Back to Journals » Therapeutics and Clinical Risk Management » Volume 12
Antiallergic effect of the atomized extract of rhizome of Curcuma longa, flowers of Cordia lutea and leaves of Annona muricata
Authors Arroyo-Acevedo J, Franco-Quino C , Ruiz-Ramírez E, Chávez-Asmat R , Anampa-Guzmán A , Raéz-González E, Cabanillas-Coral J
Received 25 June 2016
Accepted for publication 31 August 2016
Published 10 November 2016 Volume 2016:12 Pages 1643—1647
DOI https://doi.org/10.2147/TCRM.S115786
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Jorge Arroyo-Acevedo,1–3 Cesar Franco-Quino,4,5 Eliberto Ruiz-Ramirez,4,5 Roberto Chávez-Asmat,1,6 Andrea Anampa-Guzmán,7,8 Ernesto Raéz-González,3 José Cabanillas-Coral9
1Pharmacology Laboratory, Institute of Clinical Research, National University of San Marcos, Lima, Peru; 2Institute of Clinical Research, National University of San Marcos, Lima, Peru; 3Institute of Pathology, Faculty of Medicine, National University of San Marcos, Lima, Peru; 4Laboratory of Pharmacology and Physiology, Faculty of Dentistry, National University of San Marcos, Lima, Peru; 5Graduate Unit, Faculty of Pharmacy and Biochemistry, National University of San Marcos, Lima, Peru; 6Association for the Development of Student Research in Health Sciences (ADIECS), Lima, Peru; 7School of Human Medicine, Faculty of Medicine, National University of San Marcos, Lima, Peru; 8Sociedad Científica de San Fernando (SCSF), Lima, Peru; 9Faculty of Medicine, National University San Luis Gonzaga of Ica, Ica, Peru
Introduction: Allergies are a problem that greatly affects the population, and hence the use of antiallergic medications is fairly widespread. However, these drugs have many adverse effects. The use of medicinal plants could be an option, but they need to be evaluated.
Objective: This study was designed to evaluate the antiallergic effect of the atomized extract of rhizome of Curcuma longa, flowers of Cordia lutea, and leaves of Annona muricata.
Materials and methods: Twenty-four New Zealand white albino rabbits were randomized into 2 groups. Group A received the atomized extract diluted in physiological saline (APS) and group B received it diluted in Freund’s adjuvant (FA). Then, the back of each rabbit was divided into 4 quadrants. The A-I quadrant received only physiological saline. The A-I quadrants of each rabbit conformed the PS group. The following 3 quadrants received the APS in 10 µg/mL, 100 µg/mL, and 1,000 µg/mL, respectively. The B-I quadrant received only FA. The B-I quadrants of each rabbit conformed the FA group. The following 3 quadrants received the AFA in 10 µg/mL, 100 µg/mL, and 1,000 µg/mL, respectively. The occurrence of erythema and edema was recorded according to the Draize scoring system and the primary irritation index. After 72 hours, biopsies were performed.
Results: The AFA group presented significantly less erythema and edema compared to the FA group (P<0.05). The histopathologic evaluation at 72 hours showed normal characteristics in the APS group.
Conclusion: Considering the clinical and histopathological signs, we conclude that the administration of the atomized extract of rhizome of C. longa, flowers of C. lutea, and leaves of A. muricata lacks antigenic effect but could have an antiallergenic effect in a model of dermal irritation in rabbits.
