Back to Journals » Medical Devices: Evidence and Research » Volume 12
Annular closure device lowers reoperation risk 4 years after lumbar discectomy
Authors Nanda D , Arts MP, Miller LE , Köhler HP, Perrin JM, Flüh C , Vajkoczy P
Received 20 June 2019
Accepted for publication 17 August 2019
Published 4 September 2019 Volume 2019:12 Pages 327—335
DOI https://doi.org/10.2147/MDER.S220151
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Dharmin Nanda1, Mark P Arts2, Larry E Miller3, Hans-Peter Köhler4, Jason M Perrin5, Charlotte Flüh6, Peter Vajkoczy7
1Department of Neurosurgery, Isala Klinieken, Zwolle, the Netherlands; 2Department of Neurosurgery, Haaglanden Medical Center Westeinde, The Hague, the Netherlands; 3Miller Scientific Consulting, Inc., Asheville, NC, USA; 4Department of Neurosurgery, Asklepios Westklinikum Hamburg, Hamburg, Germany; 5Department of Neurosurgery, University Clinic Mannheim, Mannheim, Germany; 6Department of Neurosurgery, University Medical Center Schleswig-Holstein, Campus Kiel, Kiel, Germany; 7Department of Neurosurgery, Charité Universitätsmedizin, Berlin, Germany
Correspondence: Dharmin Nanda
Department of Neurosurgery, Isala Klinieken, Dokter van Heesweg 2, AB Zwolle 8025, The Netherlands
Tel +31 038 424 5000
Email [email protected]
Objective: To determine whether implanting an annular closure device (ACD) following a lumbar discectomy procedure in patients with large defects in the annulus fibrosus lowers the risk of reoperation after 4 years.
Methods: In a multicenter randomized trial, patients with large annular defects following single-level lumbar discectomy were intraoperatively randomized to additionally receive an ACD or no treatment (Controls). Clinical and imaging follow-up were performed at routine intervals over 4 years of follow-up. Main outcomes included reoperations at the treated lumbar level, leg pain scores on a visual analog scale, Oswestry Disability Index (ODI), and Physical Component Summary (PCS) and Mental Component Summary (MCS) scores from the SF-36 questionnaire.
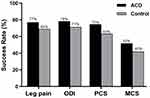
Results: Among 550 patients (ACD 272, Control 278), the risk of reoperation over 4 years was 14.4% with ACD and 21.1% with Controls (P=0.03). The reduction in reoperation risk with ACD was not significantly influenced by patient age (P=0.51), sex (P=0.34), body mass index (P=0.21), smoking status (P=0.85), level of herniation (P=0.26), leg pain severity at baseline (P=0.90), or ODI at baseline (P=0.54). All patient-reported outcomes improved in each group from baseline to 4 years (all P<0.001). The percentage of patients who achieved the minimal clinically important difference without a reoperation was proportionally higher in the ACD group compared to Controls for leg pain (P=0.07), ODI (P=0.10), PCS (P=0.02), and MCS (P=0.06).
Conclusion: The addition of a bone-anchored ACD following lumbar discectomy in patients with large post-surgical annular defects reduces the risk of reoperation and provides better long-term pain and disability relief over 4 years compared to lumbar discectomy only.
Keywords: annular closure device, annulus fibrosus, disc herniation, lumbar discectomy, randomized controlled trial, reoperation, sciatica
Introduction
Herniation of a lumbar intervertebral disc resulting in radiating buttock and leg pain is common in middle-aged adults.1 In most cases, sciatica symptoms arising from lumbar disc herniation resolve without specific treatment.2,3 However, approximately 1 in 5 affected individuals report persistent bothersome symptoms that are refractory to nonsurgical treatments.2,3 Although continuation of conservative care may be considered in these chronic cases, accumulating evidence demonstrates that lumbar discectomy provides more rapid and longer lasting symptom relief.4,5 Lumbar discectomy as described by Spengler6 involves surgical removal of the herniated disc material in the extradiscal space. This limited disc excision procedure alleviates radicular symptoms by decompression of nerve roots and reductions in herniation-induced local inflammation.7 Although lumbar discectomy is highly effective in relieving radicular symptoms in most patients,8 symptom recurrence is a challenging complication that requires a reoperation in most cases to adequately manage symptoms.9,10 Reoperations are technically demanding, more expensive,10 and less effective11,12 than primary procedures. Consequently, determination of factors that increase reoperation risk and identification of therapies that reduce this risk is of clinical and economic importance to patients, surgeons, hospitals, and health care payers.
