Back to Journals » International Journal of Nanomedicine » Volume 19
Anti-Microbial Effect of AgBr-NP@CTMAB on Streptococcus Mutans and Assessment of Surface Roughness Hardness and Flexural Strength of PMMA
Authors Huang JJ , Jia L, Zhang QJ, Li HH, Zheng DL , Zheng M
Received 17 October 2023
Accepted for publication 24 January 2024
Published 8 February 2024 Volume 2024:19 Pages 1273—1285
DOI https://doi.org/10.2147/IJN.S436613
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor R.D.K. Misra
Jing-Jing Huang,1,* Lin Jia,2,3,* Qiao-Jun Zhang,4 Hao-Hong Li,5 Da-Li Zheng,2 Ming Zheng3
1Second Department of Dental Implant, School and Hospital of Stomatology, Fujian Medical University, Fuzhou, 350001, People’s Republic of China; 2Fujian Key Laboratory of Oral Diseases, Fujian Biological Materials Engineering and Technology Center of Stomatology, School and Hospital of Stomatology, Fujian Medical University, Fuzhou, Fujian, 350004, People’s Republic of China; 3Department of Prosthodontics, School and Hospital of Stomatology, Fujian Medical University, Fuzhou, 350001, People’s Republic of China; 4Zhongshan Hospital Affiliated to Xiamen University, Xiamen, 361000, People’s Republic of China; 5College of Chemistry, Fuzhou University, Fuzhou, Fujian, 350116, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Ming Zheng, Department of Prosthodontics, School and Hospital of Stomatology, Fujian Medical University, 246 Yangqiao Middle Road, Fuzhou, 350001, People’s Republic of China, Tel +86-591-83736431, Email [email protected] Da-Li Zheng, Fujian Key Laboratory of Oral Diseases, School and Hospital of Stomatology, Fujian Medical University, 88 Jiaotong Road, Fuzhou, 350004, People’s Republic of China, Tel +86-591-83720599, Email [email protected]
Purpose: To investigate the inhibition of Streptococcus mutans (S.mutans) and its biofilm by AgBr-nanoparticles (NP) @CTMAB (cetyltrimethyl-ammonium bromide) and evaluate the changes in Polymethyl methacrylate (PMMA)’s surface roughness (Ra), microhardness, and flexural strength during prolonged immersion in AgBr-NP@CTMAB for application in the denture cleaning industry.
Patients and Methods: The antibacterial activity of AgBr-NP@CTMAB against S.mutans was measured colony formation assay, OD600 and laser confocal microscopy. Changes in the specimens’ values for surface roughness, microhardness, and flexural strength (MPa) were measured after immersion solutions for 180 or 360 days.
Results: The AgBr-NP@CTMAB solution exhibited a robust antibacterial effect on planktonic S. mutans, with a minimum bactericidal concentration of 5 μg/mL. The 10 μg/mL AgBr-NP@CTMAB solution efficiently inhibited S. mutans biofilm formation. (2) No significant difference in surface roughness after immersion in AgBr-NP@CTMAB (10 μg/mL and 20 μg/mL) comparing with distilled water (P > 0.05) and Polident had significantly higher than distilled water (P < 0.05). There was a significant decrease in the surface hardness of the PMMA specimens that were immersed in the Polident compared with those in distilled water (P < 0.05). While, no significant differences in surface hardness after immersion in the AgBr-NP@CTMAB (P > 0.05). The result of flexural strength suggested that there was no statistically significant difference (P < 0.05) between AgBr-NP@CTMAB as well as Polident and water.
Conclusion: AgBrNP@CTMAB can efficiently inhibit the growth of plankton S.mutans and biofilm formation, without affecting the flexural strength, microhardness, or surface roughness of PMMA. Therefore, AgBrNP@CTMAB holds promise as a new denture cleaning agent.
Keywords: AgBr-NP@CTMAB, S.mutans, polymethyl methacrylate, denture cleanser, biofilm
Introduction
The most commonly employed material for making moveable complete and partial dentures is polymethyl methacrylate (PMMA). Due to its favorable aesthetic outcomes, affordable production costs, and simplicity of manufacture, this sort of material is widespread.1,2 When patients neglect to maintain their dentures clean, microorganisms including Candida albicans (C. albicans) and Streptococcus mutans (S. mutans) are commonly detected in this area.3,4 Denture stomatitis (DS), a fungal infection exhibiting a high incidence rate among denture users, ranks among the most prevalent fungal infections.5 Despite the potential involvement of systemic factors in DS, the identification of microbial plaques developing on denture surfaces emerges as a pivotal factor contributing to the occurrence of DS.6 C. albicans is linked to the development of DS along with other elements including trauma, systemic disorders, poor denture hygiene.7 Notably, in the presence of sucrose, S. mutans enhances its adhesive interactions with C. albicans, culminating in the collaborative development of hypervirulent biofilms.3,4,8–10
According to certain reports, S. mutans facilitates C. albicans’ growth on the prosthesis, which causes DS.11,12 Consequently, a pivotal measure in preventing DS involves limiting the proliferation of microorganisms within denture plaque. As such, the meticulous cleansing of dentures is imperative to mitigate the risk of cross-contamination and promote optimal oral health. For patients who have trouble applying the mechanical way of cleaning, it is advised to use chemical denture cleaners for denture hygiene.13
The manufacturer of Polident recommends either a 5-minute immersion or overnight soaking to effectively eliminate the majority of bacteria.14 The solution of AgBr-NP@CTMAB shown similar antifungal ability to suppress biofilm development as Polident in a previous investigation by our lab, without noticeable residual cytotoxicity.15 AgBr-NP@CTMAB has a lot of potential as a novel ingredient in denture cleaner since its single ingredient can achieve the same efficacy as Polident, which has four active components. Despite the well-established understanding that S. mutans, a prominent contributor to dental caries, may lead to systemic illnesses such as subacute bacterial endocarditis upon entering the circulation, the specific impact of AgBr-NP@CTMAB’s antimicrobial activity on the growth of S. mutans remains unknown.3 Further investigation is warranted to elucidate this aspect of AgBr-NP@CTMAB’s functionality in promoting oral health.
