of AMPs.90 Compared to normal cell membranes, the structural characteristics of tumor cell membranes are more conducive to AMPs to exert their anticancer effects. First of all, cancer cells, unlike healthy eukaryotic cells that contain zwitterionic phosphatidylcholine and sphingomyelin in their membranes and therefore overall neutrally charged,91 carry a net negative charge due to the elevated expression of anionic molecules such as phosphatidylserine (PS) and O-glycosylated mucins in their outer membrane leaflet,92,93 which results in a greatly enhanced capacity for electrostatic interactions with AMPs. The binding affinity of some AMPs to tumor cell membranes is 10 times higher than that of normal cells, showing selective tumor cytotoxicity.94,95 Furthermore, tumor cells have more abundant microvilli on their cell surface in comparison with normal cells, and the increased overall surface area of tumor cells allows a greater number of AMP molecules to interact with the surface of cancer cells, thereby promoting AMP-mediated cytotoxicity.96,97 Collectively, the aforementioned characteristics account for the ability of some AMPs to selectively kill tumor cells without damaging healthy cells.
In addition, many AMPs exhibit strong cytotoxicity on MDR tumor cell lines. Also, certain AMPs can reverse the drug-resistance of tumor cells, thereby increasing tumor sensitivity to chemotherapeutic drugs. One of the main reasons causing tumor MDR is the over-expression of P-gp. The over-expressed P-gp could increase the negative charge on the MDR tumor cell membrane, thus enhancing AMP binding by electrostatic attraction.98,99 Then, the hydrophobic C-terminal of AMP may insert into the hydrophobic region of the cell membrane, which may change the conformation of the cell membrane and reduce the activity of P-gp by inhibiting the efflux of chemotherapy drugs, increasing the intracellular drug concentration, and reversing drug resistance of MDR tumor cells.100
Recognized Models of Selective Membrane-Disrupting Mechanism
At present, scholars have proposed several models to explain the interaction between AMPs and tumor cell membranes from different perspectives (as shown in Figure 2) including “Barrel-stave” model, “Carpet” model, “Toroidal-pore” model, and “Aggregate” model. These models are not completely independent, that is, AMPs under different concentrations and different conditions may function through complex mechanisms.
“Barrel-Stave” Model
In the “Barrel-stave” model (Figure 2A), the monomers of α-helix cationic AMPs (such as cecropin B101 and bee venom peptide102) first bind to the tumor cell membrane by electrostatic attraction. Then, the conformation of AMPs changes, that is, AMPs perpendicularly insert their hydrophobic region into the hydrophobic core of the cell membrane and form a position like “staves” in a barrel. Thereafter, the AMP monomers will self-aggregate into the α-helix polymer with their hydrophilic side constituting the barrel-like hollow channel and insert into the hydrophobic membrane cores more deeply, forming transient transmembrane pores which may disrupt the ion gradients and transmembrane potential and eventually lead to cell death.103 It is worth noting that the “barrel-stave” model cannot explain the membrane disruption effect of AMPs with fewer than 23 amino acids as AMPs need to be long enough to pass through the cell membrane in this model.104
“Carpet” Model
In the carpet model (Figure 2B), the AMPs initially interact with the tumor cell membrane through electrostatic attraction and arrange parallel to the cell surface in a “carpet”-like manner, which interfered with the accumulation of phospholipids. Subsequently, the local membrane is destabilized due to the change in curvature stress and internal osmotic pressure. Finally, a circular gap will be formed, and the cell membrane will be disintegrated. During this process, the AMPs insert into the hydrophobic core of the lipid bilayer.91 Studies suggested that the AMP magainin worked through the “carpet” model at low concentrations, while worked through a “barrel plate” model at high concentrations.105,106
“Toroidal-Pore” Model
In the “Toroidal-pore” model (Figure 2C), AMPs also originally bind to the cell membrane through electrostatic attraction and then induce continuous bending of the lipid monolayer with the hydrophilic group of AMPs interacting with the hydrophilic head of the cell membrane and then spiral insertion into the cell membrane, forming transmembrane pores.107 Different from the “barrel-stave” model, the hydrophilic parts of AMPs and hydrophilic heads of the upper and lower lipids always together face to the center of the pores and the AMPs are unnecessary to pass through the whole phospholipid bilayer; thus, this model can explain the effect of some short AMPs.104 Studies have shown that AMPs such as melittin103,108 and protegrin-1109 work through the “Toroidal-pore” model.
“Aggregate” Model
Similar to the “Toroidal-pore” model, in the “Aggregate” model (Figure 2D), the AMPs are first electrostatically attracted to the tumor cell membrane. Then, the AMPs combine with lipids to form micelle complexes, and the aggregates will insert into the cell membrane, forming dynamic transmembrane pores. The difference with the “Toroidal-pore” model is that the AMPs working through the “Aggregate” model have no specific orientation and there is usually only one AMP located in the center of the aggregate.110,111
Non-Membrane Disrupting Mechanisms of AMPs
Although the electrostatic interaction with tumor cell membrane and further disrupting action is the key of AMPs to kill the tumor cells, certain AMPs are accompanied by other mechanisms, such as inhibiting tumor DNA synthesis, destroying tumor cytoskeleton, inhibiting tumor angiogenesis, destroying tumor mitochondria, inducing tumor cell apoptosis or necrosis, and regulating immunity, as shown in Figure 3.
Inhibition and Destruction of Chromosomes and DNA in Cancer Cells
Some AMPs may accumulate in the nucleus area of cancer cells and kill cancer cells by breaking the tumor DNA, thus playing an anti-cancer role.112 For example, the AMP named Cecropin-XJ isolated from Xinjiang silkworm was found to have the ability to combine with the groove of tumor cell DNA and then insert into the DNA sequence, resulting in the disordered arrangement of base pairs and looseness of DNA double helix structure, leading to DNA damage and cell death.113 In addition, using a single-cell gel electrophoresis technique, Wang et al114 found that the CM4-component of the AMP from Bombyx mori could selectively break the DNA of K562 cancer cells and kill cells without affecting the nuclear chromatin DNA of normal human leukocytes.
Damage to Cytoskeleton in Cancer Cells
The cell cytoskeleton is a fine network structure that can not only maintain cell morphology and maintain the order of cell internal structure but also participate in important life activities such as cell movement, material transportation, information transmission, cell division, and gene expression. There is a highly developed cytoskeleton system in eukaryotic cells with microfilaments and microtubules as important components of the cytoskeleton. However, the cytoskeleton of cancer cells is underdeveloped with a reduced number of microfilaments and microtubules.115 Tubulin is the target of many anti-cancer drugs, and the cytoskeleton damage induced by AMPs can occur in both cancer cells and normal cells. Normal cells can self-heal due to their complete cytoskeleton system after being treated by AMPs, while tumor cells cytoskeleton is incomplete and cannot be repaired in time after the action of AMPs, resulting in cell death. Studies have shown that the AMP, cecropins B3, can disintegrate microtubules, disrupt the normal function of microtubules, and affect the integrity of the tumor cytoskeleton, thereby killing tumor cells.116
Inhibition of Tumor Angiogenesis
Certain AMPs have been found to inhibit tumor cell growth by inhibiting tumor angiogenesis. For example, the AMP called K6L9 can kill tumor cells through anti-angiogenesis and metastasis inhibition.117 Another example is that the AMPs, human α-defensins, can specifically bind to fibronectin (FN) to block the adhesion and transfer of integrin α5β1 mediating endothelial cells to fibronectin, and block the proliferation and apoptosis of endothelial cells mediated by vascular endothelial growth factor (VEGF), that is, human α-defensins inhibit tumor angiogenesis in many ways, thereby exerting its anti-cancer effect.118
Attack the Mitochondria Leading to Cancer Cell Apoptosis or Necrosis
Many AMPs can also induce apoptosis or necrosis of tumor cells in a variety of ways. It was found that pardaxin, a marine antimicrobial peptide, can act on the mitochondrial membrane, reduce the potential of mitochondrial membrane, release cytochrome c (cyt c) in the cytoplasm, and release a large amount of reactive oxygen species (ROS). Finally, the pardaxin activates the apoptosis proteins caspases 3 and 7, and induces apoptosis in the mitochondrial pathway, resulting in cell death.97 In addition, buforin II B, an AMP derived from histone H2A, can pass through the tumor cell membrane without damage, act on mitochondria, release cytochrome c, and activate the apoptosis protein caspase-9, and induce tumor cell apoptosis in the mitochondrial pathway.119
Regulation of Immunity
Antimicrobial peptides are a part of the body’s immune system, so many antimicrobial peptides can play their anti-tumor effects through immunomodulation. Some AMPs can chemoattract monocytes, dendritic cells (DC), T cells and neutrophils, etc, promoting the uptake and presentation of tumor cells by antigen-presenting cells and enhancing the immunogenicity of tumors.120,121 As an example, the AMP LL-37 can inhibit tumor growth by improving the phagocytic capacity of DC cells, increasing the expression and strengthening the function of phagocytic receptors, enhancing the expression of co-stimulatory molecules, promoting the immune response of Th1 cells, and increasing the secretion of various cytokines.122 Another example is Cecropin XJ. Cecropin XJ can induce hepatocellular carcinoma cell apoptosis by activating caspase-3 and poly(ADP-ribose) polymerase, downregulating the expression of B‑cell lymphoma 2 (Bcl‑2), and upregulating the expression of Bcl‑2‑associated death promoter and Bcl‑2‑associated X protein.123
Potential Clinical Application of AMPs as Antitumor Agents
Although the research on AMPs mainly focuses on their antimicrobial properties, more and more studies have shown that many AMPs possess outstanding antitumor activities in vitro and in vivo, especially in breast cancer, cervical cancer, lung cancer, etc. AMPs are natural drugs with exciting potential as a new kind of anticancer drugs, and AMPs with excellent antitumor effects are often called anticancer peptides (ACP).124 Traditional chemotherapeutics have shortcomings such as lack of tumor cell selectivity, which can easily cause serious side effects, and they also easily induce tumor cell resistance. In contrast, AMPs have significant advantages in anti-tumor as they offer a wide range of antimicrobial and anticancer spectrum, high selectivity to cancer cells, harmlessness to normal cells and important organs, and low propensity to develop drug resistance. Furthermore, AMPs also have the ability to quickly kill tumor cells and destroy and prevent primary tumor metastasis. As AMPs can act directly on tumor cell membranes, they also have significant anti-proliferation effects on drug-resistant tumors.125 Therefore, AMPs have great application prospects in cancer treatment with the following potential anti-tumor strategies:126,127 (1) AMPs used alone as anticancer agents; (2) AMPs combined with other drugs; (3) AMPs combined with nanotechnology.
Anti-Cancer Application of AMPs Alone
Antimicrobial Peptides Used Alone to Kill Common Tumors
AMPs with anticancer activities (anticancer peptides, ACP) are mainly α-helix and β-sheet peptides. The ACPs mainly include the following four antimicrobial peptide families:67 Defensins, Lactoferrins, Cecropins, and Magainins. Also, there are many types of ACPs,84 including Melitins, Cathelicidins, Dermaseptins, Temporins, Tachyplesin, etc.