A major risk factor for reoperation is a post-discectomy defect in the annulus fibrosus of sufficient size to allow reherniation of the intervertebral disc contents. In a meta-analysis by Miller and colleagues,13 the risk of reoperation in patients with a large (≥6 mm width) post-discectomy annular defect was more than double that of patients with smaller defects. In order to lower the risk of symptom recurrence and reoperation among these high-risk patients, an implantable bone-anchored device was developed to provide durable occlusion of the annular defect. A 554-patient multicenter randomized trial confirmed that lumbar discectomy followed by annular closure device (ACD) implantation lowered the risk of reoperation through 3 years compared to lumbar discectomy only in patients with large annular defects.14 Demonstration of treatment durability over longer term follow-up is crucial in this relatively young patient population and, given the recent marketing approval of this device in the United States, dissemination of current trial results will facilitate shared decision making between patients and surgeons. Thus, we report the 4-year outcomes from this randomized trial in high-risk patients who underwent lumbar discectomy with or without ACD implantation for treatment of symptomatic intervertebral disc herniation.
Methods
Trial design
This multicenter randomized trial was conducted to determine whether additional treatment with a bone-anchored ACD would lower the risk of lumbar disc reherniation and reoperation in patients with large postsurgical annular defects following lumbar discectomy. Patients with large annular defects were specifically targeted for this trial given their well-known high risk of reherniation after lumbar discectomy.13 The trial was conducted in accordance with the Declaration of Helsinki, ethics committees at each participating site (listed in Table S1) approved the study protocol, and patients provided written informed consent prior to participation. The trial was prospectively registered at ClinicalTrials.gov (NCT 01283438). The trial design15 and 3-year results14 have previously been reported.
Participants
A complete listing of inclusion and exclusion criteria was previously published.16 Preoperative imaging included magnetic resonance imaging, low-dose computed tomography at the index level, and anteroposterior/lateral and flexion/extension X-rays. Eligible patients presented with a single-level lumbar disc herniation identified on preoperative imaging that was associated with persistent leg pain (≥40 on a 0–100 visual analog scale) despite at least 6 weeks of conservative treatment and with a positive physical examination diagnostic test. Additional important eligibility criteria were a score ≥40 on the Oswestry Disability Index and disc height ≥5 mm. Patients with osteoporosis of the lumbar spine, previous surgery at the level of the herniation, spondylolisthesis with >25% slip, or active infection were not eligible for the trial.
Surgical procedure
Patients who met all preoperative eligibility criteria were treated with lumbar discectomy as described by Spengler6 and were evaluated for a final intraoperative inclusion criterion before enrollment in the trial. At completion of the lumbar discectomy procedure, surgeons used a sizing tool to measure the height and width of the defect in the annulus fibrosus. Patients with a large defect (4–6 mm height and 6–10 mm width) were enrolled in the trial and randomly allocated 1:1 to receive discectomy only (Controls) or to additionally receive a bone-anchored ACD (Barricaid, Intrinsic Therapeutics, Woburn, MA, United States) at completion of the lumbar discectomy. Patients with an annular defect outside of this size range were excluded from further participation in the trial.
In patients assigned to the ACD group, a bone-anchored ACD was implanted as a stand-alone procedure following the lumbar discectomy. A sizing trial under fluoroscopic control was first performed to determine access trajectory and device placement. The ACD was then implanted under fluoroscopic guidance by placing the occlusion component in the annular defect and securing the anchor into an adjacent vertebral body. After correct device placement was confirmed under fluoroscopic guidance, the surgical site was inspected and standard wound closure was performed.
