Back to Journals » Drug Design, Development and Therapy » Volume 14
Annatto-Derived Tocotrienol Promotes Mineralization of MC3T3-E1 Cells by Enhancing BMP-2 Protein Expression via Inhibiting RhoA Activation and HMG-CoA Reductase Gene Expression
Authors Wan Hasan WN, Chin KY , Abd Ghafar N, Soelaiman IN
Received 26 July 2019
Accepted for publication 14 February 2020
Published 3 March 2020 Volume 2020:14 Pages 969—976
DOI https://doi.org/10.2147/DDDT.S224941
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sukesh Voruganti
Wan Nuraini Wan Hasan,1 Kok-Yong Chin,1 Norzana Abd Ghafar,2 Ima Nirwana Soelaiman1
1Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia, UKM Medical Centre (UKMMC), Kuala Lumpur 56000, Malaysia; 2Department of Anatomy, Faculty of Medicine, Universiti Kebangsaan Malaysia, UKM Medical Centre (UKMMC), Kuala Lumpur 56000, Malaysia
Correspondence: Ima Nirwana Soelaiman
Department of Pharmacology, Faculty of Medicine, Universiti Kebangsaan Malaysia, UKM Medical Centre (UKMMC), Jalan Yaacob Latiff, Bandar Tun Razak, Cheras, Kuala Lumpur 56000, Malaysia
Tel +60 3 9145 5002
Fax +60 3 9145 6633
Email [email protected]
Purpose: Annatto-derived tocotrienol (AnTT) has been shown to improve bone formation in animal models of osteoporosis and promote differentiation of pre-osteoblastic cells. However, the mechanism of action of AnTT in achieving these effects is unclear. This study aims to investigate the mechanism of action of AnTT on MC3T3-E1 pre-osteoblasts via the mevalonate pathway.
Methods: Murine pre-osteoblastic cells, MC3T3-E1, were cultured with the density of 1 × 104 cells/mL and treated with 4 concentrations of AnTT (0.001– 1 μg/mL). Expression of HMG-CoA reductase (HMGR) gene was carried out using qPCR after treatment with AnTT for 21 days. RhoA activation and bone morphogenetic protein-2 (BMP-2) were measured using immunoassay after 9 and 15 days of AnTT treatment. Lovastatin was used as the positive control. Mineralized nodules were detected using Von Kossa staining after 21 days of AnTT treatment.
Results: The results showed that HMGR was up-regulated in the lovastatin group on day 9 and 21 compared to the control. Lovastatin also inhibited RhoA activation (day 9 and 15) and increased BMP-2 protein (day 15). On the other hand, AnTT at 0.001 μg/mL (day 3) and 0.1 μg/mL (day 21) significantly down-regulated HMGR gene expression compared to the control. On day 21, HMGR gene expression was significantly reduced in all groups compared to day 15. AnTT at 0.1 μg/mL significantly decreased RhoA activation on day 9 compared to the control. AnTT at 1 μg/mL significantly increased BMP-2 protein on day 15 compared to the control (P< 0.05). Mineralized calcium nodules were more abundant in AnTT treated groups compared to the control on day 21.
Conclusion: AnTT suppresses the mevalonate pathway by downregulating HMGR gene expression and inhibiting RhoA activation, leading to increased BMP-2 protein in MC3T3-E1 cells. This explains the stimulating effects of AnTT on osteoblast mineralization.
Keywords: bone, osteogenic, osteoporosis, tocotrienol, vitamin E
Introduction
Bone remodelling is regulated by osteoblast-mediated bone formation and osteoclast-mediated bone resorption. The imbalance of these two processes causes abnormalities in bone remodelling, which can produce a variety of bone disorders including osteoporosis.1 Osteoporosis is a “silent” bone degenerative disorder characterized by low bone mass and deterioration of skeletal microarchitecture, leading to bone fragility.2 Osteoporosis mainly affects postmenopausal women but it can also occur in men.3,4 It is one of the most under-recognised non-communicable health conditions affecting developing countries with increasing elderly population.5
The current therapies for osteoporosis include anti-resorptive agents (bisphosphonates, calcitonin, denosumab, estrogen + progesterone) and anabolic agents (teriparatide).6,7 These agents are effective against osteoporosis but they come with adverse side effects.8,9 The preventive agents for osteoporosis are limited to calcium with or without vitamin D. This highlights a significant gap for pharmacological prevention of osteoporosis.