ACPs usually have broad-spectrum and excellent in vitro antitumor activities, and many of them also have anti-tumor activity in vivo. For example, magainin 2 was found to have selective cytotoxicity to tumor cells, which can kill a variety of tumor cells at a peptide concentration of 5–10 times lower than the peptide concentration required to kill normal cells.128 In vivo studies have shown that administration of magainin 2 and its derivatives (MSI-136 and MSI-238) could reduce the invasion of tumor cells into soft tissue and increase the survival rate of leukemia cells in P388 mice.129 Another example is Cecropin A and B. They can lyse a variety of tumor cells at a concentration that is non-toxic to normal eukaryotic cells, and Cecropin B possesses an antitumor effect on mice bearing ascites colon adenocarcinoma cells.130,131 Table 3 summarizes the antitumor activity of AMPs from different AMP families and their antitumor mechanism.
 |
Table 3 Targeted Tumors and Relevant Antitumor Mechanism of Different AMPs |
Antimicrobial Peptides Used Alone to Kill Drug-Resistant Tumors
The chemotherapy resistance of cancer cells is one of the main reasons that limits the efficacy of chemotherapy drugs, and it seriously affects the prognosis of advanced cancer patients and the prognosis of patients with advanced cancer. Traditional anti-tumor drugs need to enter the cells to take effect, and if the uptake of drugs in the tumor tissue decreases or the drug efflux increases, the emergence of drug resistance in the tumor will occur. In contrast, many AMPs are less active in cells but act directly on tumor cell membranes, making them more effective in drug-resistant tumor cells. The most researched mechanism for the generation of MDR of tumor cells is the overexpression of the cell membrane transporter P-gp. P-gp participates in the formation of tumor cell membranes and can increase the net negative charge of the cell surface, so it is helpful for AMPs that possess positive charges to combine with the drug-resistant tumor cell membrane under electrostatic attraction.17 As a result, it is difficult for drug-resistant tumor cells to resist the action of AMPs. In recent years, a large number of studies have shown that many AMPs can kill a variety of drug-resistant tumors, and certain AMPs are more effective on drug-resistant tumor cells than normal tumor cells,162 which means that AMPs are of great value for the treatment of advanced and refractory cancers.
Gaegurins, a family of AMPs containing 6 AMPs, were isolated from Glandirana emeljanovi, which can be divided into two sub-families, Gaegurin I and Gaegurin II, according to their length and sequence. Studies have found that the derivatives of the antimicrobial peptide GN6 of the Gaegurin II sub-family can induce tumor cell cycle arrest and apoptosis in multidrug-resistant cancer cells, showing the potential to treat refractory malignant tumors.163 Besides, Temporin-1CEa, a natural AMP, was isolated and purified from the skin secretions of Rana chensinensis. Temporin-1CEa could selectively inhibit the cell proliferation of Adriamycin-resistant breast cancer MCF-7/Adr cells because the negative charges on the membrane surface of the drug-resistant breast cancer cell line MCF-7/Adr are higher than that of the ordinary MCF-7 tumor cells. At the same time, Temporin-1CEa can increase the sensitivity of MCF-7/Adr cells to the chemotherapy drug adriamycin. After 24 hours of combined administration with Temporin-1CEa, the IC50 of adriamycin decreased by 4.52 times.164 It was proved that Temporin-1CEa could co-localize with P-gP on the surface of drug-resistant cell membranes, so the anticancer mechanism of Temporin-1CEa may be acting on P-gP and affecting its efflux function, thereby reversing multidrug resistance in tumor cells.157,164
Besides, LL37, an AMP of the human-derived cathelicidin AMP family, and its derivative peptides (LL17-32 and LL13-37) have been found to increase the accumulation of mitoxantrone in ABCG2 overexpression-resistant tumor cells but does not affect normal tumor cells without ABCG2 overexpression. Similar to P-gp, ABCG2 is a drug-resistant semi-transporter on the tumor cell membrane, which also acts as a “drug pump” and is closely related to the accumulation of drugs in cancer cells. Both LL17-32 and LL13-37 could directly interact with ABCG2 in a non-competitive manner and inhibit its transport activity. Also, LL17-32 and LL13-37 could indirectly down-regulate ABCG2 protein expression in drug-resistant cells through the lysosomal degradation pathway, thus reversing multidrug resistance of tumor cells and making the drug-resistant cancer cells sensitive to chemotherapies.165
In another example, Wang et al100 gave the cationic AMP Polybia-MPI to the multi-drug resistant leukemia cell line K562/ADM that was 201 times resistant to doxorubicin and 14.7 times resistant to actinomycin. Polybia-MPI significantly inhibited the proliferation of K562/ADM cells and showed a killing effect that is similar to its effect on ordinary cells, indicating that the drug-resistant cells are sensitive to AMPs. Moreover, Banković et al17 found that NK-lysin-derived cationic peptide NK-2 could selectively inhibit the proliferation of P-gp overexpression multidrug-resistant cells, including multidrug-resistant non-small cell lung cancer cells (NCI-H460/R) and multidrug-resistant Colorectal cancer cells (DLD1-TxR). At the same time, NK-2 could also increase the sensitivity of drug-resistant cells to adriamycin by reducing the expression of P-gp on the cell surface. Additionally, NRC-03 and NRC-07, two AMPs from the pleurocidins AMP family, exhibited equal killing effect at a concentration of 50μM on normal MCF7 cells and on paclitaxel-resistant breast cancer cell line MCF7-TX400 that possess 2.6 times of P-gp expression more than the normal MCF7 cells, achieving about 60% of cell killing rate.162
In addition, many antimicrobial peptides have also been found to inhibit drug-resistant tumors and even reverse the drug resistance of tumor cells. For example, AMPs extracted from Musca domestica larva can selectively inhibit the proliferation of multi-drug resistant human hepatocellular carcinoma cell line Bel-7402/ADM. The IC50 value of these AMPs against Bel-7402/ADM cells was even lower than that of AMPs against normal Bel-7402 cells. Also, these AMPs could reverse the multi-drug resistance of tumor cells by inhibiting the relative expression of multidrug resistance gene 1 (MDR1).166 Another AMP named BMAP-28 was reported to inhibit the proliferation of multidrug-resistant CEM-VLB lymphocytic leukemia cells.84 Besides, the AMP Cecropin Bcould also have in vitro cytotoxic activity against multi-drug resistant human breast and ovarian cancer cell lines.131
The above-mentioned studies provide experimental support for AMPs to replace chemotherapeutic drugs for tumor treatment or to help solve the problem of tumor cell chemotherapy resistance, suggesting that AMPs hold great application potential in the treatment of drug-resistant tumors.
Anti-Cancer Application of AMPs Combined with Other Drugs
Traditional anti-tumor drugs need to enter cells to function effectively, but their drug utilization is low with rapid metabolism in the body and short half-life. Therefore, high-frequency administration is needed, usually leading to serious side effects. In order to achieve clinical tumor treatment, combined medication can be considered. The AMP-mediated destruction of tumor cell membranes can help chemotherapeutics better enter tumor cells, so the combined use of chemotherapeutics and AMPs may help reduce the effective dose of chemotherapeutics, thereby reducing dose-related side effects, and achieving the effect of increased efficiency and reduced toxicity. Thus, the combined use of AMPs and antitumor drugs has potential clinical application prospects.
AMPs Combined with Chemotherapy Drugs for Cancer Treatment
In 1992, Ohsaki et al146 discovered for the first time that the antimicrobial peptide Magainin analogs combined with chemotherapy drugs such as adriamycin and cisplatin showed an additive inhibitory effect on tumor cells. Since then, more and more studies have shown that AMPs can be used in combination with anti-tumor drugs to fight tumors. For example, cecropins can significantly enhance the killing effect of S-fluorouracil and Cytarabine on acute lymphoblastic leukemia cells.147 Cecropin A combined with a variety of chemotherapeutics, such as 5-fluorouracil, cytarabine, etc, showed synergistic anti-tumor activity in CCRF-SB lymphoid leukemia cells and squamous skin cancer cells.167,168
In addition, Xia’s group169 administered a Xinjiang silkworm AMP named cecropin-XJ in combination with the chemotherapy drugs such as adriamycin (ADM), cisplatin (DDP), cyclophosphamide (CTX), and carboplatin (CBP) on esophageal cancer Eca109 cells, and results showed that the cell proliferation inhibition rate and the apoptosis rate of the combined drug group were significantly higher than those of the same concentration chemotherapeutic drug group. For another example, magainin 2, an antimicrobial peptide from Xenopus skin, has been proven to penetrate the cancer cell membrane and act as an ion channel, which leads to the dissolution of cancer cells, thus significantly enhancing the cytotoxicity of chemotherapy drugs in tumor cells.170 Magainin 2 derivatives, magainin A (MAG A) and magainin G (MAG G), have also been found to exert synergistic antitumor activity after combined use with chemotherapeutics such as cisplatin (DDP), doxorubicin (DOX), and etoposide (VP-16).146
Another example is the scorpion venom peptide APBMV extracted from Buthus martensii, which has also been found to show a synergistic anti-tumor effect with a variety of chemotherapeutic drugs. After the combined use of a low concentration of APBMV (20 mg/L, cell inhibition rate is only 15.5%) and chemotherapeutic drugs such as mitomycin (MMC), DDP, fluorouracil (5-FU), and VP-16, the sensitivity of colorectal cancer LoVo cells to chemotherapeutic drugs increased and the cell inhibition rate even Increased to about 2 times that of those drugs used alone.171,172 Additionally, studies on NRC-03 and NRC-07 (two AMPs from the pleurocidin AMP family) showed that the 50% effective concentration (EC50) of cisplatin on tumor cells could be significantly reduced when used in combination with NRC-03 or NRC-07, that is, the cytotoxicity of cisplatin was increased.162
The above-mentioned studies have proven the feasibility of anti-tumor use in combination with antimicrobial peptides and chemotherapeutics and indicated that antimicrobial peptides have potential application prospects for enhancing efficacy and reducing the toxicity of chemotherapeutics.
AMPs Combined with Chemotherapy Drugs Against Drug-Resistant Tumor Cells
Importantly, the combination of AMPs and chemotherapeutic drugs has obvious advantages in the treatment of drug-resistant tumor cells. Overexpression of drug efflux protein in drug-resistant tumor cells sharply decreases the concentration of drug uptake by tumor cells, which greatly limits the effect of chemotherapeutic drugs. Unlike traditional chemotherapy drugs, the action of AMPs is not affected by the overexpression of tumor multidrug resistance genes due to their special membrane disruption mechanism. Even, certain AMPs can destroy the function of drug efflux proteins such as P-GP, allowing multidrug-resistant tumor cells to regain chemical drug sensitivity.84,173
Studies have shown that the combination of chemotherapeutic drugs (such as ADM and DDP) and the antimicrobial peptide θ-defensin can improve the sensitivity of multidrug-resistant breast cancer MDA-MB-231 cells to the chemotherapeutic drugs and effectively enhance the cytotoxic effect of such chemotherapeutic drugs.135 Besides, Johnstone et al174 found that the combined use of the chemotherapeutic drug ADM and multiple mammalian and plant-derived AMPs significantly increased the sensitivity of drug-resistant cells to ADM, thus improving the anti-tumor activity of ADM. The mechanism may relate to the membrane destruction effect of the AMPs. Another research found that NRC-03 and NRC-07, the two AMPs from the pleurocidin AMP family, can also reverse the sensitivity of multidrug-resistant breast cancer MDA-MB-231 cells to some chemotherapeutic drugs. The experimental process was to pre-treat MDA-MB-231 cells for 20 min with a low concentration (10 μM) of NRC-03 or NRC-07 20 minutes and then added a gradient concentration of cisplatin. After 72 h or 96 hours of incubation, the EC50 of cisplatin in the NRC-03 pretreatment group decreased by 5.5 times at both 72 h and 96 h, while it decreased by 1.6 times after 72 h and 1.7 times after 96 h in NRC-07 pretreatment group, respectively. Meanwhile, NRC-03 could also enhance the cytotoxicity of docetaxel (DTX) on MDA-MB-231 cells.162
The above studies indicate that AMPs can also be used as chemotherapeutic drug sensitizers, and the combination of AMPs and chemotherapeutic drugs has broad application prospects in the treatment of multidrug-resistant tumors.
