Back to Journals » Cancer Management and Research » Volume 11
Anti-tumor effects of engineered mesenchymal stem cells in colon cancer model
Authors Yang J, Lv K, Sun J, Guan J
Received 24 March 2019
Accepted for publication 13 August 2019
Published 17 September 2019 Volume 2019:11 Pages 8443—8450
DOI https://doi.org/10.2147/CMAR.S209880
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sanjeev K. Srivastava
Jianying Yang, Kui Lv, Junfeng Sun, Jianguo Guan
Department of Emergency, Anhui No. 2 Provincial People’s Hospital, Hefei, People’s Republic of China
Correspondence: Jianguo Guan
Department of Emergency, Anhui No. 2 Province People’s Hospital, 1868 Dangshan Road, North 2nd Ring Road, Hefei 230041, People’s Republic of China
Tel +86 1 385 512 5938
Fax +86 5 586 428 6088
Email [email protected]
Background: Cell-based gene therapy is considered as a promising strategy for the treatment of human malignancy. In many different types of cancer, mesenchymal stem cells (MSCs) are observed as valuable and potential anti-cancer agents. However, the exact mechanisms of MSCs involved in tumor microenvironment are not well understood.
Aim: Our aims are to elucidate the MSCs-mediated tumor microenvironment.
Materials and methods: In this study, colon cancer model was established by injecting the HT29 cells into the subcutaneous of right axilla of nude mice. We applied the human placenta-derived MSCs (hP-MSCs) armed with a double fusion gene containing the herpes simplex virus truncated thymidine kinase and firefly luciferase for treatment of colon cancer on days 10, 15, and 20 after HT29 cells injection. Molecular imaging methods were used for real-time imaging tumor progression and tracking transplanted hP-MSCs by bioluminescence imaging. Furthermore, proliferation and apoptosis-related proteins levels in colon cancer tissues were examined by immunofluorescence and Western blotting.
Results: Our results demonstrated that the administration of engineered hP-MSCs significantly inhibited the tumors and this effect was enhanced by ganciclovir application. Further analysis demonstrated the anti-tumor effect of engineered hP-MSCs in vivo depended on inhibiting tumor proliferation and inducing tumor apoptosis.
Conclusion: Collectively, this work showed that engineered hP-MSCs could inhibit colon cancer progression and metastasis by inducing tumor cell death and suppressing proliferation.
Keywords: tumor microenvironment, mesenchymal stem cells, malignant tumor, gene therapy, bioluminescence imaging
Introduction
Colon cancer is a common malignant tumor, and its morbidity and mortality are on the rise in recent years.1 It is a serious threat to people’s health and life.2 Traditional radiotherapy, chemotherapy and surgical treatment can kill tumor cells but also cause great damage to normal cells.3 Therefore, it is urgent to find a new therapeutic method that can target tumor cells without harming normal cells. Mesenchymal stem cells (MSCs) are a kind of pluripotent stem cells with multidirectional differentiation potential derived from mesoderm and mainly exist in connective tissue and interstitium of organs.4–6 Under suitable conditions, MSCs can be induced to differentiate into adipose tissue, bone, cartilage and other tissue cells.7 MSCs have low immunogenicity, immunomodulatory ability, the ability to be easily cultured and amplified in vitro and migrate to tumor or inflammatory sites, so they have been widely used in the experimental and clinical studies of inflammation and tumor diseases.8–10
Recently, MSCs have been used as a cell carrier to participate in tumor-targeted therapy,11,12 and studies have shown that gene-modified MSCs could continuously and stably produce therapeutic factors to play a role in tumor inhibition after reaching tumor sites, providing a new possibility for tumor immunotherapy.13–15 Tumor growth and development require support from the surrounding stroma. A large number of studies have shown that tumor cells could locally produce high concentrations of cytokines in tumor microenvironment, which was conducive to the migration of MSCs to tumor tissue and participate in the construction of tumor microenvironment.16,17 These cytokines mainly include: vascular endothelial growth factor, fibroblast growth factor, platelet-derived growth factor, hepatocyte growth factor, monocyte chemotactic protein-1 (MCP-1) and epidermal growth factor.18 The research showed that MCP-1 was expressed in all breast cancer cells, and it was confirmed that MCP-1 played an important role in the migration of MSCs to breast cancer.19,20