Surface roughness and flexural strength of denture base resins may change after repeated contact to different disinfectants.16 Chemical denture cleaners should, in theory, lessen or eliminate biofilm buildup while remaining compatible with the denture base materials’ mechanical and physical attributes.17,18 For detachable prosthesis, a variety of chemical cleaners are available, including acids, enzymes, antiseptics, and alkaline peroxides.19 However, a noteworthy drawback of denture cleaners lies in their potential adverse effects on the physical characteristics of denture base materials, particularly in exacerbating surface roughness. Elevated surface roughness on the denture base accelerates microbial plaque accumulation, rendering it more challenging to remove.20–22 Polident is characterized by its antifungal and antibacterial properties, coupled with an appealing scent.23,24 On the other hand, negative consequences, such as modifications to the hardness and surface roughness of acrylic resin, have been reported, particularly after Polident extended usage.25,26 Nevertheless, no research has yet been done on how AgBr-NP@CTMAB affects the flexural strength, hardness, and surface roughness of PMMA.
The objective of this study was twofold: firstly, to ascertain the efficacy of AgBr-NP@CTMAB in inhibiting the growth of S. mutans and preventing biofilm formation, and secondly, to assess the impact of AgBr-NP@CTMAB on the surface hardness, flexural strength, and roughness of PMMA in comparison to distilled water and Polident. To test these objectives, we formulated the following null hypotheses: 1) There would be no discernible difference in the antimicrobial activity against S. mutans between AgBr-NP@CTMAB and water. 2) The efficacy of AgBr-NP@CTMAB and water in combating S. mutans biofilms on PMMA resin surfaces would be comparable. 3) AgBr-NP@CTMAB would not induce any significant alterations in the surface roughness, hardness, and flexural strength of PMMA. These null hypotheses served as the basis for our systematic investigation into the antimicrobial properties of AgBr-NP@CTMAB and its potential impact on the physical characteristics of PMMA.
Materials and Methods
Synthesis of AgBr-NP@CTMAB Hybrid Material
The synthesis and physical measurements of AgBr-NP@CTMAB Hybrid Material has been reported in our previous paper.15 The macroscopical size of AgBr-NP@CTMAB can be measured as 20×80 μm according to the Scanning Electron Microscope (SEM) image as shown in the Figure 1A of this publication. Its microcosmic has been constructed as the encapsulation of AgBr nano-clusters (Ag4Br8 tetramer, about 1.6×0.44 nm) into the CTMAB cations.
 |
Figure 1 Growth curves of S. mutans by different concentrations of AgBr-NP@CTMAB. |
Bacterial Recovery and Culture
S. mutans standard strain American Type Culture Collection (ATCC) 25175, friendly provided by Fujian Key Laboratory of Oral Diseases, Fujian Medical University (Fuzhou, China), was applied as reference. S. mutans was recovered and cultured with Bovine brain heart infusion broth medium (BHI).27 The 200 µL 1×106 CFU/mL bacterial was added to the plate of the automatic growth curve analyzer, and triplicate wells were set up. A Bioscreen fully automated growth curve analyzer was used to monitor S. mutans’ dynamic development over a 24-hour incubation period at 37°C and continual medium-speed shaking. The OD600 nm value was measured at 1 h intervals and the growth curve was prepared using the OD value of S.mutans as the vertical coordinate and the incubation time as the horizontal coordinate. This systematic approach ensured precise tracking and documentation of S. mutans’ growth dynamics under the specified experimental conditions.
Filter Paper Diffusion Method
To determine the inhibition of S.mutans by AgBr-NP@CTMAB, 100 µL of 1×106 CFU/mL of S. mutans solution was pipetted onto BHI agar plates. Five sterile filter paper sheets were placed on the surface of the BHI solid medium inoculated with S. mutans for 10 minutes, and 500 μg/mL and 250 μg/mL AgBr-NP@CTMAB solution, 0.05% cetylpyridinium chloride solution (CPC), Phosphate buffered saline (PBS), and Polident were placed on 6 mm sterile filter paper. The S.mutans containing plates were filled with fully saturated filter paper, and the plates were cultured for 24 hours at 37 °C.28
Microbial Broth Dilution Assay
The test concentrations of AgBr-NP@CTMAB solution were administered to the plates containing 106 CFU/mL S. mutans in accordance with the CLSI (Clinical and Laboratory Standards Institute) standardization standards. These concentrations were 10 μg/mL, 5 μg/mL, 2.5 μg/mL, 1.25 μg/mL and 0.625 μg/mL. For 24 hours, the solutions were incubated aerobically at 37°C. There were triplicate in each group. The experiment was repeated three times. A Bioscreen automated growth curve analyzer was used to capture S. mutans’ dynamic growth as well as the growth curves.