The mevalonate pathway, responsible for cholesterol synthesis, has been the target of drug intervention for osteoporosis.10 Statins, traditionally known as cholesterol-lowering agents, have been found to promote bone formation in vitro and in vivo.11–13 Statins regulate the mevalonate pathway by inhibiting the 3-hydroxy-3-methyl-glutaryl coenzyme-A (HMG-CoA) reductase (HMGR) enzyme, the rate-limiting enzyme for the mevalonate pathway, from converting HMG-CoA into mevalonate. These will subsequently suppress the synthesis of isoprenoids, such as farnesyl pyrophosphate (FPP) and geranylgeranyl pyrophosphate (GGPP). FPP and GGPP are involved in the protein prenylation process, whereby the isoprenoids are bound covalently to the C-terminal of small G-proteins or GTPase.14 G-proteins consist of 5 families; the Rho/Rac/Cdc42, Ras, Rab, Sar1/Arf, and Ran families.15 Rho is involved in statin-induced osteogenesis. A study by Harmey et al showed that Rho-Rho kinase inhibition stimulated differentiation and calcium nodule formation of mouse calvariae cells.16 Inhibition of Rho kinase was shown to increase bone morphogenetic protein-2 (BMP-2) gene expression by pitavastatin in human osteoblasts.17 However, this stimulatory effect was abolished by mevalonate or GGPP, indicating that these effects originated from inhibition of the mevalonate pathway.17 BMP-2 plays a crucial role in the differentiation of human embryonic stem cells.18 BMP signalling regulates transcription factors including runt-related factor 2 (Runx2) and osterix (OSX) involved in the formation of osteoblasts and expression of downstream genes involved in bone formation.19
Tocotrienol is a member of the vitamin E family, along with tocopherol. It can be found in palm oil, wheat germ, rice bran, barley, and annatto bean. Both tocotrienol and tocopherol contain 4 isomers, i.e. alpha (α), beta (β), delta (δ) and gamma (γ), depending on the side chains on the chromanol ring. Tocotrienol exerts powerful neuroprotective, antioxidant, anti-cancer and lipid-lowering properties, which distinguish it from tocopherol.20 In addition, γ-tocotrienol preserved normal body composition and calcium content more effectively compared to α-tocopherol in dexamethasone-induced rats.21 Both palm tocotrienol and annatto derived-tocotrienol have been reported to protect bone in various animal models of osteoporosis.22 A previous study suggested the involvement of mevalonate pathway in the bone-sparing effects of γ-tocotrienol in ovariectomized mice.23 Annatto derived-tocotrienol (AnTT) from seeds of achiote tree native to tropical America contains 90% δ- and 10% γ-tocotrienol.24,25 In animal models of osteoporosis due to testosterone deficiency, AnTT was shown to prevent bone loss by increasing osteoblast number, osteoid volume and osteoid surface.26 Combination of AnTT and lovastatin also increased bone formation, improved bone structure and bone strength in ovariectomized rats.27,28 In cell culture studies, AnTT stimulated MC3T3-E1 differentiation and mineralization.29 However, the mechanism of action behind this osteogenic activity is still unknown.
In the present study, the mechanism of action of AnTT on the mevalonate pathway, specifically on HMGR gene, RhoA prenylated protein and BMP-2 protein, in MC3T3-E1 cells was investigated. It is hypothesized that AnTT promotes bone mineralization via the mevalonate pathway, marked by decreased expression of HMGR gene, RhoA and increased BMP-2 proteins in MC3T3-E1 cells.
Materials and Methods
Chemicals
Minimum Essential Medium Eagle – Alpha Modification (α-MEM) was obtained from Invitrogen (Carlsbad, USA). Antibiotic-antifungal (AA), foetal bovine serum (FBS) and phosphate-buffered saline (PBS) were obtained from Gibco (Waltham, USA). Sodium phosphate, ascorbic acid and silver nitrate were obtained from Sigma-Aldrich Co. (St Louis, USA). Ethanol was obtained from HmbG Chemicals (Hamburg, Germany). Lovastatin was obtained from ChemFaces (Wuhan, China).