AMPs-Based Cancer Gene Therapy
In addition to targeting common and drug-resistant tumors, AMPs in combination with other drugs also have many applications in gene therapy. It has been proven that good therapeutic effects can be obtained by transferring or intratumorally injecting AMPs or their precursor genes into tumor cells. For example, David Winder et al175 introduced an expression vector containing cecropin A gene into EJ human bladder cancer cells, which can significantly inhibit or slow down the growth of tumors in immunodeficient mice. Besides, cancer gene therapy based on melittin has also been reported. After adenovirus-mediated transfer of melittin gene to BEL-7402 human liver cancer cells under the control of α-fetoprotein promoter could significantly inhibit the proliferation of liver cancer cells in vivo and in vitro.176 For another example, Xu et al177 co-transfected L1210 mouse leukemia model with murine β-defensin 2 (β-defensin 2) gene and IL-18 gene. The co-transfection group produced a stronger anti-tumor immune response than a single transfection of β-defensin 2 gene or IL-18 gene that improved the survival rate of mice. The survival rate of mice in the co-transfection group was as high as 80%, which was higher than that of the two single transfection groups, and it showed long-term protective immunity to the surviving mice. Therefore, gene therapy of AMPs alone or in combination with other drugs may be used for the treatment of human cancer.
Modified AMPs for Cancer Treatment
In addition to the combination with chemotherapeutics, AMPs could also be modified by tumor-targeting peptides or cell-penetrating peptides to enhance the tumor-targeting and anti-tumor activities of the AMPs. Tumor-targeting peptides such as NGR, RGD, and CREKA can specifically bind to specific ligands overexpressed on the surface of tumor cell membranes, so they have high tumor cell selectivity and can be used as tumor-targeting vectors.178–180 As an example, Tachyplesin, an AMP derived from the horseshoe crab, was modified with RGD peptide, which significantly increased the enrichment of Tachyplesin in tumor blood vessels and tumor tissues. Results proved that the anti-tumor effects of RGD-Tachyplesin both in vivo and in vitro were better than those of Tachyplesin used alone. This method could enhance the effectiveness and meanwhile reduce the toxicity of AMPs.161 In another research, Ma et al181 coupled an AMP, D(KLAKLAK)2, with a tumor-targeting peptide, TMTP1, and found that the new fusion peptide effectively inhibited the growth and metastasis of prostate cancer and gastric cancer transplanted in nude mice, which was expected to become a new effective anti-tumor agent. For another example, when the AMP, MP, and the cell-penetrating peptide, Antp, were used alone, they only showed weak cytotoxicity with no obvious tumor cell killing effect. However, their fusion peptide, MPGA, exerted strong tumor cell killing activity by destroying the cell membranes.182
Anti-Cancer Application of AMPs Related Nanoparticles
Although AMPs have good prospects for tumor treatment due to their broad-spectrum anti-cancer activity and low possibilities to induce drug resistance in tumor cells, some limitations in their practical applications have emerged with the increasing research. The main problem is that AMPs are easily hydrolyzed by proteases and they have certain hemolytic toxicity in vivo. Secondly, the quantity of natural AMPs is limited, but the production cost is high. Therefore, how to rationally design and optimize AMPs to maintain their tumor cell selectivity as well as their stable anti-cancer activities is of great significance.183,184 The development of nanotechnology has inspired researchers. In the past few decades, nanotechnology has been widely used, especially in the medical field. At present, nanoparticles in medical research are generally between 1–500nm in diameter, which has unique physical, chemical, and biological properties, such as size effect, long circulation in vivo, controlled release of drugs, and prevention of drug degradation.185,186 Functionalized nanoparticles have many possibilities in overcoming the shortcomings of AMPs, which provides a huge prospect for better application of AMPs. Antimicrobial peptide nano-preparations used in anti-cancer research mainly include two aspects: (1) AMPs are used as drugs and nanomaterials are used as a drug delivery system. AMPs can be loaded onto nanocarriers for targeted delivery of the antimicrobial peptides to tumor sites. The purpose is to reduce the in vivo hemolytic toxicity and immunogenicity of exogenous AMPs, while retaining their anti-cancer activity, aiming to reduce the peptide dose required for treatment while ensuring safety;27,187 (2) Antimicrobial peptide modified nano-formulations are used as drug carriers. Many AMPs have similar properties to cell-penetrating peptides, for example, they are usually positively charged and the peptide sequence contains both multiple basic amino acids and hydrophobic amino acids, which help them to effectively interact with tumor cell membranes. Thus, AMPs can be used as cell-penetrating peptides to modify the surface of the nano-preparations so as to transfer anti-cancer chemicals to the tumor sites and finally achieve an enhanced anti-cancer effect.188–190
AMPs Worked as Effective Component
At present, there are many reports on antimicrobial peptide nano preparations. The AMP, called CA, was isolated from Plutella xylostella and was found to have good anti-liver tumor activity. Liu et al191 prepared CA-loaded nanoparticles based on poly(lactic-co-glycolic acid) (CA-PLGA-NPs), showing good activity against liver cancer HepG2 cells. As a carrier of the peptide CA, PLGA can not only improve the stability of CA in vitro but also prolong the action time of CA in the body. As a result, these nanoparticles showed good sustained-release effect, no obvious sudden-release phenomenon, and long platform release period, which could effectively reduce the dosage of peptide administrations, thereby supplementing the pharmaceutics basis for the delivery of the peptide CA. Another study incorporated Melittin, a typical AMP, into monolayer perfluorocarbon nanoparticles, which was verified to enhance the retention time of Melittin in vivo, prevent the peptide from being hydrolyzed by serum proteases, and significantly reduce the hemolytic toxicity of Melittin in vivo. More importantly, this nano-preparation retained the therapeutic effect of Melittin on melanoma models and pre-cancerous skin damage.192 In addition, Gamal’s group loaded the black snake (Walterinnesia aegyptia)-derived AMP onto silica nanoparticles. This preparation could significantly enhance the immune activity of normal lymphocytes,193 and it could induce cell cycle arrest or apoptosis in human prostate cancer cells194 and in a mouse model bearing human multiple myeloma.195 For another example, lycosin-I is an AMP isolated from the venom of Lycosa singorensis, which can interact with cell membranes and then kill tumor cells by destroying tumor cell membranes. Combining the tumor cell membrane selectivity of lycosin-I with the advantages of nano-preparation, Tan et al196 modified 60 nm gold particles with lycosin-I, which can significantly improve the transfer efficiency of the gold nanoparticles in tumor cells. At the same time, the lycosin-I-gold nanoparticles had low sensitivity to non-tumor cells and could selectively accumulate at tumor sites through blood circulation in tumor-bearing mice model, but were non-toxic to normal mice. Therefore, this nano-drug delivery system can be used as a new targeted drug delivery system in tumor treatment. With the help of the photothermal effect of gold nanorods, Tan’s research group also constructed lycosin-I modified gold nanorods. The modified gold nanorods maintained tumor selectivity of lycosin-I, and could generate heat under near-infrared (NIR) light irradiation to kill tumor cells. The results revealed the potential value of gold nanorods in tumor diagnosis and tumor photothermal treatment.
Liposome drug delivery system has been extensively studied among many different nano-preparations. At present, there are also some reports on the research of liposomes based on AMPs. For example, Temporin-1CEa, an AMP extracted from the skin secretions of Rana chensinensis, was found to have good in vitro antitumor activity.158 To solve the poor stability of Temporin-1CEa in vivo, prevent it from being hydrolyzed, and increase its tumor cell uptake rate, Wu et al197 constructed polyethylene glycol (PEG)-modified liposomes containing Temporin-1CEa (Temporin-1 CEa-liposomes) by using the reverse-phase evaporation method. PEG-modified Temporin-1 CEa liposomes showed better serum stability and higher cell uptake rate on the basis of retaining the anti-cancer effect of Temporin-1 CEa. In addition, L-K6, an AMP modified based on the AMP Temporin-1 CEb from Rana chensinensis, was proved to have good anti-cancer activity in vitro and was non-toxic to normal human cells. Besides, L-K6 was less likely to cause cancer cell resistance compared with other anticancer drugs.198 Chen et al199 used L-K6 as a drug to prepare L-K6 liposomes in order to maintain the activity of L-K6 while improving its biological stability. The L-K6 liposome drug delivery system better retained the selective anti-cancer activity of L-K6, and exhibited a similar inhibitory effect on the proliferation of human breast cancer MCF-7 cells as L-K6 while showing no obvious toxicity to human immortalized epidermal HaCaT cells. To further improve the biological stability of L-K6 liposomes, the author also prepared PEG-modified L-K6 liposomes. The results showed that the particle size of PEG-modified L-K6 liposomes was reduced, the liposome appearance was more thorough, and the serum stability was improved, which was more conducive to the accumulation of the drug to the tumor site and play a more potent anti-cancer effect. In addition, Huang et al200 designed a hybrid cytolytic peptide, α-melittin, by linking the N-terminus of melittin to the C-terminus of an amphipathic α-helical peptide via a GSG linker. α-Melittin could interact with phospholipids and self-assemble into lipid nanoparticles, which significantly enhanced the anti-cancer activity of melittin. At the same time, this α-melittin-based lipid nanoparticle efficiently shielded the positive charge of melittin within the phospholipid monolayer, resulting in the reduced cytotoxicity and a widened safe dosage range, at the same time. In addition, Yamada et al201 prepared transferrin modified liposomes combined with a pH-sensitive fusion peptide and encapsulated the wasp-derived peptide mastoparan. The liposomes could target mastoparan to the mitochondrial site of the human chronic myelogenous leukemia K562 cells, destroying the mitochondrial membrane and inducing the release of cytochrome c, thereby causing cell death.
The above studies indicate that the nano-drug delivery system is a good delivery system for peptide anti-cancer drugs, which can improve the tumor targeting of peptide anti-cancer drugs, effectively improve the stability of peptide anti-cancer drugs, and reduce their cytotoxicity, which is of great help to promote the clinical application of peptide drugs.
AMPs Modified as Functional Ligands
In addition to being used as anticancer drugs in nanoparticles, AMPs can also be modified on the surface of the drug-loaded nano-preparations as functional ligands. Currently, a large number of studies have been conducted to improve the cellular uptake of nanoparticles by covalently combining cationic or amphiphilic cell-penetrating peptides to the surface of nanoparticles. Tat is one of the most studied and applied cell-penetrating peptides. Tseng et al202 modified the surface of Adriamycin-loaded liposomes with Tat, which increased the uptake of Adriamycin in cancer cells by nearly 12 times, thus significantly enhancing the antitumor activity of Adriamycin. Similarly, Sharma’s group203 used the penetratin, a Drosophila-derived cell-penetrating peptide, to modify the Adriamycin-loaded liposomes, thus significantly improving the transfer efficiency of the liposomes in brain tumor cells. Similar to cell-penetrating peptides, most AMPs are also cationic or amphipathic, therefore, some researchers believe that cell-penetrating peptides and AMPs are both “membrane-active peptides”. The cell penetration effect of certain AMPs has also been widely reported, and many peptides have been used both as antimicrobial peptides and cell-penetrating peptides, such as insect-derived peptide Pyrrhocoricin, bovine-derived peptide Lactoferricin, Drosophila-derived peptide penetratin and so on.190,204 Therefore, it is feasible to use AMPs as the ligands to modify the surface of drug-loaded nano preparations in order to improve the cell penetration ability, tumor-targeting ability, and the tumor cell uptake rate the nanoparticles, thus promoting drug action. In summary, the AMP-based nano preparations are also a potential delivery model of anti-cancer drugs.