Liquid Dilution Assay
The MBC of S.mutans was determined by the liquid dilution method. 50 µL of the stock solution was applied to BHI agar plates and hatched aerobically 48 h at 37°C. To determine whether or not the bacteria grew the number of colonies was counted.
Crystalline Violet Staining Assay
Following AgBr-NP@CTMAB amplification overnight on S. mutans biofilm formation, the logarithmic growth phase bacterial solution was collected, resuspended in 1% sucrose BHI medium, and diluted to 1×106 CFU/mL. Various quantities of AgBr-NP@CTMAB (10 μg/mL, 5 μg/mL, 2.5 μg/mL, 1.25 μg/mL and 0.625 μg/mL) were put into 24-well plates after 1 mL 106 CFU/mL S. mutans was seeded into the plates. A negative control sterilized distilled water group was set up. Each group was done in triplicate and the experiment was repeated three times. The culture fluid was removed from the well plates after 24 hours. S. mutans was fixed in 500 µL of a 4% paraformaldehyde solution for 30 minutes. 1 mL of 0.1% crystal violet dye was added after the 4% paraformaldehyde had been taken out, and it was let to sit at room temperature for 15 minutes. The crystal violet was located, cleaned with deionized water three times, and photographed before being examined.29
Laser Confocal Microscopic Observation
A mature single-strain biofilm of S. mutans was formed after 24 hours of incubation at 37°C. The biofilms were subsequently exposed to BHI medium with AgBr-NP@CTMAB solutions at concentrations of 5 μg/mL, 10 μg/mL, and 20 μg/mL for 8 hours. The vitality of the bacterial fractions inside the biofilm was determined by carefully aspirating each group after 8 hours, washing it three times with sterile PBS, and staining it with the LIVE/DEAD BaclightTM bacterial viability kit. Each group was done in triplicate and the experiment was repeated three times. SYTO9 green fluorescent and propidium iodide PI red dyes, which stain bacteria with healthy cell membranes green and those with damaged membranes red, respectively, are included in the kit. In the same centrifuge tube, the fluorescent staining solution was produced in the following proportions: SYTO9: PI: saline 1.5: 1.5: 1000. FV3000 software was used to render the images. Using excitation wavelengths of 488 nm for SYTO-9 and 561 nm for propidium iodide, stained biofilms were seen at 100 magnification using a confocal scanning laser microscope (FV3000, OLYMPUS, Japan). For every type of solution (N = 3 biofilms per group), three sets of Z-stack pictures were acquired. A quantitative examination of the percentage of living and dead bacteria within each biofilm’s biovolume—the volume occupied by the bacteria in three dimensions—was conducted using the Image J.
Fabrication of Specimen
PMMA acrylic resin specimens (Vertex Castavaria, Vertex, the Netherlands) were prepared according to the manufacturer’s introductions using a customized steel mold. Abrasive sheets between 600 and 1000 grit were used to polish the specimens after low temperature polymerization (55 ± 5°C, 30 min; Multicure, Vertex, Zeist, Netherlands) while they were being cooled under water. The specimens were then washed with water, cleaned with an ultrasonic cleaner for 30 minutes, and then submerged in PBS for 52 ± 2 hours at 37 °C.30 40 PMMA acrylic resin specimens (64 mm x 10 mm x 3.3 mm) for flexural strength test and 80 PMMA acrylic resin specimens (10 mm x 10 mm x 2 mm) for microhardness test and the remainder for surface roughness test.
Denture Cleansers Preparation and Immersion Protocol
The PMMA specimens were soaked for 8 h a day in 10 µg/mL and 20 µg/mL AgBr-NP@CTMAB, additionally to pure water for the negative control and freshly made Polident (per the manufacturer’s recommendations) for the positive control. Following measurements, specimens for the roughness surface test were submerged in the solutions mentioned above for 180 days. One researcher used a process where samples were immersed for 360 days as part of the microhardness test and the flexural strength test.