Keywords: rabbit, irritation, erythema, edema
Introduction
Currently, natural medicine provides valuable resources to meet the requirements for the care of global health at affordable prices, and hence it is important to test the safety and efficacy comparable to conventional therapeutic drugs.1 It is important that natural products do not produce toxicity due to long-lasting therapies. Nowadays, allergies are on the rise in world population,2 and the economic development of a country is related to the onset of allergic problems.3 Food allergies and contact dermatitis have become a serious problem.4,5
Allergies are a problem that greatly affects the population,3,4 and thus the use of antiallergic medications is fairly widespread. On the other hand, these drugs have adverse effects on the central and autonomic nervous systems.6–8 The H1 first-generation antihistamines, inverse agonists of histamine receptors, are the most popular antiallergic medications and are over-the-counter drugs.7 However, due to their poor selectivity, there are many side effects such as drowsiness, sedation, and depression of the central nervous system. Being also antagonists of the muscarinic receptor, they cause mydriasis, dry mouth, constipation, and urinary retention.8
The lyophilized association of rhizome of Curcuma longa, flowers of Cordia lutea, and leaves of Annona muricata is a product that has been approved in Canada in 2012 as a natural product of health with nutraceutical and hepatoprotectant properties. However, the preparation of the lyophilized association is expensive. The atomized association is cheaper and, like the lyophilized one, it preserves the secondary metabolites. The atomized association has been proven to be innocuous when administered orally for 28 days in Holtzman rats,9 but it does not have preclinical safety data.10 This study aims to evaluate the antiallergic effect of the atomized extract of rhizome of C. longa, flowers of C. lutea, and leaves of A. muricata in a model of dermal irritation in rabbits.
Materials and methods
Preparation of the association
The rhizome of C. longa, flowers of C. lutea, and leaves of A. muricata were collected. Taxonomic identification was made at the Museum of Natural History, National University of San Marcos, Lima, Peru. The plants were dried in an oven at 40°C. The association was prepared in a 1:1:1 ratio and was subjected to a spray process using a power mill and macerated with ethanol at 70°C. The resulting solution was subjected to the atomization process to obtain a light yellow powder. The atomized association was stored in amber bottles under refrigeration (5°C) and away from light. It was reconstituted with physiological saline (PS) and Freund’s adjuvant (FA), respectively.
Experimental design
The assessment of dermal sensitization was performed according to the standards of the Organisation for Economic Cooperation and Development (OECD), Standard 406.11 Twenty-four New Zealand white rabbits weighing 1,000±100 g and 4 months of age were procured from the National Institute of Health of Peru. The animals were housed in well-ventilated, large, spacious cages in the bioterium of the Faculty of Medicine, National University of San Marcos. The animals received a balanced diet and water ad libitum. The rabbits were kept at 12 hours light/dark cycle and a temperature of 24°C±2°C with humidity 60%–75%. The experiment began with a 2-day preconditioning period.
The backs of the rabbits was depilated 24 hours before the start of the experiment, and they were randomized into 2 groups, each with 12 rabbits. Group A received the atomized extract diluted in physiological saline (APS) and group B received the atomized extract diluted in FA (AFA) by intradermal injection. Then, the back of each rabbit was divided into 4 quadrants: A-I, A-II, A-III, and A-IV. The A-I quadrant received only PS. The A-I quadrants of each rabbit conformed the PS group. The following 3 quadrants (A-II, A-III, A-IV) received the APS in 10 μg/mL, 100 μg/mL, and 1,000 μg/mL, respectively. The B-I quadrant received only FA. The B-I quadrants of each rabbit conformed the FA group. The following 3 quadrants (B-II, B-III, IV-B) that received the AFA in 10 μg/mL, 100 μg/mL, and 1,000 μg/mL, respectively, were administered 0.1 mL of each solution.
The occurrence of erythema and edema was scored and recorded according to the Draize scoring system12 on the back of the rabbits at 1, 2, 4, 6, 24, 48, and 72 hours. The erythema and eschar formation received a score from 0 to 4 and the edema also received a score from 0 to 4. The interpretation of the total scoring was slight irritation (<2), moderate irritation (2–5), and severe irritation (>5). The average of the combined scores given for the areas of intact and abraded skin was referred to as the primary irritation index.13 The interpretation of the total scoring was no irritation (0–1), slight irritation (1.1–2), moderate irritation (2.1–5), moderate to severe irritation (5.1–6), and severe irritation (6.1–8). After 72 hours, biopsies under local anesthesia (lidocaine 2%) were performed using the technique of Losange for histopathological study, which were evaluated with an electron microscope (Alpha Optics®).