Challenges and Ways Forward
Although there are many basic studies on AMPs, the potential anti-cancer mechanisms of some AMPs are still difficult to clarify. Also, there are very few in vivo and clinical experiments of AMPs, and there is a lack of effective correlation between the in vitro sensitivity tests and the animal models.124 Therefore, strengthening the research on the pharmacokinetics and pharmacodynamics of AMPs, and establishing the analysis of the correlation between in vitro stability and bioavailability in animal models may solve this problem.
Besides, the most serious barrier to the clinical use of anti-cancer AMPs remains the enzymatic degradation and inactivation of these cytotoxic peptides through interactions with negatively charged serum proteins. The hemolytic toxicity and easy hydrolysis of AMPs are also prominent problems that restrict the clinical application of AMPs. Thus, how to maintain the stable anti-cancer activity of AMPs and at the same time reduce its hemolytic toxicity as well as rational design and optimization of AMP drugs is of great significance. To solve the above problems, two ideas can be considered. One is to optimize the structure of AMPs as the structure–activity relationship is also crucial to improve the anti-cancer effects of AMPs. The methods of peptides design and modification to improve the stability, half-life time, and specificity of AMPs include amino acid substitution, cyclization, hybridization, fragmentation, modification of C- and N-terminal of a peptide by polymer or targeting molecules,205,206 etc. Amino acid substitution is the most basic modification method. Many structure–activity studies on small linear AMPs indicated that the net positive charges, hydrophobicity, amphiphilicity, and helicity were the most important factors that affect their activities.207 As we know, most AMPs are membrane activity peptides, and that the enhanced positive charges of AMPs usually lead to higher activity, likely because the enhanced positive charges of AMPs result in increased affinity to the negatively charged cancer cell membranes.208 However, higher positive charges do not always lead to better activity, and a high antimicrobial activity could be achieved within an optimal hydrophobicity window.209 Excessive hydrophobicity may lead to strong hemolytic toxicity, which may be caused by the self-binding of the peptides that may impede the peptides pass through prokaryotic cell membranes without hindering their passage through eukaryotic cell membranes.210 In summary, it is better to keep a balance between the overall positive charges, the hydrophobicity, and other factors when modifying the structures of AMPs.
Another strategy is to design a new AMP drug delivery system so as to protect AMPs from being enzymatically degraded and reduce their hemolytic toxicity. Due to the special physicochemical properties of AMPs, the development of a specific delivery system to protect AMPs can increase the therapeutic index of AMPs while also improving their feasibility of intestinal absorption and oral administration. Combining AMPs with modern nanotechnology is one of the options for developing new antimicrobial peptide delivery systems, which is of great significance to promote the clinical application of new anti-cancer drugs of AMPs. Nano-drug delivery systems can well maintain the anti-cancer activity of AMPs while avoiding their enzymatic hydrolysis and reducing their hemolytic toxicity. Alternatively, a specific ligand can be used to achieve direct tumor targeting of antimicrobial peptide nanoparticles, and the targeted delivery of AMPs to the tumor sites can reduce the amount of peptide needed to achieve a therapeutic effect, thus avoiding severe side effects. Furthermore, in order to achieve the rapid release of drugs, smart stimuli-responsive nano-drug carriers have received extensive attention for anti-cancer drug delivery. Smart nano-drug carriers mainly include tumor microenvironment-respond nano-drug carriers and external stimulus-respond nano-drug carriers. Smart Stimuli-Responsive nanocarriers can be stably transported in the body, and after reaching the tumor site, by sensing the chemical environment of the tumor site or receiving external stimuli, the loaded AMPs can be released at the necessary time and a specific site. As a result, the peptide concentration in the tumor site can be increased, the anti-cancer efficiency of the AMPs can be enhanced, and the toxic side effects on normal tissues can be reduced.
Future Prospective
Due to the severe side effects of chemotherapy drugs and the emergence of tumor cell resistance, the clinical efficacy of chemotherapy drugs is greatly reduced. Better than chemotherapy drugs, most AMPs can specifically inhibit the growth of most tumor cells, but are harmless to normal cells. Many AMPs can also promote the proliferation of white blood cells and fight tumors through the regulation of the body’s immunity.211 Due to the unique structural properties and unique anti-cancer mechanisms of AMPs, as well as its advantages in cell selectivity and resistance to drug-resistant tumors, AMPs bring new hope for the development of anti-cancer drugs. There are different perspectives of antimicrobial peptides in anti-cancer application. On the one hand, AMPs with obvious killing effect on tumors can be directly used as anti-cancer drugs. On the other hand, some AMPs can be used as adjuvant drugs for tumor treatment. Therefore, in the past few years, such AMPs have been extensively studied as auxiliary drugs for anti-cancer drugs.177,212 Additionally, some AMPs are too cytotoxic and may have a killing effect on normal cells, but when such AMPs at low-dose are combined with chemotherapy drugs, the cytotoxicity of those AMPs can be restrained and at the same time, the anti-cancer effect of chemotherapy drugs can be significantly enhanced.169,171
Besides, there are many other difficulties that need to be overcome in the process of AMPs, from basic research to clinical applications. First of all, the resources of natural AMPs are limited, and the chemical synthesis of AMPs needs high cost.213 Although genetic engineering is currently a relatively economical and practical method for large-scale production of AMPs, there are still many limitations.214 For example, the antibacterial and antiviral capabilities of AMPs make it difficult to use the commonly used bacteria and viruses as expression systems. Besides, with small molecular weight, the separation and purification of AMPs are difficult, and the gene expression yield is still insufficient. Therefore, how to reduce the production cost of AMPs is one of the problems that must be solved in the practical application of AMPs.
With the in-depth research on AMPs, the optimization of AMPs structure and the design of new nano-delivery systems for AMPs will be easier to achieve, and the application of AMPs in the clinical treatment of tumors is just around the corner.
Acknowledgments
The authors acknowledge the funding of the National Natural Science Foundation of China [No.82173989] and the support from the National Administration of Traditional Chinese Medicine [No.zyyzdxk-2023272].
Disclosure
The authors report no conflicts of interest in this work.
References
1. Hashim D, Boffetta P, Vecchia CL, et al. The global decrease in cancer mortality: trends and disparities. Ann Oncol. 2016;27(5):926. doi:10.1093/annonc/mdw027
2. Munker S, Gerken M, Fest P, et al. Chemotherapy for metastatic colon cancer: no effect on survival when the dose is reduced due to side effects. BMC Cancer. 2018;18(1):455. doi:10.1186/s12885-018-4380-z
3. Nakashima-Kamimura N, Mori T, Ohsawa I, Asoh S, Ohta S. Molecular hydrogen alleviates nephrotoxicity induced by an anti-cancer drug cisplatin without compromising anti-tumor activity in mice. Cancer Chemother Pharmacol. 2009;64(4):753–761. doi:10.1007/s00280-008-0924-2
4. Norouzi‐Barough L, Sarookhani MR, Sharifi M, et al. Molecular mechanisms of drug resistance in ovarian cancer. J Cell Physiol. 2018;233(6):4546.
5. Chatterjee S, G Damle S, K Sharma A. Mechanisms of resistance against cancer therapeutic drugs. Curr Pharm Biotechnol. 2014;15(12):1105.
6. Stone TA, Cole GB, Ravamehr-Lake D, Nguyen HQ, Deber CM. Positive charge patterning and hydrophobicity of membrane-active antimicrobial peptides as determinants of activity, toxicity, and pharmacokinetic stability. J Med Chem. 2019;62(13):6276–6286. doi:10.1021/acs.jmedchem.9b00657
7. Gupta S, Bhatia G, Sharma A, Saxena S. Host defense peptides: an insight into the antimicrobial world. J Oral Maxillofac Pathol. 2018;22(2):239.
8. Di YP. Antimicrobial peptides in host defense against drug-resistant bacterial and viral infections. Curr Med Chem. 2020;27(9):1385–1386. doi:10.2174/092986732709200327085156
9. Schröder-Borm H, Bakalova R, Andr J. The NK-lysin derived peptide NK-2 preferentially kills cancer cells with increased surface levels of negatively charged phosphatidylserine. FEBS Lett. 2005;579(27):6128–6134. doi:10.1016/j.febslet.2005.09.084
10. Oliva R, Del Vecchio P, Grimaldi A, et al. Membrane disintegration by the antimicrobial peptide (P)GKY20: lipid segregation, domain formation, budding and micellization. PCCP. 2019;21(7):3989–3998. doi:10.1039/C8CP06280C
11. Parchebafi A, Tamanaee F, Ehteram H, Ahmad E, Nikzad H, Haddad Kashani H. The dual interaction of antimicrobial peptides on bacteria and cancer cells; mechanism of action and therapeutic strategies of nanostructures. Microb Cell Fact. 2022;21(1):118. doi:10.1186/s12934-022-01848-8
12. Nguyen LT, Haney EF, Vogel HJ. The expanding scope of antimicrobial peptide structures and their modes of action. Trends Biotechnol. 2011;29(9):464–472. doi:10.1016/j.tibtech.2011.05.001
13. Maraming P, Klaynongsruang S, Boonsiri P, et al. The cationic cell-penetrating KT2 peptide promotes cell membrane defects and apoptosis with autophagy inhibition in human HCT 116 colon cancer cells. J Cell Physiol. 2019;234(12):22116–22129. doi:10.1002/jcp.28774
14. Luo X, Teng Q-X, Dong J-Y, et al. Antimicrobial peptide reverses ABCB1-mediated chemotherapeutic drug resistance. Front Pharmacol. 2020;11:1208. doi:10.3389/fphar.2020.01208
15. Kawami M, Yamada Y, Issarachot O, Junyaprasert VB, Yumoto R, Takano M. P-gp modulating effect of Azadirachta indica extract in multidrug-resistant cancer cell lines. Pharmazie. 2018;73(2):104–109. doi:10.1691/ph.2018.7116
16. Dennison SR, Wallace J, Harris F, Phoenix DA. Amphiphilic alpha-helical antimicrobial peptides and their structure/function relationships. Protein Pept Lett. 2005;12(1):31. doi:10.2174/0929866053406084
17. Banković J, Andrä J, Todorović N, et al. The elimination of P-glycoprotein over-expressing cancer cells by antimicrobial cationic peptide NK-2: the unique way of multi-drug resistance modulation. Exp Cell Res. 2013;319(7):1013.
18. Lu J, Chen ZW. Isolation, characterization and anti-cancer activity of SK84, a novel glycine-rich antimicrobial peptide from Drosophila virilis. Peptides. 2010;31(1):44–50. doi:10.1016/j.peptides.2009.09.028
19. Smolarczyk R, Cichoń T, Szala S, et al. Peptydy – nowa klasa leków przeciwnowotworowych[Peptides: a new class of anticancer drugs]. Postepy Hig Med Dosw. 2009;63(835515):360. Polish.
20. Rothan HA, Ambikabothy J, Ramasamy TS, Rashid NN, Yusof R. A preliminary study in search of potential peptide candidates for a combinational therapy with cancer chemotherapy drug. Int J Pept Res Ther. 2019;25:115–122.
21. Gallo RL, Huttner KM. Antimicrobial peptides: an emerging concept in cutaneous biology. J Invest Dermatol. 1998;111(5):739–743. doi:10.1046/j.1523-1747.1998.00361.x
22. Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415(6870):389–395. doi:10.1038/415389a
23. Lidholm DA, Gudmundsson GH, Xanthopoulos KG, Boman HG. Insect immunity: cDNA clones coding for the precursor forms of cecropins A and D, antibacterial proteins from Hyalophora cecropia. FEBS Lett. 1987;226:8.