Measurement of Surface Roughness
All specimens had their surface roughness evaluated using a profilometer (TR240, Shidai, Beijing, China). Each specimen underwent three measurements. The resolution was 0.01 mm, the interval (cutoff length) was 0.8 mm, the transverse length was 2.0 mm, and the stylus speed was 0.5 mm/s, all in accordance with the Japanese Industrial Standards JIS’13/ISO’97 standard. The choice parameter was chosen to be the specimen’s Ra (m) value. One operator recorded all of the measurements.31
Measurement of Vickers Microhardness
A microhardness tester (HXD-1000TM, Taiming, Shanghai, China) was used to determine Vickers microhardness. With a 300-gram load, the diamond indenter point was left on the samples’ surface for 30 seconds. Each sample has three locations along its diagonal line that are under pressure. The specimens’ HV was determined using the measured values.32
Flexural Strength Measurement
Using a universal testing device (AGS-X, Daojin, Suzhou, China), three-point bending tests were carried out on newly recovered specimens without drying. Specimens were positioned horizontally, 50 mm apart, on 2 stainless steel supports. Using a 5 KN load cell with a crosshead speed of 5 mm/min and a blunt round-end tip (2 mm in diameter), the load was applied at the specimen’s midway. S = 3WL/2bd2, where S is the flexural strength (MPa), W is the maximum load at fracture (Newtons), L is the distance between supports (50 mm), and b and d are the specimen width (10 mm) and thickness (3.3 mm), respectively, were used to calculate the flexural strength of each specimen.33,34
Statistical Analysis
To get representative findings, each experiment was run three times. The threshold of significance was set at p < 0.05, and data were analyzed using the T-test and one-way analysis of variance (one-way ANOVA), followed by Tukey’s multiple comparison test. At a significance level of 5%, all statistical analyses were carried out using IBM’s SPSS 27.0 software in Chicago, Illinois, USA.
Results
Results of Inhibition of S.mutans by AgBr-NP@CTMAB Using Filter Paper Method
In Figure 2, discernible inhibition circles were not observed around the negative control PBS group, indicating no significant inhibitory effect. However, evident inhibition circles were observed around the filter paper treated with the AgBr-NP@CTMAB solution. The 500 μg/mL AgBr-NP@CTMAB filter paper could achieve inhibition circles similar to the 0.05% cypermethrin, and far exceeded the inhibition circles formed by the Polindent.
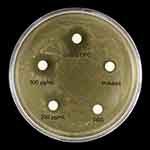 |
Figure 2 Photographs of the zone of inhibition against S.mutans by AgBr-NP@CTMAB. |
Determination of the Antibacterial Kinetics of AgBr-NP@CTMAB by Micro-Broth Dilution Method
The inhibitory efficacy of AgBr-NP@CTMAB solution on planktonic S. mutans proliferation was evaluated by growth kinetic measurements. The variation curve of OD value at 600 nm shown the inhibitory effect of altered concentrations of AgBr-NP@CTMAB solution on the proliferation of S. mutans for 24 h. As a result, better biofilm inhibition efficiency is indicated by lower OD values and vice versa. In Figure 1, the values in growth curve of AgBr-NP@CTMAB which the concentration reached or above 5 μg/mL were lower than Negative Control (NC) group, indicating the proliferation of S. mutans was plainly reduced. The growth curves of the NC group and the 0.625 μg/mL AgBr-NP@CTMAB group overlapped, showing that the ability of S. mutans to proliferate in the two groups was not substantially different. This observation underscores the concentration-dependent inhibitory effect of AgBr-NP@CTMAB on the planktonic growth of S. mutans.
Determination of the Minimum Bactericidal Concentration of AgBr-P@CTMAB by Liquid Dilution Method
- In Figure 3, the OD600 nm values were significantly various from NC group, while the OD600 nm values of 2.5 μg/mL, 1.25 μg/mL and 0.625 μg/mL AgBr-NP@CTMAB solutions were not statistically different than the negative control group.
- Each group of solutions was transferred to a centrifuge tube and photographed, and the groups of 10 μg/mL, 5 μg/mL AgBr-NP@CTMAB solution and CPC solution showed a significant reduce compared with NC group in turbidity. No significant differences in turbidity were observed among 2.5 μg/mL, 1.25 μg/mL, 0.625 μg/mL AgBr-NP@CTMAB solution and the negative control group in Figure 4.
- The growth of colonies was shown in Figure 5. Compared with the NC group, no colonies were visible in the 5 μg/mL, 10 μg/mL AgBr-NP@CTMAB and 0.1% cetylpyridinium chloride solution groups. In contrast, the AgBr-NP@CTMAB solution groups of 2.5 μg/mL, 1.25 μg/mL and 0.625 μg/mL showed colonies spreading over the whole plate, and the growth of colonies was similar to that of the NC group. No colony growth was scanned in the groups of 5 μg/mL, 10 μg/mL AgBr-NP@CTMAB solution and 0.1% cetylpyridinium chloride solution. In contrast, 2.5 μg/mL, 1.25 μg/mL, 0.625 μg/mL of AgBr-NP@CTMAB solution group, the number of colonies became dense from sparse as the concentration decreased, indicating that the bacterial inhibition ability was enhanced as AgBr-NP@CTMAB solution concentration increased.
 |
Figure 3 OD results of each group of solutions at 600 nm after 24 h incubation. (ns, P>0.05, ****P<0.0001). |
 |
Figure 4 Turbidity of the solution in each group after 24 h incubation. |
Results of the Efficacy of AgBr-NP@CTMAB on of S.mutans Biofilm Formation by Crystalline Violet Staining
The findings demonstrated that the AgBr-NP@CTMAB solution had an inhibiting effect on S. mutans’ biofilm production, and full biofilms developed in both the AgBr-NP@CTMAB solution at 0.625 μg/mL and the negative control group in Figure 6. As the AgBr-NP@CTMAB solution concentration rose, the amount of biofilm formation decreased, and when the concentration reached 10 μg/mL and above, no S.mutans biofilm formation was observed in the 24-well plates. The OD570 nm values of the solutions in each group as shown in Figure 7. Except for the 0.625 μg/mL AgBr-NP@CTMAB solution group, all the other groups showed significant differences compared with the negative control group.