Statistical analysis
Data are presented as mean ± standard deviation. Data were analyzed for homogeneity of variance by the Levene test and normality by the Wilk–Shapiro W statistics. Repeated measures analysis of variance followed by Mauchly’s sphericity test and Tukey’s multiple comparison test method was carried out to compare the mean value of different groups. A P-value of <0.05 was considered statistically significant in all cases. Data were analyzed by using SPSS version 21.
Ethical considerations
During the entire experimental process, international ethical principles for research using laboratory animals were respected. The protocol was approved by the Institute for Ethics in Health of the National University of San Marcos (Nro 0310).
Results
The results show that hypersensitivity reactions were time dependent. There was very light erythema at 1 hour, but after 72 hours, there were severe edema and erythema for the positive control, FA group.
In addition, the APS group did not show hypersensitivity reactions compared with the PS group. Only mild erythema was observed after 24 hours, but there was absence of the antigenic effect. The AFA group presented significantly less erythema and edema compared to the FA group (P<0.05). Regarding the primary dermal irritation index (PDII), it is evident that the positive control caused moderate-to-severe irritation (5.1), the APS group had no irritating effect (0.1), and the AFA group showed dose-dependent effect. The groups that received 10 μg and 100 μg had moderate irritation, but the group that received 1,000 μg showed slight irritation (Table 1).
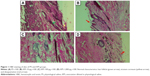
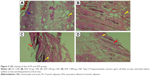
The histopathologic evaluation at 72 hours revealed normal characteristics in the APS group (Figure 1). The FA group had type I–II hypersensitivity reaction manifested by the presence of giant cells, thickening of capillary, and increased inflammation (Figure 2A). The AFA 10 μg group had moderately interstitial edema (Figure 2B), consistent with moderate irritation. The AFA 100 μg group also showed slightly interstitial edema and desquamation (Figure 2C) that coincide with its PDII (moderate irritation). However, the AFA 1,000 μg group showed slight interstitial edema (Figure 2D) and slight irritation.
Discussion
The exposure to FA induces signs of hypersensitivity dermal reactions associated with inflammation, edema, and erythema. FA was used as a vehicle that facilitates dermal sensitization in rabbit fur. This lipophilic agent causes irritation and tissue injury. Its lipophilicity helps to produce irritation and allergenicity. On the other hand, the PS vehicle was used to determine the effect of the atomized association without causing skin irritation.
The atomized extract of rhizome of C. longa, flowers of C. lutea, and leaves of A. muricata has made the exposed tissues less vulnerable. One study found that C. longa decreases the hyperactivity of airway due to allergic causes by reducing eosinophil migration and inhibiting tumor necrosis factor-α.14 It has been proposed to be useful not only in asthmatics but also in hypersensitivity induced by reactions of food.15 This favorable property could be due to the presence of certain metabolites. Curcumin, the main principle in C. longa, which has proven antiallergic and anti-inflammatory activity, could determine the effects of the association in irritation.15–17 Also, C. lutea presents some alkaloids, routines, quercetines, linoleic acid, and palmitic acid18 and has been proved to have antitumor, anti-inflammatory,19 antiallergic, and antibacterial effects.20 The other component, A. muricata, was originally used for its nutritional properties because it was an important source of various amino acids such as arginine, glutamine, and serine.21 However, it also showed antiparasitic effect,22 anti-infective activity against some strains of Staphylococcus, Pseudomonas, and Escherichia coli23,24 and antioxidant and anti-inflammatory properties.18,25,26 The presence of nobiletin, a citrus flavonoid that is associated with the suppression of mast cell degranulation,27 could explain its effects.8
The H1 antihistamines of first generation have potential hepatotoxic effects.8 On the other hand, C. longa has hepatoprotective and even nephroprotective effects.28 Moreover, the lyophilized association of rhizome of C. longa, flowers of C. lutea, and leaves of A. muricata was approved in Canada as a hepatoprotectant.29 The antiallergic activity of the atomized extract of rhizome of C. longa (A4R), flowers of C. lutea, and leaves of A. muricata could be an alternative treatment for hypersensitivity reactions without the adverse effects of conventional medicine.