Follow-up and outcomes
Clinical follow-up occurred at 6 weeks, 3 months, 6 months, and annually thereafter for 4 years; patients will continue in follow-up for 5 years. Low-dose CT, MRI, and flexion-extension X-rays were performed annually. Reoperations were defined as any repeat procedure at the index level including discectomy, supplemental fixation, fusion, or device explant. Clinical outcomes included leg pain severity on a 0 to 100 visual analog scale, Oswestry Disability Index (ODI), SF-36 Physical Component Summary (PCS) score, and SF-36 Mental Component Summary (MCS) score. The minimal clinically important difference (MCID) for each patient-reported outcome was defined as a ≥20-point decrease from baseline for leg pain,17 ≥15-point decrease from baseline for ODI,18 ≥5.7-point increase from baseline for PCS,19 and ≥6.3-point increase from baseline for MCS.19
Statistical analysis
Treatment group comparisons were performed with Student’s t-test for continuous data or Fisher’s exact test for categorical data. The risk of reoperation was analyzed with Kaplan–Meier methods and the log-rank test. Treatment-by-subgroup interactions were tested using a Cox proportional hazards model containing the main effects of treatment (ACD or Control), subgroup, and treatment-by-subgroup interaction. Longitudinal patient-reported outcomes were analyzed with mixed-model analysis of variance and adjusted for the confounding effects of reoperations.20 Statistical significance was set at P<0.05 and hypothesis testing was two-sided. Statistical analyses were performed using SAS v9.4 (SAS Institute, Cary, NC, United States).
Results
Between 2010 and 2014, 554 patients with large post-discectomy defects in the annulus fibrosus were enrolled in the trial, including 276 who were randomized to additional treatment with an ACD and 278 who received no additional treatment (Controls). The analysis excluded 4 patients in whom ACD implantation was not attempted due to anatomic constraints, resulting in a sample size of 550 patients (272 ACD, 278 Controls). Baseline patient characteristics were well-matched between treatment groups. Comparing the ACD group to Controls, mean age was 43±11 versus 44±10 years, 57% versus 62% were male, and body mass index was 26±4 kg/m2 in each group. Intervertebral disc herniation was identified at L4-L5 or L5-S1 in 97% of the cases. Mean leg pain on the visual analog scale was 81±15 in each group despite at least 6 weeks of conservative management. The ACD was successfully implanted in 267 (98.2%) patients; the occlusion component of the device was malpositioned in 4 patients and a nerve root injury occurred in 1 patient.
Compliance with clinical and imaging follow-up was 76% in the ACD group and 72% in the Control group over 4 years (Figure 1). Through 4 years, there were 49 reoperations in 37 ACD patients (0.18 per patient) and 77 reoperations in 55 Control patients (0.28 per patient). At 1 year, the risk of reoperation was 6.7% in the ACD group and 12.9% in Controls. This early benefit of ACD was maintained over 4 years of follow-up with reoperation rates of 14.4% with ACD and 21.1% with Controls (Figure 2). The corresponding hazard ratio (HR) was 0.63 (95% CI: 0.42, 0.96; P=0.03), which indicates a 37% reduction in the risk of reoperation with ACD over 4 years. The reduction in reoperation risk with ACD was not significantly influenced by patient age (P=0.51), sex (P=0.34), body mass index (P=0.21), smoking status (P=0.85), level of herniation (P=0.26), leg pain severity at baseline (P=0.90), or ODI at baseline (P=0.54) (Figure 3). Risk factors for reoperation in each treatment group are reported in Table 1. In a multivariate Cox proportional hazards model, female sex (HR =2.38; P<0.01), and smoking (HR =2.24; P<0.01) were independently associated with a higher reoperation risk in the Control group. No patient characteristic influenced the risk of reoperation in the ACD group.
 |
Table 1 Predictors of reoperation through 4 years by treatment group |
The primary indications for reoperation in each group were reherniation and lumbar/extremity pain or dysfunction. Although the risk of reoperation was lower in the ACD group, the distribution of reoperation techniques was comparable between the groups. Discectomy with or without a fusion procedure was the most common reoperation type in each group. The ACD was removed partially or entirely in 23 reoperations, 5 of which involved device removal with no additional surgeries. None of the reoperations in the ACD or Control group were attributable to morphologic changes in the vertebral endplates. After a review of all operative notes from patients treated with the ACD who underwent reoperation, no report indicated that the presence of the device interfered with surgical planning or altered the operative technique.