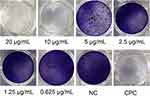 |
Figure 6 Results of crystalline violet staining for the effect of different solutions on biofilm formation of S. mutans. |
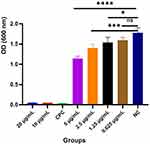 |
Figure 7 The OD570 nm results of 95% ethanol decolorization after crystal violet staining in each group. (ns, P>0.05, *P<0.05, ***P<0.001, ****P<0.0001). |
Bactericidal Effect of AgBr-NP@CTMAB on S.mutans Biofilm Observed by Laser Confocal Microscope
As shown in Figure 8, the results of bacterial viability staining in the biofilm of S.mutans after 8 h with each solution group. When concentration reached 5 μg/mL, a little red appeared in the large bright green area, and as the concentration increased, the area of red increased and the area of green decreased. The results of the live-dead staining were alike to the positive group at 20 μg/mL AgBr-NP@CTMAB, Polyclonal, with basically all bright red areas, indicating that obvious bacterial death was caused. As shown in Figure 8B. The bactericidal effect of 20 μg/mL of AgBr-NP@CTMAB on S.mutans biofilm was not significantly different from that of Polindent, according to quantitative fluorescence analysis performed using ImageJ software.
The Impression of AgBr-NP@CTMAB on Mechanical Properties of PMMA
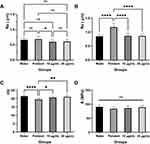
Roughness of Surface Figures 9A and B display the average values and standard deviations of denture surface roughness (Ra) prior to immersion and following solution treatment. The PMMA surface roughness significantly changed in all solutions when compared to before soaking (P < 0.05). AgBr-NP@CTMAB (10 µg/mL and 20 µg/mL) did not substantially vary from distilled water in terms of surface roughness (P > 0.05), while Polident considerably outperformed distilled water in terms of surface roughness (P < 0.05).
Figure 9C displays the average values and ranges of the surface microhardness (VHN) for PMMA specimens. According to the analysis of the two-way ANOVA, the surface microhardness of the PMMA specimens that were immersed in the Polident decreased significantly compared with inserted in distilled water (P < 0.05). A comparison of the surface microhardness of PMMA specimens following immersion in the AgBr-NP@CTMAB (10 µg/mL and 20 µg/mL) and distilled water revealed no significant changes (P > 0.05).
Figure 9D displays the average values and ranges of the flexural strength for PMMA specimens. The flexural strength of the PMMA specimens did not differ statistically significantly between AgBr-NP@CTMAB (10 µg/mL and 20 µg/mL), Polident and water.
Discussion
This study aimed to elucidate the bactericidal efficacy of AgBr-NP@CTMAB solution against S. mutans through in vitro experiments and evaluate potential impacts on the flexural strength, microhardness, and surface roughness of PMMA resin caused by AgBr-NP@CTMAB and Polident. The overarching objective was to provide insights guiding the application of AgBr-NP@CTMAB in denture cleaning. Contrary to the null hypothesis positing no differences in activity against S. mutans and biofilms between AgBr-NP@CTMAB and water, the results indicated rejection of this hypothesis. Conversely, the acceptance of the null hypothesis suggested that AgBr-NP@CTMAB solutions did not adversely affect the surface properties and mechanical strength of PMMA.
The AgBr-NP@CTMAB employed in this investigation represents an emerging composite antibacterial material distinguished by potent antibacterial capabilities, heightened chemical stability, and minimal residual toxicity. As demonstrated by Kumar in analogous studies, Ag and Ag compound nanoparticles exhibit notable antibacterial effectiveness against various Candida species and foodborne pathogenic bacteria.35 The organic antimicrobial components, characterized by quaternary ammonium salt molecules, contribute to antibacterial and antifungal activities.
S. mutans, a pivotal factor in cariogenic biofilm development, secretes extracellular polysaccharides, facilitating bacterial adhesion, maintaining biofilm structure, and serving as a key virulence factor associated with tooth cavities.36 Effectively removing plaque bacteria adhered to dentures is crucial. The Minimum Bactericidal Concentration (MBC) of AgBr-NP@CTMAB against S. mutans was determined to be 5 μg/mL, and complete inhibition of S. mutans biofilm formation occurred at a concentration of 10 μg/mL, aligning with the positive control group using 0.1% dipyridamole. To attain similar bactericidal effects on S. mutans biofilms, a concentration of 20 μg/mL of AgBr-NP@CTMAB solution was comparable to Polident. As opposed to their comparable planktonic counterparts, bacteria in biofilms are 100 to 1500 times more resistant to antimicrobial treatments.37 It explains why the AgBr-NP@CTMAB solution concentration required to achieve the bactericidal effect on biofilms is greater than that required to inhibit biofilm formation. Considering the higher resistance of bacteria in biofilms to antimicrobial treatments, the elevated concentration required for biofilm eradication underscores the potential of AgBr-NP@CTMAB as an anti-biofilm agent to prevent denture stomatitis (DS). However, it is necessary to further investigate the mechanism of its anti-biofilm activity.