Experimental Treatments
Annatto-derived tocotrienol (AnTT) was a generous gift from American River Nutrition (Hadley, USA). AnTT was prepared based on a previous study.29 Briefly, AnTT stock solution was dissolved in ethanol to a concentration of 5 mg/mL. From the stock solution, 25 µL AnTT was added to 60 µL FBS and incubated overnight. On the following day, 90 µL differentiation media and 105 µL ethanol were added to the mixture. AnTT was diluted into 0.001, 0.01, 0.1 and 1 µg/mL in differentiation media. For the control group, the same amount of ethanol as the AnTT groups was used.
For the positive control group, 20 mg lovastatin was dissolved with 1 mL ethanol. Then, lovastatin was diluted into 5 µM in differentiation media. All treatments were freshly prepared every 3 days until the end of treatment.
Cell Culture
Murine calvarial pre-osteoblast cell line, MC3T3-E1, was purchased from American Type Culture Collection (ATCC) (ATCC No CRL-2594) (Manassas, USA). The cells were cultured in growth media (α-MEM supplemented with 10% AA and 10% FBS) at 37°C and 5% carbon dioxide. The cells were seeded at the density of 1 × 104 cells/mL growth media in all experiments. On the next day, the cells were treated with AnTT (0.001–1 µg/mL) and 5 µM lovastatin prepared in differentiation media (growth media + 3 mM sodium phosphate + 50 µg/mL ascorbic acid).
HMGR Gene Expression
Pre-osteoblast cells were seeded at a density of 5 × 104 in 6-well plate. On the following day, the cells were treated with AnTT (0.001–1 µg/mL) and 5 µM lovastatin for 3, 9, 15 and 21 days. At the end of each time-point, the cells were washed with PBS and lysed using TRI-Reagent (Molecular Research Centre, Inc., Cincinnati, OH, USA). The extracted RNA converted into cDNA with a thermal cycler (Techne, Staffordsire, UK) using iScript™ cDNA Synthesis Kit (Bio-Rad Laboratories Inc., Hercules CA, USA). Expression of targeted genes was quantified by CFX96 Touch™ Real-Time Detection System (Bio-Rad Laboratories Inc., Hercules CA, USA) under the amplification condition of 40 cycles, 10 s at 95 °C for denaturation and 30 s at 56 °C for annealing. The mice primers used were: β-actin, 5ʹ-GAAGAGCTATGAGCTGCCTGA-3ʹ and 5ʹ-GCACTGTGTTGGCATAGAGGT-3ʹ; HMGR, 5ʹ-TCTTTCCGTGCTGTGTTCTG-3ʹ and 5ʹ-TTTTAACCCACGGAGAGGTG-3ʹ (First Base, Singapore Science Park II, Singapore).
RhoA Activation Assay
The RhoA activation assay measured the active form of RhoA in the cells. The cells were seeded in a 6-well plate at a density 5×104 and incubated overnight. Then, they were treated with AnTT (0.001–1 µg/mL) and 5 µM lovastatin for 9 and 15 days. The cells were harvested at the end of each time-point and assayed with G-LISA® RhoA Activation Assay Kit (Cat. BK124) (Cytoskeleton Inc. Denver, USA).
BMP-2 Protein
Murine preosteoblastic cells were seeded at a density of 5 × 104 in a 6-well plate. On the next day, the cells were treated with AnTT (0.001–1 µg/mL) and 5 µM lovastatin for 9 and 15 days. At the end of each time-point, the cells were washed with PBS. Then, the cells were lysed with freeze-thaw method 3 times in PBS. The supernatants were collected after quick centrifuged at high speed. The expression of BMP-2 was determined using an enzyme-linked immunosorbent assay (Cat. No. E-EL-M0193, Elabscience, Wuhan, China).
Assessment of Mineralization
To determine the effects of AnTT on mineralization, the extracellular matrix of the culture was assessed using Von Kossa staining. Cells were treated with AnTT for 3, 9, 15 and 21 days. At the end of each time-point, the cells were washed with deionised water and fixed with 10% buffered formalin for 10 min. Then, the cells were treated with 5% silver nitrate and incubated at room temperature for 1 hr under ultraviolet light. After the cells were washed with deionised water, positive staining for Von Kossa was visualized under the inverted microscope EVOS Cell Imaging System (Thermo Fisher Scientific).