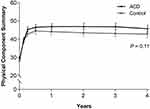
All patient-reported outcomes (leg pain, ODI, PCS, and MCS) significantly improved in each group from baseline to 4 years (all P<0.001). The magnitude of improvement from baseline tended to be greater in the ACD group versus Controls for leg pain severity (−56 vs −49, P=0.04; Figure 4), ODI (−38 vs −34, P=0.04; Figure 5), PCS (16.4 vs 14.5, P=0.11; Figure 6), and MCS (10.4 vs 6.2, P=0.02; Figure 7). At 4 years, the percentage of patients who achieved the MCID without a reoperation was proportionally higher in the ACD group compared to Controls for leg pain (P=0.07), ODI (P=0.10), PCS (P=0.02), and MCS (P=0.06) (Figure 8).
 |
Figure 4 Change in leg pain severity over 4 years of follow-up.Note: Plotted values are mean and 95% confidence interval.Abbreviation: ACD, annular closure device. |
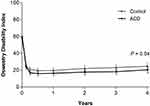 |
Figure 5 Change in Oswestry Disability Index over 4 years of follow-up.Notes: Plotted values are mean and 95% confidence interval.Abbreviation: ACD, annular closure device. |
Discussion
Patients with a large post-discectomy defect in the annulus fibrosus have a high risk for reoperation.13 In the current trial, this risk was 21.1% over 4 years of follow-up, which was reduced to 14.4% in patients who received an ACD. Interestingly, most reoperations occurred early in follow-up with reoperation rates of 6.7% with ACD and 12.0% with Controls at 1 year. Thereafter, the reoperation rate declined considerably to approximately 2.5% annually in each group. Thus, it appears that in patients with large postsurgical annular defects, the excess risk of reoperation is mainly confined to the first postoperative year, after which reoperation rates approximate that of the general lumbar discectomy population.21 Our results demonstrated the ability of an ACD to significantly reduce the risk of reoperation in the first year and to maintain this benefit through 4 years of follow-up.
Both treatment groups reported statistically significant and clinically meaningful improvements in leg pain and ODI over 4 years, with results slightly favoring patients treated with an ACD. Similarly, health-related quality of life considerably improved on the PCS and MCS in both groups, with slightly greater improvements noted for the ACD group relative to Controls. A striking observation in this study was the deleterious impact of lumbar disc herniation on preoperative physical function. At baseline, the mean PCS scores in each group were in the 10th percentile of population-based norms.22 Following lumbar discectomy, PCS scores peaked at 6 months and were maintained at this level for 4 years such that postoperative PCS values corresponded to the 41st and 34th percentiles in the general population with ACD and Control, respectively. Although cost-utility data were not a focus of this paper, the combination of lower reoperation rates and better improvements in health-related quality of life identified in the current study with the ACD suggest the potential for cost savings with routine ACD use in high-risk patients, a conclusion that has been corroborated by others.23,24
In the Control group composed of patients with large annular defects, the risk of reoperation was 21.1% at 4 years, which is double the 10.5% reoperation rate reported through 4 years in the SPORT trial.25 This finding parallels that of a meta-analysis that reported a large annular defect more than doubled the risk of reoperation.13 The reoperation rate in patients treated with lumbar discectomy only in the current trial was comparable to rates reported in previous studies of patients with large annular defects.26,27 Additionally, female sex and current smoking status were identified as independent risk factors for reoperation in the Control group. While the deleterious influence of smoking on post-discectomy reoperation rates has been reported by others,28 the association of female sex on reoperation risk is less clear as most studies report no independent association of sex.12,25,28,29 In this study, the risk of reoperation in females with large post-surgical annular defects was 30.3% through 4 years, and this risk was significantly lower (17.4%) among females treated with an ACD. Additional research to understand the mechanisms of sex differences in lumbar disc surgery is crucial for optimal treatment outcomes.
The conclusions of this trial were strengthened by the randomized trial design, large sample size, and the longest follow-up duration of any study in patients with large post-discectomy annular defects. There are also several limitations of this trial. First, the results presented here are applicable only to patients with large post-discectomy annular defects, who account for approximately 30% of all lumbar discectomy cases.13 Implantation of an ACD in patients with small annular defects cannot be justified clinically given the inherently low risk of symptom recurrence in these individuals. Additional patient characteristics that are crucial to achieving positive results include adequate disc height and non-osteoporotic bone mineral density of the lumbar spine. Second, the decision to reoperate involved shared decision making between the patient and surgeon and, therefore, there is potential for bias in the reported reoperation rates. Finally, 5-year follow-up in this study is ongoing and these long-term outcomes are anxiously awaited to provide final comparative efficacy, safety, and cost-utility results of bone-anchored ACD implantation.