Surface roughness (Ra) on denture bases emerged as a pivotal factor influencing bacterial colonization and plaque formation.38 According to earlier studies, dental plaque that has been allowed to build up on roughened detachable dentures promotes the growth of bacteria in the tiny pores of the dentures, leading to foul odors, discoloration, stomatitis of the dentures, and denture breakage.39,40 In contrast to Polident, which exhibited elevated Ra values, AgBr-NP@CTMAB demonstrated no significant increase in surface roughness compared to distilled water. The active oxygen produced by hydrogen peroxide in Polident contributed to increased Ra, while distilled water alone increased Ra over time due to PMMA’s inherent water absorption tendencies.41–44
Microhardness, a critical parameter for denture longevity,41 remained relatively unchanged after immersion in AgBr-NP@CTMAB, while Polident led to a decrease. This is consistent with previous studies indicating a reduction in surface hardness following Polident treatment.1,45,46 The flexural strength, a key mechanical property indicative of overall performance and durability,47 remained unaffected by both AgBr-NP@CTMAB and Polident. All solutions, including AgBr-NP@CTMAB and Polident, maintained flexural strength within clinically acceptable ranges, ensuring the denture’s structural integrity.48 Paranhos et al discovered acrylic resin’s flexural strength was unaffected by an overnight soaking in denture cleaning solutions mimicking a year and a half of usage, which is consistent with the findings of this investigation.49
When interpreting the results of this investigation, the following constraints need to be taken into account: In this experiment, a single-strain biofilm model of S.mutans was constructed in vitro, which did not restore the multi-strain biofilm morphology under the complex environment of humidity, pH, and saliva in the oral cavity, so it needs to be further explored whether the experimental drugs also have good bactericidal and antibacterial effects on multi-strain biofilms.
Conclusion
AgBrNP@CTMAB effectively combats S. mutans plankton growth and prevents the development of S. mutans biofilm, also has good bactericidal effect on S.mutans biofilm. In terms of surface roughness, microhardness, and flexural strength, it may be utilized as a denture cleaner for PMMA without risk. This shows that AgBr-NP@CTMAB has a lot of potential as a new denture cleanser ingredient. Polident should be used with caution for increasing surface roughness and decreasing hardness.
Abbreviations
S.mutans, Streptococcus mutans; NP, Nanoparticle; CTMAB, Cetyltrimethylammonium bromide; MBC, Minimum bactericidal concentration; OD, Optical density; PMMA, Polymethyl methacrylate; C. albicans, Candida albicans,; DS, Denture stomatitis; CFU, Colony forming unit; BHI, Brain heart infusion; CPC, cetylpyridinium chloride solution; SEM, Scanning Electron Microscope; ATCC, American Type Culture Collection; CLSI, Clinical and Laboratory Standards Institute; PBS, Phosphate buffered saline; JIS, Japanese Industrial Standards; NC, Negative Control; CLSI, Clinical and laboratory standards institute.
Data Sharing Statement
This article has all the data that were created or evaluated during this investigation.
Acknowledgment
Startup financing from Fujian Medical University (Grant number: 2019QH1130) provided financial assistance for this work. The study’s design, data collection, analysis, and interpretation, as well as the writing of the publication, were all done independently from the sponsoring organizations.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Ozyilmaz OY, Akin C. Effect of cleansers on denture base resins’ structural properties. J Appl Biomater Funct Mater. 2019;17(1):1–9.
2. Kurt A, Erkose-Genc G, Uzun M, et al. The effect of cleaning solutions on a denture base material: elimination of Candida albicans and alteration of physical properties. J Prosthodont. 2018;27(6):577–583. doi:10.1111/jopr.12539
3. Lemos JA, Palmer SR, Zeng L, et al. The biology of S.mutans. Microbiol Spectr. 2019;7(1):1–18. doi:10.1128/microbiolspec.GPP3-0051-2018
4. Yassin SA, German MJ, Rolland SL, Rickard AH, Jakubovics NS. Inhibition of multispecies biofilms by a fluoride-releasing dental prosthesis copolymer. J Dent. 2016;48:62–70. doi:10.1016/j.jdent.2016.03.001
5. Young BC, Jose A, Cameron DA, et al. Attachment of Candida albicans to denture base acrylic resin processed by three different methods. Int J Prosthodont. 2009;22(5):488–499.