Statistical Analysis
Statistical analysis was performed using SPSS software for Windows, version 20 (IBM Corporation, Armonk, NY, USA). The difference in the variables of interest among the study groups was analysed using one-way analysis of variance (ANOVA) with Turkey post hoc pairwise comparison. For gene expression analysis, mixed-design ANOVA with small effect analysis was used. A P-value less than 0.05 (P<0.05) was considered statistically significant.
Results
Effects of AnTT on HMGR Gene
In this study, MC3T3-E1 cells were treated with AnTT (0.001–1 µg/mL) and 5 µM lovastatin (positive control) for 3, 9, 15 and 21 days (Figure 1). There were significant time (P<0.05) and treatment (P<0.05) effects on HMGR gene expression. In terms of time, for the control group, HMGR gene expression was significantly reduced on day 9 and day 21 compared to day 3 and day 15, respectively (P<0.05). HMGR gene expression for cells treated with AnTT at 0.001, 0.1 and 1 µg/mL reduced on day 9 and 21 compared to day 3 and 15, respectively (P<0.05). In terms of treatment, HMGR gene expression of cells treated with 0.01 µg/mL of AnTT reduced on day 9 compared to the control (P<0.05). HMGR gene expression was significantly decreased in all groups on day 21 compared to day 3 (P<0.05). For the lovastatin group, HMGR gene expression was up-regulated on day 9 and day 21 compared to the control (P<0.05). On day 3, 0.001 µg/mL AnTT significantly down-regulated HMGR gene expression compared to the control (P<0.05). On day 21, 0.1 µg/mL AnTT significantly down-regulated HMGR gene expression compared to the control (P<0.05).
Effects of AnTT on RhoA Activation
In order to confirm the involvement of the mevalonate pathway, RhoA activation assay was carried out to determine whether Rho was geranylgeranylated after AnTT treatment. MC3T3-E1 cells were treated with AnTT (0.001–1 µg/mL) and 5 µM lovastatin (positive control) for 9 and 15 days (Figure 2). RhoA activation was significantly decreased in the lovastatin group on day 9 and 15 compared to control (P<0.05). On day 9, RhoA activation was significantly decreased in the 0.1 µg/mL AnTT group compared to the control (P<0.05).
Effects of AnTT on BMP-2 Protein
In this study, MC3T3-E1 cells were treated with AnTT (0.001–1 µg/mL) and 5 µM lovastatin (positive control) for 9 and 15 days (Figure 3). In terms of time, BMP-2 protein levels of the control, 0.001, 0.1, 1 µg/mL AnTT and lovastatin groups were significantly increased on day 15 compared to day 9 (P<0.05). In terms of treatment, BMP-2 protein level of the lovastatin group was significantly increased compared to the control on day 15 (P<0.05). On day 15, 1 µg/mL AnTT significantly increased BMP-2 protein compared to the control (P<0.05).
Effects of AnTT on Mineralization
AnTT (0.001–1 µg/mL) were treated for 9 and 15 days and stained using Von Kossa technique to determine the degree of mineralization (Figure 4). Positive staining for Von Kossa staining appeared in dark brown or black in the culture. On day 3 and 9, all groups were not stained. On day 15, positive staining appeared in all groups. On day 21, the cultures were stained more intensively in 0.01, 0.1 and 1 µg/mL AnTT groups compared to the control. These data indicated that AnTT promoted osteoblast mineralization.
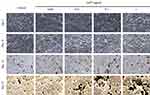 |
Figure 4 Von Kossa staining on MC3T3-E1 cells after AnTT treatment for day 3, day 9, day 15 and day 21. |
Discussion
Osteoblasts play a crucial role in bone formation. Osteoblasts secrete proteins to form the bone matrix and later mineralise it. Previous studies showed that statins promote osteoblast differentiation via inhibition of the mevalonate pathway, which leads to suppression of prenylated proteins including RhoA. This will modulate signalling pathways including BMP-2, to promote bone formation. The present study showed that AnTT down-regulated HMG-CoA reductase gene expression, which leads to inhibition of RhoA prenylated protein. This indicated that AnTT, acting as a HMG-CoA reductase suppressor, inhibited the mevalonate pathway. Besides that, AnTT also increased BMP-2 protein level involved in the expression of transcription factors Runx2 and Osx critical in osteoblast differentiation. This translated to increased mineralization in osteoblast culture as observed in the current study. Therefore, AnTT could serve as a potential bone anabolic agent via inhibition of the mevalonate pathway.