24. Gudmundsson GH, Lidholm DA, Asling B, Gan RB, Boman HG. The cecropin locus. Cloning and expression of a gene cluster encoding three antibacterial peptides in Hyalophora cecropia. J Biol Chem. 1991;266(18):11510–11517. doi:10.1016/S0021-9258(18)98986-6
25. Vizioli J, Richman AM, Uttenweiler-Joseph S, Blass C, Bulet P. The defensin peptide of the malaria vector mosquito Anopheles gambiae: antimicrobial activities and expression in adult mosquitoes. Insect Biochem Mol Biol. 2001;31(3):241–248. doi:10.1016/S0965-1748(00)00143-0
26. Bals R, Koczulla AR, Von DG. The human peptide antibiotic LL-37/hCAP-18 is an inducer of angiogenesis. J Clin Invest. 2003;111(11):1665–1672.
27. Braun K, Pochert A, Lindén M, et al. Membrane interactions of mesoporous silica nanoparticles as carriers of antimicrobial peptides. J Colloid Interface Sci. 2016;475:161–170. doi:10.1016/j.jcis.2016.05.002
28. Friedrich CL, Rozek A, Patrzykat A, Hancock REW. Structure and mechanism of action of an indolicidin peptide derivative with improved activity against gram-positive bacteria. J Biol Chem. 2001;276(26):24015–24022. doi:10.1074/jbc.M009691200
29. Staubitz P, Peschel A, Nieuwenhuizen WF, et al. Structure–function relationships in the tryptophan‐rich, antimicrobial peptide indolicidin. J Pept Sci. 2010;7:552.
30. Himanshu K, Kaznessis YN. Structure of the antimicrobial beta-hairpin peptide protegrin-1 in a DLPC lipid bilayer investigated by molecular dynamics simulation. Biochim Biophys Acta. 2007;1768(3):509–520. doi:10.1016/j.bbamem.2006.11.015
31. Guerrero E, Saugar JM, Matsuzaki K, Rivas L. Role of positional hydrophobicity in the leishmanicidal activity of magainin 2. Antimicrob Agents Chemother. 2004;48(8):2980. doi:10.1128/AAC.48.8.2980-2986.2004
32. Tomoya T, Epand RF, Epand RM, et al. Position-dependent hydrophobicity of the antimicrobial magainin peptide affects the mode of peptide−lipid interactions and selective toxicity. Biochemistry. 2002;41(34):10723–10731. doi:10.1021/bi0256983
33. Mangoni ML, Papo N, Barra D, et al. Effects of the antimicrobial peptide temporin L on cell morphology,__membrane permeability and viability of Escherichia coli. Biochem J. 2004;380:859–865. doi:10.1042/bj20031975
34. Simmaco M, Mignogna G, Canofeni S, Miele R, Mangoni ML, Temporins BD. Antimicrobial peptides from the European red frog rana temporaria. Eur J Biochem. 1996;243(3):788–792.
35. Brocal I, Falco A, Mas V, et al. Stable expression of bioactive recombinant pleurocidin in a fish cell line. Appl Microbiol Biotechnol. 2006;72(6):1217–1228. doi:10.1007/s00253-006-0393-7
36. Falco MO-V A, Chico V, Brocal I, Perez L, Coll JM, Estepa A. Antimicrobial peptides as model molecules for the development of novel antiviral agents in aquaculture. Mini Rev Med Chem. 2009;9(10):1.
37. Syvitski RT, Burton I, Mattatall NR, Douglas SE, Jakeman DL. Structural characterization of the antimicrobial peptide pleurocidin from winter flounder. Biochemistry. 2005;44(19):7282. doi:10.1021/bi0504005
38. Du ZQ, Jin YH. Molecular characterization and antibacterial activity analysis of two novel penaeidin isoforms from pacific white shrimp, litopenaeus vannamei. Appl Biochem Biotechnol. 2015;177(8):1607–1620. doi:10.1007/s12010-015-1840-7
39. Destoumieux D, Bulet P, Loew D, Van Dorsselaer A, Rodriguez J, Bachere E. Penaeidins, a new family of antimicrobial peptides isolated from the shrimp Penaeus vannamei (Decapoda). J Biol Chem. 1997;272(45):28398. doi:10.1074/jbc.272.45.28398
40. Charlet S, Chernysh S, Philippe H, Hetru C, Hoffmann JA, Bulet P. Innate immunity. Isolation of several cysteine-rich antimicrobial peptides from the blood of a mollusc, Mytilus edulis. J Biol Chem. 1996;271(36):21808–21813. doi:10.1074/jbc.271.36.21808
41. Epple P, Bohlmann AH. An Arabidopsis thaliana thionin gene is inducible via a signal transduction pathway different from that for pathogenesis-related proteins. Plant Physiol. 1995;109(3):813–820. doi:10.1104/pp.109.3.813
42. Vignutelli A, Wasternack C, Apel K, Bohlmann H. Systemic and local induction of an Arabidopsis thionin gene by wounding and pathogens. Plant J Cell Mol Biol. 2010;14(3):285–295. doi:10.1046/j.1365-313X.1998.00117.x
43. Berrocal-Lobo M, Segura A, Moreno M, Lopez G, Garcia-Olmedo F, Molina A. Snakin-2, an antimicrobial peptide from potato whose gene is locally induced by wounding and responds to pathogen infection. Plant Physiol. 2002;128(3):951–961. doi:10.1104/pp.010685
44. Mao Z, Zheng J, Wang Y, et al. The new CaSn gene belonging to the snakin family induces resistance against root-knot nematode infection in pepper. Phytoparasitica. 2011;39(2):151–164. doi:10.1007/s12600-011-0149-5
45. Hiroo Y, Koji O, Kazuhiko T, Nobuo I. Mode of antibacterial action by gramicidin S. J Biochem. 1986;100(5):1253.
46. Gause GF, Brazhnikova MG. Gramicidin S and its use in the treatment of infected wounds. Nature. 1944;154(3918):703. doi:10.1038/154703a0
47. Kaletta C, Entian KD. Nisin, a peptide antibiotic: cloning and sequencing of the nisA gene and posttranslational processing of its peptide product. J Bacteriol. 1989;171(3):1597–1601. doi:10.1128/jb.171.3.1597-1601.1989
48. Garcíagarcer MJ, Elferink MGL, Driessen AJM, Konings WN. In vitro pore-forming activity of the lantibiotic nisin. Role of protonmotive force and lipid composition. Eur J Biochem. 2010;212(2):417–422. doi:10.1111/j.1432-1033.1993.tb17677.x
49. Wang Y-P, Lai R. Insect antimicrobial peptides: structures, properties and gene regulation. Zool Res. 2010;31(1):27–34. doi:10.3724/SP.J.1141.2010.01027
50. Sitaram N, Nagaraj R. Host-defense antimicrobial peptides: importance of structure for activity. Curr Pharm Des. 2002;8(9):727–742. doi:10.2174/1381612023395358
51. Lohner K. New strategies for novel antibiotics: peptides targeting bacterial cell membranes. Gen Physiol Biophys. 2009;28(2):105. doi:10.4149/gpb_2009_02_105
52. Jenssen H, Hamill P, Hancock REW. Peptide antimicrobial agents. Clin Microbiol Rev. 2006;19(3):491–511. doi:10.1128/CMR.00056-05
53. Zhang S, Zhu L, Yu J, et al. Evaluating the potential of a loop-extended scorpion toxin-like peptide as a protein scaffold. Protein Eng Des Sel. 2016;12:12.
54. Irudayam SJ, Berkowitz ML. Influence of the arrangement and secondary structure of melittin peptides on the formation and stability of toroidal pores. Biochim Biophys Acta. 2011;1808(9):2258–2266. doi:10.1016/j.bbamem.2011.04.021
55. Bechinger B, Zasloff M, Opella SJ. Structure and orientation of the antibiotic peptide magainin in membranes by solid-state nuclear magnetic resonance spectroscopy. Protein Sci. 1993;2(12):2077–2084. doi:10.1002/pro.5560021208
56. Taylor K, Barran PE, Dorin JR. Structure-activity relationships in beta-defensin peptides. Biopolymers. 2008;90(1):1–7. doi:10.1002/bip.20900
57. Lele DS, Talat S, Kumari S, Srivastava N, Kaur KJ. Understanding the importance of glycosylated threonine and stereospecific action of Drosocin, a Proline rich antimicrobial peptide. Eur J Med Chem. 2015;92:637–647. doi:10.1016/j.ejmech.2015.01.032
58. Ju YL, Yang ST, Kim HJ, Lee SK, Kim JI. Different modes of antibiotic action of homodimeric and monomeric bactenecin, a cathelicidin-derived antibacterial peptide. Bmb Rep. 2009;42(9):586. doi:10.5483/BMBRep.2009.42.9.586
59. Son WS, Kim JS, Kim HE, Park SH, Lee BJ. Structural studies on the antimicrobial peptide Brevinin 1E by spectroscopic methods. Spectrosc Int J. 2003;17(2–3):127–138.
60. Thaker HD, Cankaya A, Scott RW, Tew GN. Role of amphiphilicity in the design of synthetic mimics of antimicrobial peptides with gram-negative activity. ACS Med Chem Lett. 2013;4(5):481. doi:10.1021/ml300307b
61. Liu Y, Jasensky J, Chen Z. Molecular interactions of proteins and peptides at interfaces studied by sum frequency generation vibrational spectroscopy. Langmuir. 2012;28(4):2113–2121.
62. Michael Z. Antimicrobial peptides of multicellular organisms: my perspective. Adv Exp Med Biol. 2019;1117:3–6. doi:10.1007/978-981-13-3588-4_1
63. Brogden KA, Ackermann M, McCray PB, Tack BF. Antimicrobial peptides in animals and their role in host defences. Int J Antimicrob Agents. 2003;22(5):465–478. doi:10.1016/s0924-8579(03)00180-8
64. Otte JM, Zdebik AE, Brand S, et al. Effects of the cathelicidin LL-37 on intestinal epithelial barrier integrity. Regul Pept. 2009;156(1–3):104–117. doi:10.1016/j.regpep.2009.03.009
65. Yang D, Biragyn A, Hoover DM, Lubkowski J, Oppenheim JJ. Multiple roles of antimicrobial defensins, cathelicidins, and eosinophil-derived neurotoxin in host defense. Annu Rev Immunol. 2004;22(1):181–215. doi:10.1146/annurev.immunol.22.012703.104603
66. Pan CY, Lin CN, Chiou MT, Yu CY, Chien CH. The antimicrobial peptide pardaxin exerts potent anti-tumor activity against canine perianal gland adenoma. Oncotarget. 2015;6(4):2290–2301. doi:10.18632/oncotarget.2959
67. Roudi R, Syn NL, Roudbary M. Antimicrobial peptides as biologic and immunotherapeutic agents against cancer: a comprehensive overview. Front Immunol. 2017;8:1320.
68. Leite ML, da Cunha NB, Costa FF. Antimicrobial peptides, nanotechnology, and natural metabolites as novel approaches for cancer treatment. Pharmacol Ther. 2018;183:160–176.
