Back to Journals » Journal of Pain Research » Volume 14
Animal Models of Complex Regional Pain Syndrome Type I
Authors Liu Y, Liang Y, Gao M, Li Y, Zhao T, Zhao Y
Received 8 August 2021
Accepted for publication 28 October 2021
Published 4 December 2021 Volume 2021:14 Pages 3711—3721
DOI https://doi.org/10.2147/JPR.S333270
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Qi Fang
Yu Liu,1 Ying Liang,1 Min Gao,1 Yingchun Li,1 Tingting Zhao,2 Yani Zhao1
1Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Taiyuan, Shanxi, 030032, People’s Republic of China; 2Shaanxi University of Traditional Chinese Medicine, Xi’an, Shaanxi, 712046, People’s Republic of China
Correspondence: Ying Liang
Third Hospital of Shanxi Medical University, Shanxi Bethune Hospital, Shanxi Academy of Medical Sciences, Tongji Shanxi Hospital, Taiyuan, No. 99, Longcheng Street, Xiaodian District, Taiyuan, Shanxi, 030032, People’s Republic of China
Tel +86-13934225687
Email [email protected]
Abstract: Complex regional pain syndrome (CRPS) is a chronic pain disorder characterized by spontaneous or evoked regionally-confined pain which is out of proportion to the initial trauma event. The disease can seriously affect the quality of the patients’ life, increase the psychological burden, and cause various degrees of disability. Despite the awareness of CRPS among medical practitioners for over a century, its pathogenesis remains unclear, and the available treatment is still unsatisfactory. Effective animal models are the foundation of disease research, which is helpful in understanding the pathogenesis and an in-depth exploration of the appropriate therapeutic approaches. Currently, researchers have established a series of animal models of the disease. There are four main CRPSI animal models: chronic post-ischemic pain (CPIP) model, tibial fracture/cast immobilization model, passive transfer-trauma model, and the needlestick-nerve-injury (NNI) model. The modeling methods of these models are constantly improving over time. In preclinical studies, the interpretation of experimental results and the horizontal comparison between similar studies may be affected by the nature of the experimental animal breeds, sex, diet, and psychology. There is need to facilitate the choice of appropriate animal models and avoid the interference of the factors influencing animal models on the interpretation of research results. The review will provide a basic overview of the influencing factors, modeling methods, and the characteristics of CRPSI animal models.
Keywords: CRPS, reflex sympathetic dystrophy, animal model, mouse, rat, sex, diet, psychology
Introduction
Complex regional pain syndrome (CRPS) is a chronic and painful disease that usually occurs in the extremities. It is mainly characterized by local spontaneous or induced pain whose degree and duration are not proportional to the initial injury event.1 Sensory, motor, autonomic, and trophic abnormalities were also noted to be components of this syndrome. Contingent on the presence of a peripheral nerve injury, it can be classified into CRPSI (without peripheral nerve injury, also known as reflex sympathetic dystrophy) and CRPSII (peripheral nerve injury, also known as causalgia). The incidence of CRPSI is much higher than CRPSII; A retrospective epidemiological analysis of 1043 CRPS patients reported that the incidence of CRPSI was 88%, and that of CRPSII was 12%.2 In the 1950s, John Bonica described CRPSI in three stages: acute/early stage, dystrophic stage, and the atrophy/late stage.3 However, clinical practice has shown that not all CRPSI patients develop sequentially in this staging pattern, and the signs and symptoms of one stage may occur at any other stage.4 Currently, CRPS is often classified into two subtypes: “cold” and “warm.” Acute CRPS is often manifested as warm CRPS characterized by local inflammatory symptoms, while chronic CRPS is often associated with cold CRPS characterized by cold skin and trophic changes in the soft tissue or the bone.5 The course and outcome of CRPSI are highly variable. Many patients can recover within a year. However, a considerable number of patients have persistent symptoms, and some patients experience chronic pain and disability.6 “Cold” CRPS is more likely to exhibit a longer disease course and develop into worse functional outcome.7 Corresponding to the complex clinical manifestations and the disease course, CRPSI may not be a single factor-induced disease. Presently, many factors have been found to be related to the occurrence of CRPSI, such as inflammation, immunity, nerve injury, ischemia-reperfusion injury, central and peripheral sensitization, functional changes in the sympathetic nervous system, disuse, functional and anatomical changes in the central nervous system, psychological factors, and genetics.8 Nevertheless, the detailed pathophysiological process of CRPSI is still unclear. CRPSI can lead to a poor quality of life, pose a threat to the mental health of the patient, and the personal and social identity.9 The main treatments for the condition are physical therapy, pharmacotherapy, and interventional techniques, however, the current treatment effect is not satisfactory.10
Animal models are the basis of disease research. Establishing an appropriate animal model is conducive to an in-depth understanding of its pathogenesis and the development of an appropriate and effective treatment plan. So far, researchers have established a series of animal disease models. The establishment methods, the advantages and the disadvantages of CRPSII animal models have been reported.11 However, to the best of the author’s knowledge, there is no summary report on the influencing factors and the establishment and the characteristics of CRPSI animal models. Moreover, to facilitate the choice of an appropriate animal model, to enable researchers to consider and avoid the interference of the factors influencing animal model on the interpretation of the research results. Therefore, this paper focuses on the factors influencing CRPSI animal models, the modeling methods and the model characteristics of four CRPSI animal models, namely, chronic post-ischemic pain (CPIP) model, tibial fracture/cast immobilization model, passive transfer-trauma model, and the needlestick-nerve-injury (NNI) model.