Some studies have shown that MSCs could deliver several oncolytic viruses, antiangiogenic factors, and substrates expressing prodrug enzyme conjugates to produce toxic effects.21 MSCs expressing simplex virus thymidine kinase showed strong anti-tumor effects in pancreatic cancer mouse model after intraperitoneal injection of substrates ganciclovir (GCV).22 Moreover, the application of MSCs carrying HGF and NK4 antagonists to the lung metastasis model of colon cancer showed a significant increase in survival time,23 which was consistent with the induction of apoptosis of tumor cells by inhibiting tumor-related angiogenesis.22,24
In this study, we explored, by in vitro and in vivo investigations, the feasibility and efficacy of engineered MSCs-double fusion (DF) as cellular vehicles of the therapeutic gene herpes simplex virus truncated thymidine kinase (HSV-ttk) in the treatment of human colon cancer (HT29). We hypothesized that in vivo imaging technology could better guide the presentation of MSCs and monitor the behavior of MSCs in vivo in real time. Based on this hypothesis, MSCs derived from human placenta were used as cell vectors to mediate the treatment of colon cancer in animal models. We used the dual reporter genes renilla luciferase (Rluc) and firefly luciferase (Fluc) in the same animal to detect tumor development and mesenchymal cell survival, respectively.
Materials and methods
Cell culture
The HT29 cell line from ATCC (Manassas, VA, USA) was maintained at 37°C in a humidified 5% CO2 incubator and cultured in DMEM/F12 medium (Gibco, Grand Island, NY, USA) supplemented with 10% FBS (HyClone, Logan, UT, USA) and 1% penicillin/streptomycin (Gibco). Human placenta (hP)-MSCs were isolated as in previously described protocols25 and cultured in DMEM/F12 medium (Gibco) with 10% FBS (HyClone) and 1% penicillin/streptomycin (Gibco).
Lentivirus transduction
Fluc-HSV-ttk and Rluc-green fluorescence protein (GFP) reporter plasmids were obtained from Addgene. 293T cells were transfected with Fluc-HSV-ttk or Rluc-eGFP reporter and packaging vectors psPAX2 and Pmd2.G to produce virus for stable cell line generation. The viruses were collected by removing the cell media at 48 hrs after transfection. Infection was performed with 1 mL of lentivirus supplemented with 5 µg/mL polybrene (Sigma, St Louis, MO, USA) in six-well plates for 2 days. hP-MSCs were transduced with the lentiviral vector carrying an ubiquitin promoter driving Fluc and HSV-ttk reporter gene. The system of herpes simplex virus thymidine kinase-glycoguanine system (HSV1-TK/GCV) has been the most widely used in cancer suicide gene therapy. Moreover, HT29 cells were labeled with the lentiviral vector carrying an ubiquitin promoter driving Rluc and enhanced GFP (eGFP) DF reporter gene.
Establishment of tumor model
Male Nu/Nu nude mice (8–10 weeks old) were obtained from Laboratory Animal Center of the Academy of Military Medical Science (Beijing, China) and kept in an SPF (Specific Pathogen Free) environment. All experimental procedures were approved by the Animal Care and Use Committee of Anhui No. 2 Province People’s Hospital, Hefei, China, and were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals. HT29 cells (HT29-DF) transfected with DF gene (Flu-eGFP) were prepared into a 200 μL single-cell suspension (the cell concentration was 5x106/mL) with PBS and injected into the subcutaneous of right axilla of nude mice. The drinking water and feed of mice were treated with sterility, and the feeding environment was laminar flow. Tumor formation was observed 10 days after transplantation, and the mice were divided into 1) PBS group (the tail vein was injected with 200 μL PBS), 2) the MSCs-DF group (200 μL MSCs-DF were injected into the tail vein), and 3) the MSCs-DF+GCV group (200 μL MSCs-DF+GCV were injected into the tail vein).
Bioluminescence imaging
Fluc and Rluc with different cell numbers of activity and the fate of transplanted cells were confirmed by bioluminescence imaging (BLI) (IVIS Lumina II System; Xenogen Corporation, Hopkinton, MA, USA). For BLI in vivo, each mouse was imaged for 1–3 mins using the IVIS Luminar Imaging System at different time point. D-Luciferin (150 mg/kg) and coelenterazine (2.5 mg/kg) were intraperitoneally injected into mice for evaluating Fluc and Rluc expression, respectively. The signal intensity was analyzed by utilizing the software of living image. Average radiance of peak BLI signal was quantified by from a fixed-area region of interest over abdomen.