69. Hancock REW, Diamond G. The role of cationic antimicrobial peptides in innate host defences. Trends Microbiol. 2000;8(9):402–410. doi:10.1016/s0966-842x(00)01823-0
70. Suarez-Carmona M, Hubert P, Delvenne P, Herfs MD. “Simple” antimicrobial peptides or broad-spectrum molecules? Cytokine Growth Factor Rev. 2015;26(3):361–370. doi:10.1016/j.cytogfr.2014.12.005
71. Batista Araujo J, Sastre de Souza G, Lorenzon EN. Indolicidin revisited: biological activity, potential applications and perspectives of an antimicrobial peptide not yet fully explored. World J Microbiol Biotechnol. 2022;38(3):39. doi:10.1007/s11274-022-03227-2
72. Scavello F, Amiche M, Ghia J-E. Recent advances in multifunctional antimicrobial peptides as immunomodulatory and anticancer therapy: chromogranin a-derived peptides and dermaseptins as endogenous versus exogenous actors. Pharmaceutics. 2022;14(10):2014. doi:10.3390/pharmaceutics14102014
73. Bhargava A, Osusky M, Hancock RE, Forward BS, Kay WW, Misra S. Antiviral indolicidin variant peptides: evaluation for broad-spectrum disease resistance in transgenic Nicotiana tabacum. Plant Sci. 2007;172(3):515–523. doi:10.1016/j.plantsci.2006.10.016
74. Jang WS, Edgerton M. Salivary histatins: structure, function, and mechanisms of antifungal activity. Candida Candidiasis. 2011;185–194.
75. Matejuk A, Leng Q, Begum MD, et al. Peptide-based antifungal therapies against emerging infections. Drugs Future. 2010;35(3):197. doi:10.1358/dof.2010.35.3.1452077
76. Pistolic J, Cosseau C, Li Y, et al. Host defence peptide LL-37 induces IL-6 expression in human bronchial epithelial cells by activation of the NF-kappaB signaling pathway. J Innate Immun. 2009;1(3):254–267. doi:10.1159/000171533
77. Robinson WE, McDougall B, Tran D, Selsted ME. Anti-HIV-1 activity of indolicidin, an antimicrobial peptide from neutrophils. J Leukoc Biol. 1998;63(1):94–100. doi:10.1002/jlb.63.1.94
78. Klotman ME, Chang TL. Defensins in innate antiviral immunity. Nat Rev Immunol. 2006;6(6):447–456. doi:10.1038/nri1860
79. Guo C, Cong P, He Z, et al. Inhibitory activity and molecular mechanism of protegrin-1 against porcine reproductive and respiratory syndrome virus in vitro. Antivir Ther. 2015;20(6):573–582. doi:10.3851/IMP2918
80. Pretzel J, Mohring F, Rahlfs S, Becker K. Antiparasitic peptides. In: Yellow Biotechnology I: Insect Biotechnologie in Drug Discovery and Preclinical Research. Springer; 2013:157–192.
81. Tornesello AL, Borrelli A, Buonaguro L, Buonaguro FM, Tornesello ML. Antimicrobial peptides as anticancer agents: functional properties and biological activities. Molecules. 2020;25(12):2850. doi:10.3390/molecules25122850
82. Hirsch T, Spielmann M, Zuhaili B, et al. Human beta-defensin-3 promotes wound healing in infected diabetic wounds. J Gene Med. 2009;11(3):220–228. doi:10.1002/jgm.1287
83. Tomioka H, Nakagami H, Tenma A, et al. Novel anti-microbial peptide SR-0379 accelerates wound healing via the PI3 kinase/Akt/mTOR pathway. PLoS One. 2014;9(3):e92597. doi:10.1371/journal.pone.0092597
84. Mader JS, Hoskin DW. Cationic antimicrobial peptides as novel cytotoxic agents for cancer treatment. Expert Opin Invest Drugs. 2006;15(8):933–946. doi:10.1517/13543784.15.8.933
85. Feix SJB. Peptide–membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim Biophys Acta. 2006;1758(9):1245–1256.
86. Hoskin DW, Ramamoorthy A. Studies on anticancer activities of antimicrobial peptides. Biochim Biophys Acta. 2008;1778(2):357–375. doi:10.1016/j.bbamem.2007.11.008
87. Yang P, Ramamoorthy A, Chen Z. Membrane orientation of MSI-78 measured by sum frequency generation vibrational spectroscopy. Langmuir. 2011;27(12):7760.
88. Papo N, Braunstein A, Eshhar Z, Shai Y. Suppression of human prostate tumor growth in mice by a cytolytic d -, l -amino acid peptide. Cancer Res. 2004;64(16):5779–5786. doi:10.1158/0008-5472.CAN-04-1438
89. Benincasa M, Runti G, Mardirossian M, Scocchi M. Non-membrane permeabilizing modes of action of antimicrobial peptides on bacteria. Curr Top Med Chem. 2016;16(1):76–88. doi:10.2174/1568026615666150703121009
90. He K, Ludtke SJ, Worcester DL, Huang HW. Neutron scattering in the plane of membranes: structure of alamethicin pores. Biophys J. 1996;70(6):2659–2666. doi:10.1016/S0006-3495(96)79835-1
91. Powers JPS, Hancock REW. The relationship between peptide structure and antibacterial activity. Peptides. 2003;24(11):0–1691. doi:10.1016/j.peptides.2003.08.023
92. Dobrzyńska I, Szachowicz-Petelska B, Sulkowski S, Figaszewski Z. Changes in electric charge and phospholipids composition in human colorectal cancer cells. Mol Cell Biochem. 2005;276(1–2):113–119. doi:10.1007/s11010-005-3557-3
93. Yoon WH, Park HD, Lim K, Hwang BD. Effect of O-glycosylated mucin on invasion and metastasis of HM7 human colon cancer cells. Biochem Biophys Res Commun. 1996;222(3):0–699. doi:10.1006/bbrc.1996.0806
94. Price JAR, Pethig R, Lai CN, Becker FF, Szent-Györgyi A. Changes in cell surface charge and transmembrane potential accompanying neoplastic transformation of rat kidney cells. Biochim Biophys Acta. 1987;898(2):129–136. doi:10.1016/0005-2736(87)90031-9
95. Papo N, Shahar M, Eisenbach L, Shai Y. A novel lytic peptide composed of DL-amino acids selectively kills cancer cells in culture and in mice. J Biol Chem. 2003;278(23):21018–21023. doi:10.1074/jbc.M211204200
96. Chan SC, Hui L, Chen HM. Enhancement of the cytolytic effect of anti-bacterial cecropin by the microvilli of cancer cells. Anticancer Res. 1998;18(6A):4467–4474.
97. Domagala W, Koss LG. Surface configuration of human tumor cells obtained by fine needle aspiration biopsy. Scan Electron Microsc. 1980;3(3):101–108.
98. Pohl A, Lage H, Müller P, Pomorski T, Herrmann A. Transport of phosphatidylserine via MDR1 (multidrug resistance 1)P-glycoprotein in a human gastric carcinoma cell line. Biochem J. 2002;365(1):259–268. doi:10.1042/bj20011880
99. Yu Hang L, Shi Liang Y, Guo Gan X, Ha Pan S, Qiu Teng Y, An R-H. Externalization of phosphatidylserine via multidrug resistance 1 (MDR1)/P-glycoprotein in oxalate-treated renal epithelial cells: implications for calcium oxalate urolithiasis. Int Urol Nephrol. 2016;48(2):175–181. doi:10.1007/s11255-015-1155-1
100. Wang KR, Yan JX, Zhang BZ, Song JJ, Jia PF, Wang R. Novel mode of action of polybia-MPI, a novel antimicrobial peptide, in multi-drug resistant leukemic cells. Cancer Lett. 2009;278(1):65–72. doi:10.1016/j.canlet.2008.12.027
101. Oren Z, Shai Y. Mode of action of linear amphipathic alpha-helical antimicrobial peptides. Biopolymers. 1998;47(6):451–463. doi:10.1002/(SICI)1097-0282(1998)47:6<451::AID-BIP4>3.0.CO;2-F
102. Priyadarshini D, Ivica J, Separovic F, de Planque MRR. Characterisation of cell membrane interaction mechanisms of antimicrobial peptides by electrical bilayer recording. Biophys Chem. 2022;281:106721. doi:10.1016/j.bpc.2021.106721
103. Yang L, Harroun TA, Weiss TM, Ding L, Huang HW. Barrel-stave model or toroidal model_ a case study on melittin pores. Biophys J. 2001;81(3):1475–1485. doi:10.1016/S0006-3495(01)75802-X
104. Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacol Rev. 2003;55(1):27–55. doi:10.1124/pr.55.1.2
105. Ludtke SJ, He K, Heller WT, Harroun TA, Yang L, Huang HW. Membrane pores induced by magainin. Biochemistry. 1996;35(43):13723–13728. doi:10.1021/bi9620621
106. Wang YQ, Cai JY, Ma S, Zhang X. Effects of the antimicrobial peptide magaininIIon Escherichia coli observed by atomic force microscopy. J Chin Electron Microsc Soc. 2006;25(1):52–56.
107. Matsuzaki K, Murase O, Fujii N, Miyajima K. An antimicrobial peptide, magainin 2, induced rapid flip-flop of phospholipids coupled with pore formation and peptide translocation †. Biochemistry. 1996;35(35):11361–11368. doi:10.1021/bi960016v
108. Park SC, Kim JY, Shin SO, et al. Investigation of toroidal pore and oligomerization by melittin using transmission electron microscopy. Biochem Biophys Res Commun. 2006;343(1):222–228. doi:10.1016/j.bbrc.2006.02.090
109. Yamaguchi S, Hong T, Waring A, Lehrer RI, Hong M. Solid-state NMR investigations of peptide-lipid interaction and orientation of a beta-sheet antimicrobial peptide, protegrin. Biochemistry. 2002;41(31):9852–9862. doi:10.1021/bi0257991
110. Wu M, Maier E, Benz R, Hancock REW. Mechanism of interaction of different classes of cationic antimicrobial peptides with planar bilayers and with the cytoplasmic membrane of Escherichia coli ? Biochemistry. 1999;38(22):7235–7242. doi:10.1021/bi9826299
111. Hari L. Antimicrobial peptides in action. Journal of the American Chemical Society. 2006;128(37):12156–12161.
112. Aarbiou J, Tjabringa GS, Verhoosel RM, et al. Mechanisms of cell death induced by the neutrophil antimicrobial peptides α-defensins and LL-37. Inflamm Res. 2006;55(3):119–127. doi:10.1007/s00011-005-0062-9
113. Liu ZY, Xu T, Zheng ST, Zhang LT, Zhang FC. Study on the interaction mechanism of antimicrobial peptide Cecropin-XJ in Xinjiang silkworm and Staphylococcus aureus DNA by spectra. Spectrosc Spectral Anal. 2008;28(3):612–616.
114. Wang F, Zhang SQ, Dai ZY. Studies on the action of the CM4-ABP anti K562 cancer cells by SCGE. Prog Biochem Biophys. 1998;1998:1.