In the present study, AnTT was found to down-regulate HMGR gene in preosteoblastic cells. A previous study demonstrated that γ- and δ-tocotrienol stimulated HMGR degradation, however only δ-tocotrienol completely blocked nuclear SREBP-2 processing in the SV589 human fibroblast cells.30 The protein SREBP-2 is involved in the regulation of cholesterol biosynthesis enzymes including HMGR gene.31 Another study showed that δ-tocotrienol down-regulated SREBP-2 target gene, including HMGR in Chinese-hamster ovary cell lines, CHO and prostate cancer cell lines, LNCaP.32 These studies suggested that AnTT, which contains γ- and δ-tocotrienol, could inhibit the mevalonate pathway by down-regulating HMGR at the transcription level.
This study also demonstrated that AnTT inhibited RhoA activation in MC3T3-E1 cells in a manner comparable to the positive control, lovastatin. A previous study showed that HMG-CoA inhibitor, simvastatin blocked HMG-CoA/GGPP/RhoA-dependent pathway in mouse embryonic stem cell lines.33 Similarly, Ohnaka et al found that pitavastatin stimulated BMP-2 and osteocalcin gene by inhibiting Rho-kinase activity and inactivation of Rho via the mevalonate pathway in human osteoblasts.17 In human airway smooth muscle cells, 50 µM γ-tocotrienol inhibited RhoA activation that leads to a reduction of cell proliferation and migration.34 On the other hand, the combination of statin and γ-tocotrienol inhibited HMG-CoA reductase activity and RhoA activation in human colon cancer HCT116 and HT29 cells.35 Since AnTT inhibited HMGR in this study, it is suggested that the production of isoprenoids and prenylated G-protein like RhoA would be suppressed.
The current study also showed that AnTT increased BMP-2 protein level in pre-osteoblastic cells in a manner comparable to lovastatin. Mevastatin and simvastatin were shown to activate BMP-2 promoter, leading to increased BMP-2 mRNA and protein expressions in human osteosarcoma cell line.36 A previous animal study also showed that the combination of AnTT and lovastatin increased the expression of BMP-2 mRNA in the bones of ovariectomized rats.37 This paper investigated the effects of annatto tocotrienol via BMP2 but it cannot be ruled out that it may act through other BMPs and transforming growth factor signaling in bone metabolism.38–41
In this study, AnTT promoted osteoblast mineralization in MC3T3-E1 cells. This validated the observation previously obtained in a cellular study, whereby AnTT enhanced differentiation and mineralization (marked by Alizarin Red staining) of MC3T3-E1 cells.29 A study by Deng et al showed that γ-tocotrienol increased calcium nodule formation in mice bone marrow cells. This effect was abolished by co-treatment with mevalonate, implicating the involvement of mevalonate pathway in the mineralization induced by γ-tocotrienol.23 In animal studies, AnTT alone and in combination with lovastatin were shown to improve the structural properties of femoral trabecular bone in ovariectomized rats.28 Similarly, AnTT preserved trabecular bone microarchitecture in an animal model of osteoporosis induced by testosterone deficiency and metabolic syndrome.37,42
Several limitations should be acknowledged in this study. We only studied the mevalonate pathway partially, focusing on the upstream (HMGR) and down-stream (RhoA) section of the pathway. The effects of AnTT on the other steps of the pathway remain unknown, especially on the production of isoprenoids such, as FPP and GGPP. Inhibition of the mevalonate pathway by statins has been widely shown to deplete FPP and GGPP levels.43 We also did not study the activity of HMGR to correlate with the gene expression level due to technical issue. Nevertheless, this is the first time the effects of AnTT on the mevalonate pathway were elucidated in pre-osteoblast cells. Annatto tocotrienol, which is shown in this study to promote mineralization by modulating BMP and HMG-CoA reductase, will likely to have multifaceted effects in other physiological and pathological context like cardiovascular diseases, neurodegenerative diseases or cancer.44–49 Our work warrants further investigation of mechanism and synthetic modification to improve tocotrienols to enhance its pharmacological and therapeutic application in a disease-specific manner.