The limitations of this study include the lack of determination of the metabolites present in the atomized extract of rhizome of C. longa, flowers of C. lutea, and leaves of A. muricata. In addition, its exact mechanism is unclear.
Conclusion
Considering the clinical and histopathological signs, we conclude that the administration of the atomized extract of rhizome of C. longa, flowers of C. lutea, and leaves of A. muricata lacks antigenic effect but could have an antiallergenic effect in a model of dermal irritation in rabbits.
Acknowledgments
The authors are grateful to the staff of Sabell Peru for the technical assistance rendered.
Disclosure
The authors report no conflicts of interest in this work.
References
Efferth T. Perspectives for globalized natural medicines. Chin J Nat Med. 2011;9(1):1–6. | ||
Andhare RN, Raut MK, Naik SR. Evaluation of antiallergic and anti-anaphylactic activity of ethanolic extract of Sansevieria trifasciata leaves (EEST) in rodents. J Ethnopharmacol. 2012;142(3):627–633. | ||
Rossel GM, Araya QM. [Food allergy in childhood]. Alergia alimentaria en la infancia. Revista Médica Clínica Las Condes. 2011;22(2):184–189. Spanish. | ||
Sato A, Zhang T, Yonekura L, Tamura H. Antiallergic activities of eleven onions (Allium cepa) were attributed to quercetin 4′-glucoside using QuEChERS method and Pearson’s correlation coefficient. J Funct Foods. 2015;14:581–589. | ||
Villamarin, EA, Sánchez, N. [Food allergy]. Alergia Alimentaria. Rev Gastrohnup. 2010;12:S27–S34. Spanish. | ||
Bae M-J, Shin HS, Choi D-W, Shon D-H. Antiallergic effect of Trigonella foenum-graecum L. extracts on allergic skin inflammation induced by trimellitic anhydride in BALB/c mice. J Ethnopharmacol. 2012;144(3):514–522. | ||
Church M, Maurer M, Simons F, et al. Risk of first-generation H1-antihistamines: a GA2LEN position paper. Allergy. 2010;65(4):459–466. | ||
Simons FER, Simons KJ. Histamine and H 1-antihistamines: celebrating a century of progress. J Allergy Clin Immunol. 2011;128(6):1139–1150. | ||
Arroyo-Acevedo J, Franco-Quino C, Chavez-Asmat R, Anampa-Guzman A, Rojas-Armas J, Cabanillas-Coral J. [Study of toxicity to 28 days, of the atomized extract of the Rhizome of Curcuma longa (a4r), Cordia Lutea flowers (a4f) and leaves ofAnnona Muricata (a4l) in a Murine Model]. Estudio detoxicidad a 28 dias del extractoatomizado de rizoma de Curcumalonga (A4R), lores de Cordia lutea (A4F) y hojas de Annona muricata (A4L) en un modelo murino. Revista Peruana de Medicina Integrativa. 2016;1(1):31–37. Spanish. | ||
Cabanillas J [webpage on the Internet]. Composition for Treating Hepatitis Containing an Extract of Cordia lutea Lowers, Annona muricata Leaves, and Curcuma longa Roots. [Internet]. Canada: SABELL CORP; CA2701190 (A1); 2008. Available from: http://europepmc.org/patents/PAT/CA2701190. Accessed September 15, 2016. | ||
Organisation for Economic Cooperation and Development (OECD), Standard 406. Skin Sensitization, 1992. | ||
Draize J, Woodard G, Calevery H. Methods for the study of irritation and toxicity of substances applied topically to the skin and mucous membranes. J Pharmacol Exp Therap. 1944;82:377–390. | ||