115. Jaynes JM. Lytic peptides, use for growth, infection and cancer. Schweiz Pat. 2000;1:38.
116. Chen HM, Leung KW, Thakur NN, Tan A, Jack RW. Distinguishing between different pathways of bilayer disruption by the related antimicrobial peptides cecropin B, B1 and B3. Eur J Biochem. 2003;270(5):911–920. doi:10.1046/j.1432-1033.2003.03451.x
117. Theansungnoen T, Maijaroen S, Jangpromma N, et al. Cationic antimicrobial peptides derived fromCrocodylus siamensisLeukocyte extract, revealing anticancer activity and apoptotic induction on human cervical cancer cells. Protein J. 2016;35(3):202–211. doi:10.1007/s10930-016-9662-1
118. Chavakis T, Cines DB, Rhee JS, et al. Regulation of neovascularization by human neutrophil peptides (α-defensins): a link between inflammation and angiogenesis. FASEB J. 2004;18(11):1306–1308. doi:10.1096/fj.03-1009fje
119. Lee HS, Chan BP, Kim JM, et al. Mechanism of anticancer activity of buforin IIb, a histone H2A-derived peptide. Cancer Lett. 2008;271(1):0–55. doi:10.1016/j.canlet.2008.05.041
120. Bhat S, Milner S. Antimicrobial peptides in burns and wounds. Curr Protein Peptide Sci. 2007;8(5):506–520. doi:10.2174/138920307782411428
121. Hiemstra PS, Amatngalim GD, Does AMVD, Taube C. Antimicrobial peptides and innate lung defenses: role in infectious and noninfectious lung diseases and therapeutic applications. Chest. 2016;149(2):545–551. doi:10.1378/chest.15-1353
122. Davidson DJ, Currie AJ, Reid GS, et al. The cationic antimicrobial peptide LL-37 modulates dendritic cell differentiation and dendritic cell-induced T cell polarization. J Immunol. 2004;172(2):1146–1156. doi:10.4049/jimmunol.172.2.1146
123. Xia L, Wu Y, Ma JI, Yang J, Zhang F. The antibacterial peptide from Bombyx mori cecropinXJ induced growth arrest and apoptosis in human hepatocellular carcinoma cells. Oncol Lett. 2016;12(1):57–62. doi:10.3892/ol.2016.4601
124. Deslouches B, Di YP. Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. Oncotarget. 2017;8(28):46635.
125. Balandin SV, Emelianova AA, Kalashnikova MB, Kokryakov VN, Shamova OV, Ovchinnikova TV. Molecular mechanisms of antitumor effect of natural antimicrobial peptides. Russ J Bioorganic Chem. 2016;42(6):575–589. doi:10.1134/S1068162016060029
126. Saravanan D, Mohammed Al-Kassim H. A Review Of Potential Anticancers From Antimicrobial Peptides. Int J Pharm Pharm Sci. 2015;7(4):19–26.
127. Papo N, Shai Y. Host defense peptides as new weapons in cancer treatment. Cell Mol Life Sci Cmls. 2005;62(7–8):784–790. doi:10.1007/s00018-005-4560-2
128. Jacob L, Zasloff M. Potential therapeutic applications of magainins and other antimicrobial agents of animal origin. Ciba Found Symp. 1994;186:197. doi:10.1002/9780470514658.ch12
129. Baker MA, Maloy WL, Zasloff M, Jacob LS. Anticancer efficacy of Magainin2 and analogue peptides. Cancer Res. 1993;53(13):3052.
130. Chen HM, Wei W, Smith D, Chan SC. Effects of the anti-bacterial peptide cecropin B and its analogs, cecropins B-1 and B-2, on liposomes, bacteria, and cancer cells. Biochim Biophys Acta. 1997;1336(2):171–179. doi:10.1016/S0304-4165(97)00024-X
131. Moore A, Devine D, Bibby MC. Preliminary experimental anticancer activity of cecropins. Pept Res. 1994;7(5):265–269.
132. Selsted ME, Harwig SS, Ganz T, Schilling JW, Lehrer RI. Primary structures of three human neutrophil defensins. J Clin Invest. 1985;76(4):1436–1439. doi:10.1172/JCI112121
133. Pausch T, Adolph S, Felix K, et al. Antimicrobial peptide human neutrophil peptide 1 as a potential link between chronic inflammation and ductal adenocarcinoma of the pancreas. Pancreas. 2018;47(5):561–567. doi:10.1097/MPA.0000000000001054
134. Nishimura M, Abiko Y, Kurashige Y, et al. Effect of defensin peptides on eukaryotic cells: primary epithelial cells, fibroblasts and squamous cell carcinoma cell lines. J Dermatol Sci. 2004;36(2):87–95. doi:10.1016/j.jdermsci.2004.07.001
135. Strzelecka P, Czaplinska D, Sadej R, Wardowska A, Pikula M, Lesner A. Simplified, serine-rich theta-defensin analogues as antitumour peptides. Chem Biol Drug Des. 2017;90(1):52–63. doi:10.1111/cbdd.12927
136. Bensch KW, Raida M, M/igert H-J, Schulz-Knappe P, Forssmann W-G. hBD-I_ a novel fl-defensin from human plasma. FEBS Lett. 1995;368:331–335. doi:10.1016/0014-5793(95)00687-5
137. Baindara P, Gautam A, Raghava GPS, Korpole S. Anticancer properties of a defensin like class IId bacteriocin Laterosporulin10. Sci Rep. 2017;7:46541. doi:10.1038/srep46541
138. Gerashchenko OL, Zhuravel EV, Skachkova OV, et al. Biologic activities of recombinant human-beta-defensin-4 toward cultured human cancer cells. Exp Oncol. 2013;35(2):76.
139. Bellamy WR, Takase M, Wakabayashi H, Kawase K, Tomita M. Antibacterial spectrum of lactoferricin B, a potent bactericidal peptide derived from the N-terminal region of bovine lactoferrin. J Appl Bacteriol. 1993;73(6):472–479. doi:10.1111/j.1365-2672.1992.tb05007.x
140. Eliassen LT, Berge G, Leknessund A, Wikman M, Ø R. The antimicrobial peptide, Lactoferricin B, is cytotoxic to neuroblastoma cells in vitro and inhibits xenograft growth in vivo. Int J Cancer. 2006;119(3):493–500. doi:10.1002/ijc.21886
141. Yerly VC, Jorge RG, Yadi UAP, et al. Antibacterial synthetic peptides derived from bovine lactoferricin exhibit cytotoxic effect against MDA-MB-468 and MDA-MB-231 breast cancer cell lines. Molecules. 2017;22(10):1.
142. Suttmann H, Retz M, Paulsen F, Harder J, Lehmann J. Antimicrobial peptides of the cecropin-family show potent antitumor activity against bladder cancer cells. BMC Urol. 2008;8(1):5. doi:10.1186/1471-2490-8-5
143. Cerón JM, Contreras-Moreno J, Puertollano E, Gád C, Puertollano MA, Pablo MAD. The antimicrobial peptide cecropin A induces caspase-independent cell death in human promyelocytic leukemia cells. Peptides. 2010;31(8):0–1503. doi:10.1016/j.peptides.2010.05.008
144. Liu S, Yang H, Wan L, Cai HW, Lu XF. Enhancement of cytotoxicity of antimicrobial peptide magainin II in tumor cells by bombesin-targeted delivery. Acta Pharmacol Sin. 2011;32(1):79–88. doi:10.1038/aps.2010.162
145. Liu S, Yang H, Wan L, Cheng J, Lu X. Penetratin-mediated delivery enhances the antitumor activity of the cationic antimicrobial peptide magainin II. Cancer Biother Radiopharm. 2013;28(4):289–297. doi:10.1089/cbr.2012.1328
146. Bepler G, Carney DN, Nau MM, Gazdar AF, Minna JD. Antitumor activity of magainin analogues against human lung cancer cell lines. Cancer Res. 1992;52(13):3534–3538.
147. Kim R, Emi M, Tanabe K. Role of mitochondria as the gardens of cell death. Cancer Chemother Pharmacol. 2006;57(5):545–553. doi:10.1007/s00280-005-0111-7
148. Gauldie J, Hanson JM, Shipolini RA, Vernon CA. The structures of some peptides from bee venom. FEBS J. 2008;83(2):405–410.
149. Sui SF, Wu H, Guo Y, Chen KS. Conformational changes of melittin upon insertion into phospholipid monolayer and vesicle. J Biochem. 1994;116(3):482. doi:10.1093/oxfordjournals.jbchem.a124550
150. Risso A, Braidot E, Sordano MC, et al. BMAP-28, an antibiotic peptide of innate immunity, induces cell death through opening of the mitochondrial permeability transition pore. Mol Cell Biol. 2002;22(6):1926–1935. doi:10.1128/mcb.22.6.1926-1935.2002
151. Skerlavaj B, Gennaro R, Bagella L, Merluzzi L, Risso A, Zanetti M. Biological characterization of two novel cathelicidin-derived peptides and identification of structural requirements for their antimicrobial and cell lytic activities. J Biol Chem. 1996;271(45):28375–28381. doi:10.1074/jbc.271.45.28375
152. Ren SX, Jin S, Cheng ASL, et al. FK-16 derived from the anticancer peptide LL-37 induces caspase-independent apoptosis and autophagic cell death in colon cancer cells. PLoS One. 2013;8:1.
153. Zanetti M. Cathelicidins, multifunctional peptides of the innate immunity. J Leukoc Biol. 2004;75(1):39–48. doi:10.1189/jlb.0403147
154. Xu N, Yuan HY, Wang FL, Liao XY, Chang YP. Effect of hCAP-18/LL-37 gene transfection on proliferation and apoptosis of Lewis cells. J Jilin Univ Med Ed. 2009;35(6):1040–1043.
155. Zoggel HV, Carpentier G, Santos CD, et al. Antitumor and angiostatic activities of the antimicrobial peptide dermaseptin B2. PLoS One. 2012;7(9):1.
156. Dong Z, Hu H, Yu X, et al. Novel frog skin-derived peptide dermaseptin-PP for lung cancer treatment: in vitro/vivo evaluation and anti-tumor mechanisms study. Front Chem. 2020;8:476. doi:10.3389/fchem.2020.00476
157. Che W, Li-Li T, Song L, et al. Rapid cytotoxicity of antimicrobial peptide tempoprin-1CEa in breast cancer cells through membrane destruction and intracellular calcium mechanism. PLoS One. 2013;8(4):e60462. doi:10.1371/journal.pone.0060062
158. Wang C, Zhou Y, Li S, et al. Anticancer mechanisms of temporin-1CEa, an amphipathic α-helical antimicrobial peptide, in Bcap-37 human breast cancer cells. Life Sci. 2013;92(20–21):1004–1014. doi:10.1016/j.lfs.2013.03.016
159. Wang C, Chen YW, Zhang L, et al. Melanoma cell surface-expressed phosphatidylserine as a therapeutic target for cationic anticancer peptide, temporin-1CEa. J Drug Target. 2016;24(6):548–556.
160. Ouyang GL, Li QF, Peng XX, Liu QR, Hong SG. Effects of tachyplesin on proliferation and differentiation of human hepatocellular carcinoma SMMC-7721 cells. World J Gastroenterol. 2002;8(6):1053–1058. doi:10.3748/wjg.v8.i6.1053
161. Chen Y, Xu X, Hong S, Chen J, Zhang L. RGD-tachyplesin inhibits tumor growth. Cancer Res. 2001;61(6):2434–2438.
162. Hilchie AL, Doucette CD, Pinto DM, Patrzykat A, Douglas S, Hoskin DW. Pleurocidin-family cationic antimicrobial peptides are cytolytic for breast carcinoma cells and prevent growth of tumor xenografts. Breast Cancer Res. 2011;13(5):1–16. doi:10.1186/bcr3043
163. Kim S, Kim SS, Bang YJ, Kim SJ, Lee BJ. In vitro activities of native and designed peptide antibiotics against drug sensitive and resistant tumor cell lines. Peptides. 2003;24(7):945–953. doi:10.1016/S0196-9781(03)00194-3
164. Jing H. Effects of Antimicrobial Peptides Temporin-1CEa on Multidrug Resistance of MCF-7/Adr Cells. Liaoning Normal University; 2016. Chinese.
165. To KKW, Ren SX, Wong CCM, Cho CH. Reversal of ABCG2-mediated multidrug resistance by human cathelicidin and its analogs in cancer cells. Peptides. 2013;40:13–21. doi:10.1016/j.peptides.2012.12.019
166. Shan GUO, Rui-jun ZHAO, Jing-xia C. Effect of antimicrobial peptides on proliferation and multidrug resistance in multidrug resistant human hepatocellular carcinoma Bel-7402/ADM cells. Journal Article. Chin J Public Health. 2019;35(01):48–52. Chinese.