Conclusion
The addition of a bone-anchored ACD to lumbar discectomy in patients with large post-surgical annular defects reduces the risk of reoperation and provides for better long-term pain and disability relief over 4 years compared to lumbar discectomy only.
Abbreviations
ACD, annular closure device; HR, hazard ratio; MCID, minimal clinically important difference; MCS, Mental Component Summary; ODI, Oswestry Disability Index; PCS, Physical Component Summary.
Data sharing statement
All authors have agreed to the submission and publication of this manuscript. The authors confirm that requests for data underlying the findings described in this manuscript may be made to the corresponding author starting 1 year following publication of this article.
Acknowledgment
There were no contributors to the article other than the authors. No writing assistance regarding this study was received.
Author contributions
All authors contributed to conception and design, LM contributed to data analysis, DN, MA, HK, JP, CF, and PV contributed to data interpretation, and all authors contributed to drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Mark P Arts reports consultancy with Intrinsic Therapeutics; personal fees from Zimmer-Biomet, EIT, and Silony, outside the submitted work; and receipt of royalties from EIT. Larry Miller reports consultancy with Intrinsic Therapeutics. The authors report no other conflicts of interest in this work.
References
1. Frymoyer JW. Back pain and sciatica. N Engl J Med. 1988;318:291–300. doi:10.1056/NEJM198802043180506
2. Vroomen PC, de Krom MC, Knottnerus JA. Predicting the outcome of sciatica at short-term follow-up. Br J Gen Pract. 2002;52:119–123.
3. Vroomen PC, de Krom MC, Wilmink JT, Kester AD, Knottnerus JA. Lack of effectiveness of bed rest for sciatica. N Engl J Med. 1999;340:418–423. doi:10.1056/NEJM199902113400602
4. Peul WC, van Houwelingen HC, van den Hout WB, et al. Surgery versus prolonged conservative treatment for sciatica. N Engl J Med. 2007;356:2245–2256. doi:10.1056/NEJMoa064039
5. Kreiner DS, Hwang SW, Easa JE, et al. An evidence-based clinical guideline for the diagnosis and treatment of lumbar disc herniation with radiculopathy. Spine J. 2014;14:180–191. doi:10.1016/j.spinee.2013.08.003
6. Spengler DM. Lumbar discectomy. Results with limited disc excision and selective foraminotomy. Spine (Phila Pa 1976). 1982;7:604–607.
7. de Souza Grava AL, Ferrari LF, Defino HL. Cytokine inhibition and time-related influence of inflammatory stimuli on the hyperalgesia induced by the nucleus pulposus. Eur Spine J. 2012;21:537–545. doi:10.1007/s00586-011-2027-8
8. Asch HL, Lewis PJ, Moreland DB, et al. Prospective multiple outcomes study of outpatient lumbar microdiscectomy: should 75 to 80% success rates be the norm? J Neurosurg. 2002;96:34–44.
9. Parker SL, Mendenhall SK, Godil SS, et al. Incidence of low back pain after lumbar discectomy for herniated disc and its effect on patient-reported outcomes. Clin Orthop Relat Res. 2015;473:1988–1999.