Conclusion
AnTT treatment down-regulates HMGR gene expression, thus inhibiting the mevalonate pathway, leading to reduced RhoA activation downstream of the mevalonate pathway. AnTT also increases BMP-2 protein expression. The suppression of mevalonate pathway may partially explain the anabolic effect of AnTT on osteoblast mineralization.
Acknowledgments
We express our gratitude to American River Nutrition (USA) for providing the annatto-derived tocotrienol, DeltaGold®. This research was funded by ERGS/1/2013/SKK03/UKM/01/1 grant provided by the Ministry of Education, Malaysia, and GUP-2017-012 grant by Universiti Kebangsaan Malaysia. We would like to thank Puan Nur Farhana Mohd Fozi, Puan Nurul Hafizah Abas and James Jam Jolly from Pharmacology Department, UKM for technical assistance.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Rachner TD, Khosla S, Hofbauer LC. Osteoporosis: now and the future. Lancet. 2011;377(9773):1276–1287. doi:10.1016/S0140-6736(10)62349-5
2. WHO. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis: report of a WHO study group [meeting held in Rome from 22 to 25 June 1992]. 1994. 10.3168/jds.S0022-0302(94)77044-2
3. Rosen CJ. Postmenopausal osteoporosis. N Engl J Med. 2005;353(6):595–603. doi:10.1056/NEJMcp043801
4. Willson T, Nelson SD, Newbold J, Nelson RE, LaFleur J. The clinical epidemiology of male osteoporosis: a review of the recent literature. Clin Epidemiol. 2015;7:65.
5. Dhanwal DK, Dennison EM, Harvey NC, Cooper C. Epidemiology of hip fracture: worldwide geographic variation. Indian J Orthop. 2011;45(1):15. doi:10.4103/0019-5413.73656
6. Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–170. doi:10.1016/j.jsbmb.2013.09.008
7. Russow G, Jahn D, Appelt J, Märdian S, Tsitsilonis S, Keller J. Anabolic therapies in osteoporosis and bone regeneration. Int J Mol Sci. 2019;20(1):83. doi:10.3390/ijms20010083
8. Liberman UA, Weiss SR, Bröll J, et al. Effect of oral alendronate on bone mineral density and the incidence of fractures in postmenopausal osteoporosis. N Engl J Med. 1995;333(22):1437–1444. doi:10.1056/NEJM199511303332201
9. Cejka D, Benesch T, Krestan C, et al. Effect of teriparatide on early bone loss after kidney transplantation. Am J Transplant. 2008;8(9):1864–1870. doi:10.1111/ajt.2008.8.issue-9
10. Wan Hasan W, Kok-yong C, Jolly J, Abd NG, Ima-Nirwana S. Identifying potential therapeutics for osteoporosis by exploiting the relationship between mevalonate pathway and bone metabolism. Endocr Metab Immune Disord Drug Targets. 2018;18:450–457.
11. Qiao LJ, Kang KL, Heo JS. Simvastatin promotes osteogenic differentiation of mouse embryonic stem cells via canonical Wnt/β-catenin signaling. Mol Cells. 2011;32(5):437–444. doi:10.1007/s10059-011-0107-6
12. Oxlund H, Andreassen TT. Simvastatin treatment partially prevents ovariectomy-induced bone loss while increasing cortical bone formation. Bone. 2004;34(4):609–618. doi:10.1016/j.bone.2003.12.014
13. Mundy G, Garrett R, Harris S, et al. Stimulation of bone formation in vitro and in rodents by statins. Science. 1999;286(5446):1946–1949. doi:10.1126/science.286.5446.1946
14. Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65(1):241–269. doi:10.1146/annurev.bi.65.070196.001325
15. Matozaki T, Nakanishi H, Takai Y. Small G-protein networks:: their crosstalk and signal cascades. Cell Signal. 2000;12(8):515–524. doi:10.1016/S0898-6568(00)00102-9