167. Hui L, Leung K, Chen HM. The combined effects of antibacterial peptide cecropin A and anti-cancer agents on leukemia cells. Anticancer Res. 2002;22(5):2811–2816.
168. Do N, Weindl G, Grohmann L, et al. Cationic membrane-active peptides - anticancer and antifungal activity as well as penetration into human skin. Exp Dermatol. 2014;23(5):326–331. doi:10.1111/exd.12384
169. Xia L, Zhang F. Effect of Cecropin-XJ and it’ s combination with chemotherapeutic agents on the proliferation and apoptosis of human esophageal cancer Eca109 cells. Chin J Cancer Biother. 2014;21(4):402–407.
170. Francesca M, Marina G, Elena R. Conjugation of photosensitisers to antimicrobial peptides increases the efficiency of photodynamic therapy in cancer cells. Photochem Photobiol Sci. 2015;2015:1.
171. Wang X, Donghui LI. Effect of APBMV on large intestine cancer LoVo cells and its synergetic effect with chemotherapy drugs. Qianw J Med. 2003;2003:1.
172. Wang X, Donghui LI, Gao C. Antitumor effect of ant icancer polypeptide from buthus martensii venom: experiment in vivo. Qianw J Med. 2003;2003:1.
173. Zhang B, Shi W, Li J, et al. Synthesis and biological evaluation of novel peptides based on antimicrobial peptides as potential agents with antitumor and multidrug resistance‐reversing activities. Chem Biol Drug Des. 2017;2017:1.
174. Johnstone S, Gelmon K, Mayer LD, Hancock RE, Bally MB. In vitro characterization of the anticancer activity of membrane-active cationic peptides. I. Peptide-mediated cytotoxicity and peptide-enhanced cytotoxic activity of doxorubicin against wild-type and P-glycoprotein over-expressing tumor cell lines. Anticancer Drug Des. 2000;15(2):151–160.
175. Winder D, Günzburg WH, Erfle V, Salmons B. Expression of antimicrobial peptides has an antitumour effect in human cells. Biochem Biophys Res Commun. 1998;242(3):0–612. doi:10.1006/bbrc.1997.8014
176. Ling C-Q, Li B, Zhang C. Inhibitory effect of recombinant adenovirus carrying melittin gene on hepatocellular carcinoma. Ann Oncol. 2005;16(1):109–115. doi:10.1093/annonc/mdi019
177. Xu B, Dong CY, Zhang F, Lin YM, Wu KF, Ma XT. Synergistic antileukemia effect of combinational gene therapy using murine b-defensin 2 and IL-18 in L1210 murine leukemia model. Gene Ther. 2007;14(15):1181–1187. doi:10.1038/sj.gt.3302966
178. Zou M, Zhang L, Xie Y, Xu W. NGR-based strategies for targeting delivery of chemotherapeutics to tumor vasculature. Anticancer Agents Med Chem. 2012;12(3):1.
179. Chakravarty R, Chakraborty S, Dash A. Molecular imaging of breast cancer: role of RGD peptides. Mini Rev Med Chem. 2015;15(13):1073–1094. doi:10.2174/1389557515666150909144606
180. Me M, Myrberg H, El-Andaloussi S, l L. Design of a tumor homing cell-penetrating peptide for drug delivery. Int J Pept Res Ther. 2009;15(1):11–15. doi:10.1007/s10989-008-9156-x
181. Ma XY, Shu L, Luo DF, Liu RH, Ling X. Antitumor and antimetastatic effect of antimicrobial peptide conjugated with tumor homing peptide TMTP1 on the transplanted prostate cancer and gastric cancer in nude mice. Zhonghua Zhong Liu Za Zhi. 2013;35(10):737–741.
182. Liu S, Yang H, Cai H, Wan L, Lu X. Enhancement of cytotoxicity of cantionic antimicrobial peptide in tumor cells by conjugation to cell-penetrating peptide. J Biomed Eng. 2011;28(1):110.
183. Jing H, Cuixia C, Shengzhong Z, et al. Designed antimicrobial and antitumor peptides with high selectivity. Biomacromolecules. 2011;12(11):3839–3843. doi:10.1021/bm201098j
184. Urban P, Jose Valle-Delgado J, Moles E, Marques J, Diez C, Fernandez-Busquets X. Nanotools for the delivery of antimicrobial peptides. Curr Drug Targets. 2012;13(9):1158–1172. doi:10.2174/138945012802002302
185. Strand A. Values in nanomedical research: a discussion based on the NANOCAN project on nanoparticles in cancer therapy and diagnosis. Nanoethics. 2017;11(3):259–271. doi:10.1007/s11569-017-0295-4
186. Becker C, Bopp T, Jonuleit H. Treg cells as potential cellular targets for functionalized nanoparticles in cancer therapy. Nanomedicine. 2016;11(20):2699–2709. doi:10.2217/nnm-2016-0197
187. Swenson S, Costa F, Minea R, et al. Intravenous liposomal delivery of the snake venom disintegrin contortrostatin limits breast cancer progression. Mol Cancer Ther. 2004;3(4):499–511. doi:10.1158/1535-7163.499.3.4
188. Tian W, Li B, Zhang X, Dang W, Chen T. Suppression of tumor invasion and migration in breast cancer cells following delivery of siRNA against Stat3 with the antimicrobial peptide PR39. Oncol Rep. 2012;28(4):1362–1368. doi:10.3892/or.2012.1911
189. Ines N. Antimicrobial and cell-penetrating peptides: how to understand two distinct functions despite similar physicochemical properties. Adv Exp Med Biol. 2019;1117:93–109. doi:10.1007/978-981-13-3588-4_7
190. Splith K, Neundorf I. Antimicrobial peptides with cell-penetrating peptide properties and vice versa. Eur Biophys J. 2011;40(4):387–397. doi:10.1007/s00249-011-0682-7
191. Liu CY, Xiao YZ, Li RS, Li XR, Yuan HL. Preparation and in vitro evaluation of antibacterial peptides from Plutella xylostella poly(lactic-co-glycolic acid) nanoparticles. Chin Traditional Herbal Drugs. 2015;46(3):348–352.
192. Soman NR, Baldwin SL, Hu G, et al. Molecularly targeted nanocarriers deliver the cytolytic peptide melittin specifically to tumor cells in mice, reducing tumor growth. J Clin Invest. 2009;119(9):2830–2842. doi:10.1172/JCI38842
193. Badr G, Al-Sadoon MK, El-Toni AM, Daghestani M. Walterinnesia aegyptia venom combined with silica nanoparticles enhances the functioning of normal lymphocytes through PI3K/AKT, NFκB and ERK signaling. Lipids Health Dis. 2012;11:1.
194. Badr G, Al-Sadoon MK, Rabah DM, Sayed D. Snake (Walterinnesia aegyptia) venom-loaded silica nanoparticles induce apoptosis and growth arrest in human prostate cancer cells. Apoptosis. 2012;18(3):1.
195. Al-Sadoon MK, Rabah DM, Badr G. Enhanced anticancer efficacy of snake venom combined with silica nanoparticles in a murine model of human multiple myeloma: molecular targets for cell cycle arrest and apoptosis induction. Cell Immunol. 2013;284(1–2):129–138. doi:10.1016/j.cellimm.2013.07.016
196. Tan H, Huang Y, Xu J, et al. Spider toxin peptide lycosin-I functionalized gold nanoparticles for in vivo tumor targeting and therapy. Toxicon. 2019:158. doi:10.1016/j.toxicon.2019.07.005
197. Di WU, Zhao Y, Ren HD, Xin-Hong SI, Zhang L, Wang C. Construction of cationic anticancer peptide temporin-1CEa liposomes and evaluation of anti-breast cancer activity in vitro. Chin J Biochem Pharmaceutics. 2016;6 :22–26.
198. Wang C, Dong S, Zhang L, et al. Cell surface binding, uptaking and anticancer activity of L-K6, a lysine/leucine-rich peptide, on human breast cancer MCF-7 cells. Entific Rep. 2017;7(1):8293.
199. Chen Y-W. Preparation, Characterization and Anti-Cancer Activity Evaluation of L-K6 Antimicrobial Peptide-Liposomal Drug System. Liaoning Normal University; 2016. Chinese.
200. Huang C, Jin H, Qian Y, et al. Hybrid melittin cytolytic peptide-driven ultrasmall lipid nanoparticles block melanoma growth in vivo. ACS Nano. 2013;7(7):5791–5800. doi:10.1021/nn400683s
201. Yamada Y, Shinohara Y, Kakudo T, et al. Mitochondrial delivery of mastoparan with transferrin liposomes equipped with a pH-sensitive fusogenic peptide for selective cancer therapy. Int J Pharm. 2005;303(1–2):1–7. doi:10.1016/j.ijpharm.2005.06.009
202. Tseng Y-L, Liu -J-J, Hong R-L. Translocation of liposomes into cancer cells by cell-penetrating peptides penetratin and tat: a kinetic and efficacy study. Mol Pharmacol. 2002;62(4):864. doi:10.1124/mol.62.4.864
203. Sharma G, Modgil A, Zhong T, Sun C, Singh J. Influence of short-chain cell-penetrating peptides on transport of doxorubicin encapsulating receptor-targeted liposomes across brain endothelial barrier. Pharm Res. 2014;31(5):1194–1209. doi:10.1007/s11095-013-1242-x
204. Henriques S, Melo M, Castanho M. Cell-penetrating peptides and antimicrobial peptides: how different are they? Biochem J. 2006;399(1):1–7. doi:10.1042/BJ20061100
205. Hu C, Chen X, Zhao W, Chen Y, Huang Y. Design and modification of anticancer peptides. Drug Des. 2016;5(3):1–10. doi:10.4172/2169-0138.1000138
206. Giangaspero A, Sandri L, Tossi A. Amphipathic, α‐helical antimicrobial peptides. Pept Sci. 2015;55(21):4–30.
207. Diao Y, Han W, Zhao H, et al. Designed synthetic analogs of the alpha-helical peptide temporin-La with improved antitumor efficacies via charge modification and incorporation of the integrin alphavbeta3 homing domain. J Pept Sci. 2012;18(7):476–486. doi:10.1002/psc.2420
208. Dathe M, Nikolenko H, Meyer J, Beyermann M, Bienert M. Optimization of the antimicrobial activity of magainin peptides by modification of charge. FEBS Lett. 2001;501(2–3):146–150. doi:10.1016/S0014-5793(01)02648-5
209. Chen Y, Guarnieri MT, Vasil AI, Vasil ML, Mant CT, Hodges RS. Role of peptide hydrophobicity in the mechanism of action of α-helical antimicrobial peptides. Antimicrob Agents Chemother. 2007;51(4):1398–1406.
210. Huang Y, Huang J, Chen Y. Alpha-helical cationic antimicrobial peptides: relationships of structure and function. Protein and Cell. 2010;1(2):143–152. doi:10.1007/s13238-010-0004-3
211. Ganz T. The role of antimicrobial peptides in innate immunity. Integr Comp Biol. 2003;43(2):300–304. doi:10.1093/icb/43.2.300
212. Ma XT, Xu B, An LL, Dong CY, Wu KF. Vaccine with beta-defensin 2-transduced leukemic cells activates innate and adaptive immunity to elicit potent antileukemia responses. Cancer Res. 2006;66(2):1169. doi:10.1158/0008-5472.CAN-05-2891
213. Zhang X, Jin L, Wang Z, Wang Q. Fusion expression of antimicrobial peptides in Escherichia coli. Chin J Biotechnol. 2014;30(8):1172.
214. Fanhong K, Weixia Z, Yong C, Hongfa W. Advanced on Pichia pastoris expression of antimicrobial peptides. J Pathogen Biol. 2009;4(9):700–702.
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.