Immunofluorescence staining assay
At day 28 after transplantation of HT29-DF cells, the mice were euthanized and tumor samples were harvested and fixed. Immunofluorescence staining assay was performed to detect the proliferation protein of tumor cells by using Ki-67 Monoclonal Antibody (Invitrogen, Carlsbad, CA, USA). In addition, the apoptosis of tumor cells was detected by the Apoptosis Assay Kit (Beyotime, Shanghai, China). 594 goat anti-rabbit IgG (Invitrogen) was applied appropriately. The cell nuclei were counter-stained with DAPI. The images were analyzed by Image J software (National Institutes of Health, Bethesda, MD, USA).
Western blotting
For protein analysis, all tumor sample extracts of tumor (50–100 mg) were lysed with (radio-immunoprecipitation assay) RIPA lysis buffer (Cowei, Beijing, China) containing proteinase inhibitors on ice for at least 0.5 hrs. BCA Protein Assay Kit (Promega, Madison, WI, USA) was used to quantify the concentration of proteins. Three independent replicates of 30 μg sample were applied to detect the protein expression level with a primary antibody. The primary antibodies used include PCNA (1:1000, Abcam, Cambridge, UK), Caspase-3 (1:2000, Cell Signaling Technology, Boston, MD, USA), and GAPDH (1:1000, Abcam).
Statistical analysis
All experiments were repeated at least three times and data are expressed as mean ± standard error of measurement (SEM). An independent Student's t-test was used for two-group comparisons, and one-way ANOVA for multiple-group comparison. For statistical analysis, GraphPad Prism software version 5.0 (GraphPad Software, Inc., La Jolla, CA, USA) was performed to analyze the quantitative data. P<0.05 was defined as significant difference.
Detailed methods for the determination of vector incorporation rate can be found in the Supplementary materials.
Results
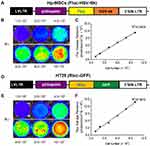
Characterization of transplanted cells
The transgene of interest containing the Fluc and HSV-ttk DF reporter gene was introduced into early passage hP-MSCs (Figure 1A). The phenotype of MSCs and MSCs-DF was presented in Figure S1 and the vector incorporation rate was determined in MSCs-DF (Figure S2). The activity of Fluc was detected by BLI to demonstrate the ability of tracking the cell fate in vivo. BLI results showed a good correlation between cell numbers and firefly signal intensity in MSCs-DF cells (Figure 1B and C). Moreover, in order to track the tumor cells, we transduced HT29 cells with DF reporter gene consisting of Rluc and eGFP (Figure 1D). And likewise, the correlation between cell number and Rluc activity of HT29-DF was detected by BLI. The results suggested that the renilla signal intensity could be used to assess the tumor growth in vivo (Figure 1E and F).
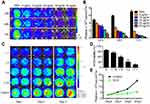
MSCs-DF/GCV mediated cytotoxicity and bystander effect for HT29 cells in vitro
To detect the efficiency of the HSV-ttk suicide gene system, we treated MSCs-DF with different concentrations of GCV (0, 5, 10, 20, 30 and 40 μg/mL). When the fusion degree of MSCs-DF reached 80%, we started to give GCV. The number of MSCs-DF cells could be detected by BLI technology at 24, 48 and 72 hrs, respectively (Figure 2A). We found that the number of cells were significantly decreased with increasing GCV concentration, and when the concentration of GCV reached 40 μg/mL, more than 70% of the cells had died after 72 hrs of GCV treatment (Figure 2B). At the same time, we also analyzed the effect of suicide gene efficiency over time. We tested the number of MSCs-DF cells for three consecutive days after GCV administration by BLI. We found that the number of MSCs-DF cells expressing suicide genes continued to decline over time.