6. Jingjing HUANG, Hao YU, Ming ZHENG, et al. Antifungal effect of tea extracts on Candida albicans. Dent Mater J. 2020;39(4):664–669. doi:10.4012/dmj.2019-014
7. Alfouzan AF, Tuwaym M, Aldaghri EN, et al. Efficacy of denture cleansers on microbial adherence and surface topography of conventional and CAD/CAM-processed denture base resins. Polymers. 2023;15(2):460. doi:10.3390/polym15020460
8. Sánchez-Vargas LO, Estrada-Barraza D, Pozos-Guillen AJ, et al. Biofilm formation by oral clinical isolates of Candida species. Arch Oral Biol. 2013;58(10):1318–1326. doi:10.1016/j.archoralbio.2013.06.006
9. Abrantes P, Africa CWJ. Measuring S.mutans, Streptococcus sanguinis and Candida albicans biofilm formation using a real-time impedance-based system. J Microbiol Methods. 2020;169:105815. doi:10.1016/j.mimet.2019.105815
10. Falsetta ML, Klein MI, Colonne PM, et al. Symbiotic relationship between S.mutans and Candida albicans synergizes virulence of plaque biofilms in vivo. Infect Immun. 2014;82(5):1968–1981. doi:10.1128/IAI.00087-14
11. Nikawa H, Egusa H, Makihira S, et al. Alteration of the coadherence of Candida albicans with oral bacteria by dietary sugars. Oral Microbiol Immunol. 2001;16(5):279–283. doi:10.1034/j.1399-302x.2001.016005279.x
12. Rocha EP, Francisco SB, Del Bel Cury AA, et al. Longitudinal study of the influence of removable partial denture and chemical control on the levels of Streptococcus mutans in saliva. J Oral Rehabil. 2003;30(2):131–138. doi:10.1046/j.1365-2842.2003.01015.x
13. Preisser AM, Gad M, ArRejaie A, et al. Impact of denture cleansing solution immersion on some properties of different denture base materials: an in vitro Study. J Prosthodont. 2019;28(8):913–919. doi:10.1111/jopr.12649
14. Hayran Y, Sarikaya I, Aydin A, Tekin YH. Determination of the effective anticandidal concentration of denture cleanser tablets on some denture base resins. J Appl Oral Sci. 2018;26:e20170077. doi:10.1590/1678-7757-2017-0077
15. Qiao-Jun Z, Yue L, Wen-Ting Z, et al. Synthesis, antifungal activity, and cytotoxicity of AgBr-NP@CTMAB hybrid and its application in PMMA. Int j Nanomed. 2021;16:3091–3103. doi:10.2147/IJN.S290673
16. Petersen PE, Bourgeois D, Bratthall D, et al. Oral health information systems – towards measuring progress in oral health promotion and disease prevention. Bull World Health Organ. 2005;83(83):686–693.
17. Felipucci DN, Davi LR, Paranhos HF, et al. Effect of different cleansers on the surface of removable partial denture. Braz Dent J. 2011;22(5):392–397. doi:10.1590/S0103-64402011000500008
18. Ural C, Sanal FA, Cengiz S. Effect of different denture cleansers on surface roughness of denture base materials. Clin Dent Res. 2011;35:14–20.
19. Alhotan A, Elraggal A, Yates J, et al. Effect of different solutions on the colour stability of nanoparticles or fibre reinforced PMMA. Polymers. 2022;14(8):1521. doi:10.3390/polym14081521
20. Vieira AP, Senna PM, Silva WJ, et al. Long term efficacy of denture cleansers in preventing Candida spp. Biofilm recolonization on liner surface. Braz Oral Res. 2010;24(3):342–348. doi:10.1590/S1806-83242010000300014
21. Tan H, Woo A, Kim S, et al. Effect of denture cleansers, surface finish, and temperature on Molloplast B resilient liner color, hardness, and texture. J Prosthodont. 2000;9(3):148–155. doi:10.1053/jopr.2000.18551
22. Harrison Z, Johnson A, Douglas CWI. An in vitro study into the limited range of denture cleaners on surface roughness and removal of Candida albicans from conventional heat cured acrylic resin denture resin base material. J Oral Rehabil. 2004;31(5):460–467. doi:10.1111/j.1365-2842.2004.01250.x
23. Arruda CN, Sorgini DB, Oliveira Vde C, et al. Effects of denture cleansers on heat-polymerized acrylic resin: a five-year simulated period of use. Braz Dent J. 2015;26(4):404–408. doi:10.1590/0103-6440201300120
24. Nikawa H, Yamamoto T, Hamada T, et al. Cleansing efficacy of commercial denture cleansers: ability to reduce Candida albicans biofilm activity. Int J Prosthodont. 1995;8:527–534.