16. Harmey D, Stenbeck G, Nobes CD, Lax AJ, Grigoriadis AE. Regulation of osteoblast differentiation by Pasteurella multocida toxin (PMT): a role for Rho GTPase in bone formation. J Bone Miner Res. 2004;19(4):661–670. doi:10.1359/JBMR.040105
17. Ohnaka K, Shimoda S, Nawata H, et al. Pitavastatin enhanced BMP-2 and osteocalcin expression by inhibition of Rho-associated kinase in human osteoblasts. Biochem Biophys Res Commun. 2001;287(2):337–342. doi:10.1006/bbrc.2001.5597
18. Pera MF, Andrade J, Houssami S, et al. Regulation of human embryonic stem cell differentiation by BMP-2 and its antagonist noggin. J Cell Sci. 2004;117(7):1269–1280. doi:10.1242/jcs.00970
19. Chen G, Deng C, Li Y-P. TGF-β and BMP signaling in osteoblast differentiation and bone formation. Int J Biol Sci. 2012;8(2):272. doi:10.7150/ijbs.2929
20. Sen CK, Khanna S, Roy S. Tocotrienols: vitamin E beyond tocopherols. Life Sci. 2006;78(18):2088–2098. doi:10.1016/j.lfs.2005.12.001
21. Ima-Nirwana S, Suhaniza S. Effects of tocopherols and tocotrienols on body composition and bone calcium content in adrenalectomized rats replaced with dexamethasone. J Med Food. 2004;7(1):45–51. doi:10.1089/109662004322984699
22. Chin K-Y, Ima Nirwana S. The biological effects of tocotrienol on bone. Drug Des Devel Ther. 2015;9:2049–2061. doi:10.2147/DDDT.S79660
23. Deng L, Ding Y, Peng Y, et al. γ-Tocotrienol protects against ovariectomy-induced bone loss via mevalonate pathway as HMG-CoA reductase inhibitor. Bone. 2014;67:200–207. doi:10.1016/j.bone.2014.07.006
24. Tan B, Foley J. Tocotrienols and geranylgeraniol from Bixa orellana byproducts. Google Patents. 2002.
25. Tan B, Llobrera J. Annatto extract compositions including tocotrienols and tocopherols and methods of use. Google Patents. 2009.
26. Chin K-Y, Abdul-Majeed S, Fozi NFM, Ima-Nirwana S. Annatto tocotrienol improves indices of bone static histomorphometry in osteoporosis due to testosterone deficiency in rats. Nutrients. 2014;6(11):4974–4983. doi:10.3390/nu6114974
27. Abdul-Majeed S, Mohamed N, Soelaiman I-N. Effects of tocotrienol and lovastatin combination on osteoblast and osteoclast activity in estrogen-deficient osteoporosis. Evid Based Complementary Alternat Med. 2012;2012. doi:10.1155/2012/960742
28. Abdul-Majeed S, Mohamed N, Soelaiman I-N. The use of delta-tocotrienol and lovastatin for anti-osteoporotic therapy. Life Sci. 2015;125:42–48. doi:10.1016/j.lfs.2014.12.012
29. Wan Hasan WN, Ghafar NA, Chin K-Y, Ima-Nirwana S. Annatto-derived tocotrienol stimulates osteogenic activity in preosteoblastic MC3T3-E1 cells: a temporal sequential study. Drug Des Devel Ther. 2018;12:1715. doi:10.2147/DDDT.S168935
30. Song B-L, DeBose-Boyd RA. Insig-dependent ubiquitination and degradation of 3-hydroxy-3-methylglutaryl coenzyme A reductase stimulated by δ-and γ-tocotrienols. J Biol Chem. 2006;281(35):25054–25061. doi:10.1074/jbc.M605575200
31. Sakakura Y, Shimano H, Sone H, et al. Sterol regulatory element-binding proteins induce an entire pathway of cholesterol synthesis. Biochem Biophys Res Commun. 2001;286(1):176–183. doi:10.1006/bbrc.2001.5375
32. Krycer JR, Phan L, Brown AJ. A key regulator of cholesterol homoeostasis, SREBP-2, can be targeted in prostate cancer cells with natural products. Biochem J. 2012;446(2):191–201. doi:10.1042/BJ20120545
33. Lee MH, Cho YS, Han YM. Simvastatin suppresses self‐renewal of mouse embryonic stem cells by inhibiting RhoA geranylgeranylation. Stem Cells. 2007;25(7):1654–1663. doi:10.1634/stemcells.2006-0753