Next, we evaluated the bystander effect of the suicide gene system on colon cancer cells. We mixed different proportions of MSCs-DF cells with HT29-DF cells in different quantitative gradients and treated with 40 μg/mL GCV. Changes of HT29-DF cells viability in each proportional group were measured after 1, 2 and 3 days, respectively. Since Rluc is expressed in HT29-DF cells, the cell viability can be reflected by analyzing the detection of Rluc signal in HT29-DF cells (Figure 2C). Statistical analysis of the results of imaging revealed that as the proportion of MSCs-DF/HT29-DF cells increased, the viability of HT29-DF cells decreased accordingly. When the ratio of the number of cells reached 1:1, the cell viability of HT29-DF was <20% of the cell viability of the control group (Figure 2D). Meanwhile, we further analyzed the effect of suicide gene on the bystander effect of HT29-DF cells over time. The results showed that the proliferation of HT29-DF cells was inhibited as the GCV treatment time increased compared with the control (treated with PBS) group (Figure 2E).
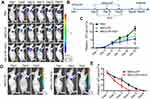
Effects of MSCs-DF on tumor growth
To evaluate the effects of transplanted MSCs-DF on the growth of colon cancer, we introduced Fluc imaging to monitor tumor progression. The outline of MSCs-DF in the treatment of tumor is shown (Figure 3B). 1×106 HT29-DF cells were injected into the subcutaneous of right axilla of Nu/Nu nude mice to establish a colon cancer model. After 10 days, 1×106 MSCs-DF were injected into mouse by the tail vein at 10, 15 and 20 days, respectively. GCV (30 mg/kg) or PBS were intraperitoneally injected into mice at days 11–13, 16–18, 21–23 after the injection of MSCs-DF. Rluc activity was detected by BLI to track tumor development. The results showed that tumor growth was inhibited to a certain extent after transplantation of MSCs-DF, while the use of GCV promoted tumor inhibition (Figure 3A and C). These findings are consistent with previous reports.10 In addition, the detection of Fluc signal was used to evaluate the fate of transplanted MSCs-DF in vivo. Imaging signals showed that the transplanted MSCs-DF could migrate to the tumor site, and its survival was gradually reducing with the passage of time. The use of GCV could significantly reduce the survival of MSCs-DF (Figure 3D and E).
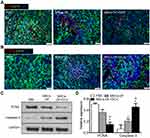
Effects of MSCs-DF on tumor proliferation and apoptosis
To further demonstrate the inhibition effect of MSCs on tumor growth, we performed immunofluorescence staining on tissues on day 28. The staining of the proliferating protein Ki67 showed that the transplanted MSCs-DF could inhibit the proliferation of the tumor, and this effect was enhanced in the MSCs-DF+GCV group (Figure 4A). Statistical analysis of TUNEL staining results showed that the positive rate of TUNEL staining in the mice transplanted with MSCs-DF+GCV was significantly higher than that of the other groups (Figure 4B). These results suggested that the inhibitory effect of transplanted MSCs-DF on tumors was achieved not only by affecting tumors proliferation but also by promoting tumor apoptosis. In addition, we used Western blot to detect the expression of PCNA and Caspase-3. The results showed that the cell proliferation protein PCNA was slightly decreased in the MSCs-DF group compared with the control group, while the expression was significantly reduced in MSCs-DF+GCV group. In contrast, the MSCs-DF group was able to up-regulate the expression of the apoptotic protein Caspase-3, which was further enhanced in the MSCs-DF+GCV group (Figure 4C and D). The results were consistent with the results of immunofluorescence staining. This further illustrated the effect of MSCs on the proliferation of colon cancer cells, mainly through the bystander effect.
Discussion
In this study, we investigated the relationship between engineered hP-MSCs and tumor formation by using the bioluminescence in vivo imaging system for visual detection. The results showed that systemic transplantation of engineered MSCs could inhibit tumor growth by inducing apoptosis of tumor cells. Meanwhile, MSCs carrying suicide genes (HSV-ttk) could further kill tumors through bystander effect. The distribution of transplanted MSCs in mice and the tumor response to them could be monitored in real time by Fluc and Rluc imaging, respectively. The use of non-invasive imaging methods to detect disease progression and the distribution of transplanted cells in vivo will be beneficial to the clinical treatment of MSCs. Because molecular imaging can visually describe biological processes in humans and other organisms, and can penetrate to cells and even molecules, it provides a non-invasive real-time monitoring method for the treatment of diseases. This holds a great potential for the treatment of disease.