25. Schwindling FS, Rammelsberg P, Stober T. Effect of chemical disinfection on the surface roughness of hard denture base materials: a systematic literature review. Int J Prosthodont. 2014;27(3):215–225. doi:10.11607/ijp.3759
26. Jeyapalan K, Kumar JK, Azhagarasan NS. Comparative evaluation of the effect of denture cleansers on the surface topography of denture base materials: an in-vitro study. J Pharm Bioallied Sci. 2015;7(6):S548–S553. doi:10.4103/0975-7406.163536
27. Quan C, Lin H, Xiao H, Zhao J. Inhibitory effect of carboxylated nanodiamond on oral pathogenic bacteria Streptococcus mutans. J Clin Lab Anal. 2021;35(8):1–9. doi:10.1002/jcla.23872
28. Ma Y, Xu M, Liu H, et al. Antimicrobial compounds were isolated from the secondary metabolites of Gordonia, a resident of intestinal tract of Periplaneta americana. AMB Express. 2021;11(1):111. doi:10.1186/s13568-021-01272-y
29. Meng J, Xu J, Huang C, Chen J. Rcs phosphorelay responses to truncated lipopolysaccharide-induced cell envelope stress in Yersinia enterocolitica. Molecules. 2020;25(23):5718. doi:10.3390/molecules25235718
30. Freitasfernandes FS, Cavalcanti YW, Ricomini Filho AP, et al. Effect of daily use of an enzymatic denture cleanser on Candida albicans biofilms formed on polyamide and poly(methyl methacrylate) resins: an in vitro study. J Prosthet Dent. 2014;112(6):1349–1355. doi:10.1016/j.prosdent.2014.07.004
31. Zamperini CA, Machado AL, Vergani CE, et al. Adherence in vitro of Candida albicans to plasma treated acrylic resin. Effect of plasma parameters, surface roughness and salivary pellicle. Arch Oral Biol. 2010;55(10):763–770. doi:10.1016/j.archoralbio.2010.06.015
32. Chen R, Han Z, Huang Z, et al. Antibacterial activity, cytotoxicity and mechanical behavior of nano-enhanced denture base resin with different kinds of inorganic antibacterial agents. Dent Mater J. 2017;36(6):693–699. doi:10.4012/dmj.2016-301
33. Gad MM, Fouda SM, ArRejaie AS, et al. Comparative effect of different polymerization techniques on the flexural and surface properties of acrylic denture bases. J Prosthodont. 2019;28(4):458–465. doi:10.1111/jopr.12605
34. Al-Harbi FA, Abdel-Halim MS, Gad MM, et al. Effect of nanodiamond addition on flexural strength, impact strength, and surface roughness of PMMA denture base. J Prosthodont. 2019;28(1):417–425. doi:10.1111/jopr.12969
35. Kumar PJ, Kwang-Hyun B. Antibacterial activity and synergistic antibacterial potential of biosynthesized silver nanoparticles against foodborne pathogenic bacteria along with its anticandidal and antioxidant effects. Front Microbiol. 2017;08(1):167–281.
36. Choi O, K CS, Kim J, et al. In vitro antibacterial activity and major bioactive components of Cinnamomum verum essential oils against cariogenic bacteria, Streptococcus mutans and Streptococcus sobrinus. Asian Pac J Trop Biomed. 2016;6:308–314.
37. Hirasawa M, Takada K. Multiple effects of green tea catechin on the antifungal activity of antimycotics against Candida albicans. J Antimicrob Chemother. 2004;53:225–229. doi:10.1093/jac/dkh046
38. Yamashita Y, Nishi Y, Murakami M, et al. Impact of surface changes and microbial adhesion on mucosal surface finishing of resin denture bases by shot blast polishing using viscoelastic media. Materials. 2022;15(6):2275. doi:10.3390/ma15062275
39. Shankar T, Gowd S, Suresan V, et al. Denture hygiene knowledge and practices among complete denture wearers attending a postgraduate dental institute. J Contemp Dent Pract. 2017;18(8):714–721. doi:10.5005/jp-journals-10024-2113
40. Porwal A, Khandelwal M, Punia V, et al. Effect of denture cleansers on color stability, surface roughness, and hardness of different denture base resins. J Indian Prosthodont Soc. 2017;17(1):61–67. doi:10.4103/0972-4052.197940
41. Machado AL, Breeding LC, Vergani CE, et al. Hardness and surface roughness of reline and denture base acrylic resins after repeated disinfection procedures. J Prosthet Dent. 2009;102(2):115–122. doi:10.1016/S0022-3913(09)60120-7
42. Oliveira LV, Mesquita MF, Henriques GE, et al. The compatibility of denture cleansers and resilient liners. J Appl Oral Sci. 2006;14(4):286–290. doi:10.1590/S1678-77572006000400014
43. Ferracane JL. Hygroscopic and hydrolytic effects in dental polymer networks. Dent Mater. 2006;22(3):211–222. doi:10.1016/j.dental.2005.05.005
44. Paranhos Hde F, R DL, Peracini A, et al. Comparison of physical and mechanical properties of microwave-polymerized acrylic resin after disinfection in sodium hypochlorite solutions. Brazilian Dental J. 2009;20(4):331–335. doi:10.1590/S0103-64402009000400012
45. Ahmad N, Jafri Z, Khan ZH, et al. Evaluation of nanomaterials to prevent oral Candidiasis in PMMA based denture wearing patients. A systematic analysis. J Oral Biol Craniofac Res. 2020;10(2):189–193. doi:10.1016/j.jobcr.2020.04.012
46. Durkan R, Ayaz EA, Bagis B, et al. Comparative effects of denture cleansers on physical properties of polyamide and polymethyl methacrylate base polymers. Dent Mater J. 2013;32(3):367–375. doi:10.4012/dmj.2012-110
47. Cunha TR, Regis RR, Bonatti MR, et al. Influence of incorporation of fluoroalkyl methacrylates on roughness and flexural strength of a denture base acrylic resin. J Appl Oral Sci. 2009;17(2):103–107. doi:10.1590/S1678-77572009000200006
48. Rocha MM, Carvalho AM, Coimbra FCT, et al. Complete denture hygiene solutions: antibiofilm activity and effects on physical and mechanical properties of acrylic resin. J Appl Oral Sci. 2021;29:e20200948. doi:10.1590/1678-7757-2020-0948
49. Peracini A, Davi LR, de Queiroz Ribeiro N, et al. Effect of denture cleansers on physical properties of heat‑polymerized acrylic resin. J Prosthodont Res. 2010;54(2):78–83. doi:10.1016/j.jpor.2009.11.004
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.