Lansdown ABG. An appraisal of methods for detecting primary skin irritants. J Soc Cosmet Chem. 1972;23:739–772. | ||
Oh S-W, Cha J-Y, Jung J-E, et al. Curcumin attenuates allergic airway inflammation and hyper-responsiveness in mice through NF-κB inhibition. J Ethnopharmacol. 2011;136(3):414–421. | ||
Shin HS, See H-J, Jung SY, et al. Turmeric (Curcuma longa) attenuates food allergy symptoms by regulating type 1/type 2 helper T cells (Th1/Th2) balance in a mouse model of food allergy. J Ethnopharmacol. 2015;175:21–29. | ||
Orellana-Paucar AM, Serruys A-SK, Afrikanova T, et al. Anticonvulsant activity of bisabolene sesquiterpenoids of Curcuma longa in zebrafish and mouse seizure models. Epilepsy Behav. 2012;24(1):14–22. | ||
Araujo C, Leon L. Biological activities of Curcuma longa L. Mem Inst Oswaldo Cruz. 2001;96(5):723–728. | ||
Mayevych I, Cabanillas J. Chemical composition of Cordia lutea L.: absence of pyrrolizidine alkaloids. Nat Prod Chem Res. 2015;3:194. | ||
Parisotto EB, Michielin EM, Biscaro F, Ferreira SR, Wilhelm Filho D, Pedrosa RC. The antitumor activity of extracts from Cordia verbenacea DC obtained by supercritical fluid extraction. J Supercrit Fluids. 2012;61:101–107. | ||
Hernandez T, Canales M, Teran B, et al. Antimicrobial activity of the essential oil and extracts of Cordia curassavica (Boraginaceae). J Ethnopharmacol. 2007;111(1):137–141. | ||
Egydio APM, Santa Catarina C, Floh EIS, dos Santos DYAC. Free amino acid composition of Annona (Annonaceae) fruit species of economic interest. Ind Crops Prod. 2013;45:373–376. | ||
Vila-Nova NS, de Morais SM, Falcão MJC, et al. Different susceptibilities of Leishmania spp. promastigotes to the Annona muricata acetogenins annonacinone and corossolone, and the Platymiscium floribundum coumarin scoparone. Exp Parasitol. 2013;133(3):334–338. | ||
Coria-Téllez AV, Montalvo-Gónzalez E, Yahia EM, Obledo-Vázquez EN. Annona muricata: a comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity. Arabian J Chem. 2016. | ||
Gupta A, Mahajan S, Sharma R. Evaluation of antimicrobial activity of Curcuma longa rhizome extract against Staphylococcus aureus. Biotechnol Rep. 2015;6:51–55. | ||
Das S. Effect of antioxidants and nutrients in patients with allergy and asthma. J Allergy ClinImmunol. 2014;134(3):763. | ||
Jamkhande PG, Wattamwar AS. Annona reticulata Linn (Bullock’s heart): plant profile, phytochemistry and pharmacological properties. J Tradit Complement Med. 2015;5(3):144–152. | ||
Onishi S, Nishi K, Yasunaga S, et al. Nobiletin, a polymethoxy flavonoid, exerts anti-allergic effect by suppressing activation of phosphoinositide 3-kinase. J Funct Food. 2014;6:606–614. | ||
Wang H, Su G, Chen G, Bai J, Pei Y. 1 H NMR-based metabonomics of the protective effect of Curcuma longa and curcumin on cinnabar-induced hepatotoxicity and nephrotoxicity in rats. J Funct Foods. 2015;17:459–467. | ||
Coral JGC, inventor. Herbal compositions and methods for treating hepatic disorders. Google Patents WO 2009043176 A9; 2009. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.