Factors Influencing CRPSI Animal Models
Varieties and Strains of Experimental Animals
Rats and mice have been widely used in animal experiments, and their suitability for animal models is due to low price, easy feeding, fast reproduction rate, and solid reproductive ability. In addition, rats also have a relatively large size, easy to operate, strong anti-infection ability, and easy survivability after modeling, and easy to observe pathological changes after modeling. Due to the advantages above, they are also the main experimental animals in CRPSI animal experiments. The mouse strains used in CRPSI animal models mainly include C57BL/6 mice and Swiss mice, while the rat strains used in CRPSI animal models mainly include SD rats, Wistar rats, and Long Evans rats. In the Budapest criteria, hyperalgesia (to pinprick) and/or allodynia (to light touch and/or temperature sensation and/or deep somatic pressure and/or joint movement) are important signs for the diagnosis of CRPS.12 Therefore, for the CRPSI animal models, the sensory threshold of the rodents to different nociceptive stimuli is a vital parameter to test the effectiveness of the models. However, it is important to note that different species and strains of rodents (rats and mice) have different responses to nociceptive stimuli, which also increases the difficulty of horizontal comparisons between CRPSI-related animal studies.13 There are some differences in the anatomical structure of different species and strains. The differences in the anatomical structure lead to the complexity of the accurate implementation of the same model in the replication and transfer between different species and strains.13 For example, in the tibial fracture/cast immobilization model of CRPSI, the duration of cast immobilization in rats is different from that in mice.14,15
Gender of Experimental Animals
In clinical practice, pain symptoms are more common in women.16 A large-scale epidemiological studies covering both developed and developing countries reported that the prevalence of women in chronic pain diseases was 45%, which was higher than that of men (31%).17 Meyer-Friessem et al investigated the gender differences of pain intensity and pain threshold in CRPSI and neuropathic pain, and found that women had lower pain threshold among healthy subjects, CRPSI and neuropathic pain patients.18 The prevalence of CRPSI in women is about 2 to 4 times higher than that in men.10 Preclinical experiments have shown that gender differences in different CRPSI animal models affect the response to nociceptive stimulation and a series of biochemical indicators. Tang et al19 found that in the CPIP model, the emergence time of mechanical allodynia is earlier among females than males (days 2 and 3 after reperfusion respectively), the degree of mechanical allodynia in female mice is stronger too, and the levels of oxidative stress and inflammation in female mice were significantly higher than those in male mice during the initiation of CRPSI, which they thought might be responsible for the gender-related pain behavior in the model. Tajerian et al20 found a lower nociceptive threshold, longer duration of hyperalgesia, and more obvious chronic central neural plasticity in female mice in the tibial fracture/cast immobilization model. Moreover, five weeks after fracture, the spatial/fear memory deficits were also found in female mice, which were not observed in males. In this model, N-methyl-D-aspartic acid (NMDA) receptor subunit NR2B, which is widely involved in central sensitization and chronic pain, only decreased in males, but not in females, and this is considered to be related to the aforementioned characteristics. In the tibial fracture/cast immobilization mice, the nociceptive sensitization after fracture initially depends on innate and adaptive immune inflammatory mechanisms, but innate inflammation gradually resolves over time, and nociceptive sensitization is entirely dependent on adaptive immunity.21 Guo et al22 found significant gender differences in the process of nociceptive innate immune response and acquired immunity in the mouse model of tibial fracture/cast immobilization. Nociceptive sensitization dependent on the spinal microglia was only found in male mice, but not in females. Female (wild-type and muMT lacking B cells), and male (wild-type and muMT lacking B cells) mice were treated with tibia fracture and 3 weeks cast immobilization. After 3 weeks both males and females exhibited allodynia and unweighting in the wild-type mice. In the muMT mice, the males showed reduced allodynia and unweighting 3 weeks after the fracture, while the females did not show any reduced pain behavior until after 7 weeks. This indicated that compared with males, nociceptive acquired immunity was delayed in female mice after fracture/cast immobilization. Due to the effects of hormone circulation, the estrus, male rodents seemed to be more popular in CRPSI preclinical experiments. Nevertheless, both clinical and animal investigations of CRPSI reported gender differences. Accordingly, in the CRPSI animal experiment, female animals are irreplaceable, and the experimental conclusions drawn based on male animals may not be representative of the population due to particular deviations.