In this paper, we explored the feasibility of engineered MSCs as gene delivery vectors for the treatment of colon cancer. MSCs and HT29 cells were transfected with the DF reporter (Fluc-HSV-ttk) and the DF reporter (Rluc-eGFP), respectively. Because Fluc and Rluc have different substrates, luciferase imaging can be used to simultaneously monitor the growth process of tumor and the transplantation efficiency of MSCs in vivo. Therefore, this traceable strategy provides a broader platform for new approaches to the treatment of diseases.
Previous studies have reported that MSCs could inhibit tumor growth by secreting cytokines such as interleukin, interferon-gamma and DKK-1/3.26 In our study, engineered MSCs expressing the suicide gene ttk were used to systematically treat tumor model mice. We observed a decrease in the growth rate of tumors injected with MSCs, and the effect was more pronounced with the administration of prodrug GCV, but this did not completely inhibit tumor growth. We found that the introduction of suicide genes into MSCs resulted in a tumor-specific prodrug transfer vector. Meanwhile, tumor growth may be inhibited by DKK-1 secreted by MSCs.26
Considering the anti-tumor effect of hP-MSCs used in this study, it is possible to apply them in the treatment of tumor-forming conditions. We believe that different sources of MSCs, different cell delivery pathways and individual differences will be issues requiring special attention in cancer treatment in the future. Since MSCs are tumor-oriented, it will undoubtedly provide a new strategy for the treatment of tumors in the future. However, the role of MSCs in tumor microenvironment is so complex that it is difficult to show the specific role of different sources of MSCs in different tumor models. Therefore, the specific role of MSCs in tumor microenvironment needs further extensive research in the future.
Conclusion
In summary, the results of this study provide data for the further development of MSCs as a carrier for targeted tumor therapy. The biological properties of engineered MSCs-DF combined with appropriate transgenic/prodrug may produce highly effective tumor cytotoxicity and may be used in the treatment of tumors in vivo. These cells possess many of the characteristics necessary for optimal cell delivery, which may prove beneficial in clinical studies.
Disclosure
The authors declare no conflicts of interest in this work.
References
1. Deng S, Zhou Z, de Hoog GS, et al. Evaluation of two molecular techniques for rapid detection of the main dermatophytic agents of tinea capitis. Br J Dermatol. 2015;173(6):1494–1500. doi:10.1111/bjd.14156
2. Conde J, Oliva N, Zhang Y, Artzi N. Local triple-combination therapy results in tumour regression and prevents recurrence in a colon cancer model. Nat Mater. 2016;15(10):1128–1138. doi:10.1038/nmat4707
3. Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63(1):11–30. doi:10.3322/caac.21166
4. Kucerova L, Matuskova M, Hlubinova K, Altanerova V, Altaner C. Tumor cell behaviour modulation by mesenchymal stromal cells. Mol Cancer. 2010;9:129. doi:10.1186/1476-4598-9-254
5. Ren G, Chen X, Dong F, et al. Concise review: mesenchymal stem cells and translational medicine: emerging issues. Stem Cells Transl Med. 2012;1(1):51–58. doi:10.5966/sctm.2011-0019
6. Song C, Xiang J, Tang J, et al. Thymidine kinase gene modified bone marrow mesenchymal stem cells as vehicles for antitumor therapy. Hum Gene Ther. 2011;22(4):439–449. doi:10.1089/hum.2010.116
7. Kuroda Y, Dezawa M. Mesenchymal stem cells and their subpopulation, pluripotent muse cells, in basic research and regenerative medicine. Anat Rec (Hoboken). 2014;297(1):98–110. doi:10.1002/ar.22798
8. Bianco P, Cao X, Frenette PS, et al. The meaning, the sense and the significance: translating the science of mesenchymal stem cells into medicine. Nat Med. 2013;19(1):35–42. doi:10.1038/nm.3028