34. Harada T, Yamasaki A, Chikumi H, et al. γ-Tocotrienol reduces human airway smooth muscle cell proliferation and migration. Pulm Pharmacol Ther. 2015;32:45–52. doi:10.1016/j.pupt.2015.04.003
35. Yang Z, Xiao H, Jin H, Koo PT, Tsang DJ, Yang CS. Synergistic actions of atorvastatin with γ-tocotrienol and celecoxib against human colon cancer HT29 and HCT116 cells. Int J Cancer. 2010;126(4):852–863. doi:10.1002/ijc.24766
36. Sugiyama M, Kodama T, Konishi K, Abe K, Asami S, Oikawa S. Compactin and simvastatin, but not pravastatin, induce bone morphogenetic protein-2 in human osteosarcoma cells. Biochem Biophys Res Commun. 2000;271(3):688–692. doi:10.1006/bbrc.2000.2697
37. Chin K-Y, Abdul-Majeed S, Mohamed N, Ima-Nirwana S. The effects of tocotrienol and lovastatin co-supplementation on bone dynamic histomorphometry and bone morphogenetic protein-2 expression in rats with estrogen deficiency. Nutrients. 2017;9(2):143. doi:10.3390/nu9020143
38. Tsuji K, Cox K, Bandyopadhyay A, Harfe BD, Tabin CJ, Rosen V. BMP4 is dispensable for skeletogenesis and fracture-healing in the limb. J Bone Joint Surg Am Vol. 2008;90(Suppl 1):14–18. doi:10.2106/JBJS.G.01109
39. Shu B, Zhang M, Xie R, et al. BMP2, but not BMP4, is crucial for chondrocyte proliferation and maturation during endochondral bone development. J Cell Sci. 2011;124(Pt 20):3428–3440. doi:10.1242/jcs.083659
40. Tsuji K, Cox K, Gamer L, Graf D, Economides A, Rosen V. Conditional deletion of BMP7 from the limb skeleton does not affect bone formation or fracture repair. J Orthop Res. 2010;28(3):384–389. doi:10.1002/jor.20996
41. Bandyopadhyay A, Tsuji K, Cox K, Harfe BD, Rosen V, Tabin CJ. Genetic analysis of the roles of BMP2, BMP4, and BMP7 in limb patterning and skeletogenesis. PLoS Gnet. 2006;2(12):e216. doi:10.1371/journal.pgen.0020216
42. Wong SK, Chin K-Y, Suhaimi FH, Ahmad F, Ima-Nirwana S. Exploring the potential of tocotrienol from Bixa orellana as a single agent targeting metabolic syndrome and bone loss. Bone. 2018;116:8–21. doi:10.1016/j.bone.2018.07.003
43. Weivoda MM, Hohl RJ. Effects of farnesyl pyrophosphate accumulation on calvarial osteoblast differentiation. Endocrinology. 2011;152(8):3113–3122. doi:10.1210/en.2011-0016
44. Lim DA, Tramontin AD, Trevejo JM, Herrera DG, Garcia-Verdugo JM, Alvarez-Buylla A. Noggin antagonizes BMP signaling to create a niche for adult neurogenesis. Neuron. 2000;28(3):713–726. doi:10.1016/S0896-6273(00)00148-3
45. Gajera CR, Emich H, Lioubinski O, et al. LRP2 in ependymal cells regulates BMP signaling in the adult neurogenic niche. J Cell Sci. 2010;123(Pt 11):1922–1930. doi:10.1242/jcs.065912
46. Kempf T, Sinning JM, Quint A, et al. Growth-differentiation factor-15 for risk stratification in patients with stable and unstable coronary heart disease: results from the AtheroGene study. Circul Cardiovas Genet. 2009;2(3):286–292. doi:10.1161/CIRCGENETICS.108.824870
47. Kempf T, Wollert KC. Growth differentiation factor-15: a new biomarker in cardiovascular disease. Herz. 2009;34(8):594–599. doi:10.1007/s00059-009-3317-3
48. Siegel PM, Massague J. Cytostatic and apoptotic actions of TGF-beta in homeostasis and cancer. Nat Rev Cancer. 2003;3(11):807–821. doi:10.1038/nrc1208
49. Park Y, Kim JW, Kim DS, et al. The Bone Morphogenesis Protein-2 (BMP-2) is associated with progression to metastatic disease in gastric cancer. Cancer Res Treat. 2008;40(3):127–132. doi:10.4143/crt.2008.40.3.127
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