10. Ambrossi GL, McGirt MJ, Sciubba DM, et al. Recurrent lumbar disc herniation after single-level lumbar discectomy: incidence and health care cost analysis. Neurosurgery. 2009;65:
11. Abdu RW, Abdu WA, Pearson AM, Zhao W, Lurie JD, Weinstein JN. Reoperation for recurrent intervertebral disc herniation in the spine patient outcomes research trial: analysis of rate, risk factors, and outcome. Spine (Phila Pa 1976). 2017;42:1106–1114. doi:10.1097/BRS.0000000000002088
12. Fritzell P, Knutsson B, Sanden B, Strömqvist B, Hägg O. Recurrent versus primary lumbar disc herniation surgery: patient-reported outcomes in the Swedish Spine Register Swespine. Clin Orthop Relat Res. 2015;473:1978–1984. doi:10.1007/s11999-014-3596-8
13. Miller LE, McGirt MJ, Garfin SR, Bono CM. Association of annular defect width after lumbar discectomy with risk of symptom recurrence and reoperation: systematic review and meta-analysis of comparative studies. Spine (Phila Pa 1976). 2018;43:E308–E315. doi:10.1097/BRS.0000000000002501
14. Kienzler JC, Klassen PD, Miller LE, et al. Three-year results from a randomized trial of lumbar discectomy with annulus fibrosus occlusion in patients at high risk for reherniation. Acta Neurochir (Wien). 2019. doi:10.1007/s00701-019-03948-8
15. Klassen PD, Hes R, Bouma GJ, et al. A multicenter, prospective, randomized study protocol to demonstrate the superiority of a bone-anchored prosthesis for anular closure used in conjunction with limited discectomy to limited discectomy alone for primary lumbar disc herniation. Int J Clin Trials. 2016;3:120–131. doi:10.18203/2349-3259.ijct20162794
16. Thome C, Klassen PD, Bouma GJ, et al. Annular closure in lumbar microdiscectomy for prevention of reherniation: a randomized clinical trial. Spine J. 2018;18:2278–2287. doi:10.1016/j.spinee.2018.05.003
17. Ostelo RW, de Vet HC. Clinically important outcomes in low back pain. Best Pract Res Clin Rheumatol. 2005;19:593–607. doi:10.1016/j.berh.2005.03.003
18. Fairbank JC, Pynsent PB. The oswestry disability index. Spine (Phila Pa 1976). 2000;25:
19. Ware JE
20. Bouma GJ, Barth M, Miller LE, et al. Challenges in the analysis of longitudinal pain data: practical lessons from a randomized trial of annular closure in lumbar disc surgery. Pain Res Treat. 2019;2019:3498603.
21. Weinstein JN, Lurie JD, Tosteson TD, et al. Surgical versus nonoperative treatment for lumbar disc herniation: four-year results for the Spine Patient Outcomes Research Trial (SPORT). Spine (Phila Pa 1976). 2008;33:2789–2800. doi:10.1097/BRS.0b013e31818ed8f4
22. Maglinte GA, Hays RD, Kaplan RM. US general population norms for telephone administration of the SF-36v2. J Clin Epidemiol. 2012;65:497–502. doi:10.1016/j.jclinepi.2011.09.008
23. Ament JD, Thaci B, Yang Z, et al. Postoperative direct healthcare costs of lumbar discectomy are reduced with the use of a novel annular closure device in high-risk patients. Spine J. 2019;19:1170–1179. doi:10.1016/j.spinee.2019.02.010
24. Ament J, Thaci B, Yang Z, et al. Cost-effectiveness of a bone-anchored annular closure device versus conventional lumbar discectomy in treating lumbar disc herniations. Spine (Phila Pa 1976). 2019;44:5–16. doi:10.1097/BRS.0000000000002746
25. Leven D, Passias PG, Errico TJ, et al. Risk factors for reoperation in patients treated surgically for intervertebral disc herniation: a subanalysis of eight-year SPORT data. J Bone Joint Surg Am. 2015;97:1316–1325. doi:10.2106/JBJS.N.01287
26. Kim KT, Lee DH, Cho DC, Sung J-K, Kim Y-B. Preoperative risk factors for recurrent lumbar disk herniation in L5-S1. J Spinal Disord Tech. 2015;28:E571–E577. doi:10.1097/BSD.0000000000000041
27. Zhou B, Wang K, Hong X, et al. Adjacent level disc degeneration: a prognostic factor for recurrent lumbar disc herniation after transforaminal endoscopic lumbar discectomy in 409 cases. Int J Clin Exp Med. 2016;9:21854–21859.
28. Andersen SB, Smith EC, Stottrup C, Carreon LY, Andersen MO. Smoking is an independent risk factor of reoperation due to recurrent lumbar disc herniation. Global Spine J. 2018;8:378–381. doi:10.1177/2192568217730352
29. Hakkinen A, Kiviranta I, Neva MH, Kautiainen H, Ylinen J. Reoperations after first lumbar disc herniation surgery; a special interest on residives during a 5-year follow-up. BMC Musculoskelet Disord. 2007;8:2. doi:10.1186/1471-2474-8-2
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.