9. Fazekasova H, Lechler R, Langford K, Lombardi G. Placenta-derived MSCs are partially immunogenic and less immunomodulatory than bone marrow-derived MSCs. J Tissue Eng Regen Med. 2011;5(9):684–694. doi:10.1002/term.362
10. Ryu CH, Park KY, Kim SM, et al. Valproic acid enhances anti-tumor effect of mesenchymal stem cell mediated HSV-TK gene therapy in intracranial glioma. Biochem Biophys Res Commun. 2012;421(3):585–590. doi:10.1016/j.bbrc.2012.04.050
11. Nowakowski A, Drela K, Rozycka J, Janowski M, Lukomska B. Engineered mesenchymal stem cells as an anti-cancer trojan horse. Stem Cells Dev. 2016;25(20):1513–1531. doi:10.1089/scd.2016.0120
12. Stuckey DW, Shah K. Stem cell-based therapies for cancer treatment: separating hope from hype. Nat Rev Cancer. 2014;14(10):683–691. doi:10.1038/nrc3798
13. Lou G, Song X, Yang F, et al. Exosomes derived from miR-122-modified adipose tissue-derived MSCs increase chemosensitivity of hepatocellular carcinoma. J Hematol Oncol. 2015;8:122. doi:10.1186/s13045-015-0220-7
14. Barcellos-de-Souza P, Gori V, Bambi F, Chiarugi P. Tumor microenvironment: bone marrow-mesenchymal stem cells as key players. Biochim Biophys Acta. 2013;1836(2):321–335. doi:10.1016/j.bbcan.2013.10.004
15. Zischek C, Niess H, Ischenko I, et al. Targeting tumor stroma using engineered mesenchymal stem cells reduces the growth of pancreatic carcinoma. Ann Surg. 2009;250(5):747–753. doi:10.1097/SLA.0b013e3181bd62d0
16. Han I, Yun M, Kim EO, Kim B, Jung MH, Kim SH. Umbilical cord tissue-derived mesenchymal stem cells induce apoptosis in PC-3 prostate cancer cells through activation of JNK and downregulation of PI3K/AKT signaling. Stem Cell Res Ther. 2014;5. doi:10.1186/scrt443
17. Kucerova L, Zmajkovic J, Toro L, Skolekova S, Demkova L, Matuskova M. Tumor-driven molecular changes in human mesenchymal stromal cells. Cancer Microenviron. 2015;8(1):1–14. doi:10.1007/s12307-014-0151-9
18. Hung SC, Deng WP, Yang WK, et al. Mesenchymal stem cell targeting of microscopic tumors and tumor stroma development monitored by noninvasive in vivo positron emission tomography imaging. Clin Cancer Res. 2005;11(21):7749–7756. doi:10.1158/1078-0432.CCR-05-0876
19. Dwyer RM, Potter-Beirne SM, Harrington KA, et al. Monocyte chemotactic protein-1 secreted by primary breast tumors stimulates migration of mesenchymal stem cells. Clin Cancer Res. 2007;13(17):5020–5027. doi:10.1158/1078-0432.CCR-07-0731
20. Bussard KM, Mutkus L, Stumpf K, Gomez-Manzano C, Marini FC. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016;18(1):84. doi:10.1186/s13058-016-0740-2
21. Kikuchi H, Yagi H, Hasegawa H, et al. Therapeutic potential of transgenic mesenchymal stem cells engineered to mediate anti-high mobility group box 1 activity: targeting of colon cancer. J Surg Res. 2014;190(1):134–143. doi:10.1016/j.jss.2014.02.047
22. Cavarretta IT, Altanerova V, Matuskova M, Kucerova L, Culig Z, Altaner C. Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth. Mol Ther. 2010;18(1):223–231. doi:10.1038/mt.2009.237
23. Mizuno S, Nakamura T. HGF-MET cascade, a key target for inhibiting cancer metastasis: the impact of NK4 discovery on cancer biology and therapeutics. Int J Mol Sci. 2013;14(1):888–919. doi:10.3390/ijms14010888
24. Yan C, Song X, Yu W, et al. Human umbilical cord mesenchymal stem cells delivering sTRAIL home to lung cancer mediated by MCP-1/CCR2 axis and exhibit antitumor effects. Tumour Biol. 2016;37(6):8425–8435. doi:10.1007/s13277-015-4746-7
25. Cao H, Yang J, Yu J, et al. Therapeutic potential of transplanted placental mesenchymal stem cells in treating Chinese miniature pigs with acute liver failure. BMC Med. 2012;10:56. doi:10.1186/1741-7015-10-56
26. Torsvik A, Bjerkvig R. Mesenchymal stem cell signaling in cancer progression. Cancer Treat Rev. 2013;39(2):180–188. doi:10.1016/j.ctrv.2012.03.005
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.