Diet and Psychology of Experimental Animals
Diet and psychology play crucial roles in the regulation of nociceptive behavior in experimental animals. Malnutrition, changes in dietary components, and specific dietary patterns can affect the sensitivity to chronic pain. In chronic inflammatory pain models, it has been observed that high fat diet, high carbohydrate diet, iron deficiency diet and western diet pattern can promote mechanical hyperalgesia and thermal hyperalgesia.23 Similarly, it was found that diet can affect pain behavior in the CRPS1 animal model. A significant correlation was found between the degree of nociceptive sensitivity and the blood glucose level in CPIP rats. Hyperglycemia can regulate the degree of nociceptive sensitivity by promoting oxidative stress, inflammation and thrombosis, and up-regulating kinin B1 receptor.24 Compared with rats with a regular diet or intraperitoneal injection of glucose to keep their blood sugar at normal or high levels during ischemic injury, rats with low blood sugar levels induced by fasting or insulin injection have lower degrees of mechanical allodynia and cold allodynia.24 Cucinello-Ragland et al25 investigated the mechanical nociceptive threshold of female rats exposed to alcohol diet in the CRPS1 cast immobilization model and found that alcohol consumption aggravated CRPS1-induced pain. Besides diet, changes in the psychological factors can also affect the pain behavior in rodents. An example is sickle cell disease. Similar to CRPS, a number of patients with sickle cell disease experience chronic pain. Tran et al26 observed that companionship reduces mechanical hyperalgesia, cold hyperalgesia, and thermal hyperalgesia in sickle mice and the withdrawal of companionship led to restored hyperalgesia level. This suggests that loneliness contributes to sustain or worsen hyperalgesia. Therefore, some attention should be paid to the psychological factors of experimental animals in CRPSI animal experiments. During the recovery period of animal surgery, it is often necessary to raise animals in a single cage. However, it is important to note that long-term single-cage feeding may lead to emotional changes in animals and then affect the experimental results to a certain extent.
Animal Models of CRPS I
Animal models are fundamental for the prospective exploration of the pathogenesis and the effective treatment of the disease. CRPS was proposed as early as during the American Civil War, and it has been known for over a century. At the early stages, the animal models of CRPS were mainly peripheral nerve injury models in accordance with the pathological characteristics of CRPSII.11 Until 2004, researchers have successively proposed more reasonable CRPSI animal models, and over time, there have been consistent significant improvements in the modeling methods. Table 1 summarizes the modeling methods and the characteristics of CRPSI animal models.
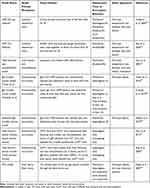 |
Table 1 Summary of Animal Models for CRPSI |
The Chronic Post-Ischemic Pain (CPIP) Model
Ring Method
Ischemia-reperfusion injury of limbs can cause pathological changes in deep micro vessel tissues, which in turn leads to signs consistent with clinical CRPS.27 Based on the long-term ischemia-reperfusion injury of rat’s hind paw, Coderre et al28 established the rat CPIP model. Under anesthesia, a 3cm3 syringe (the tube was cut off) was used to slide a Nitrile 70 Durometer O-ring with a 5.5 mm internal diameter to the proximal medial ankle joint of the rat’s hind limb. After 3 hours, the O-ring is removed, for the reperfusion of the hind limb. Typical symptoms such as edema, elevated skin temperature, mechanical allodynia (maintained for at least four weeks), mechanical hyperalgesia (maintained for at least four weeks), and the cold hyperalgesia (maintained for at least four weeks) appeared in the hind limbs of rats with O-ring relieving. Hu et al29 made minor changes to the model. They replaced the 3cm3 syringe with the 1.5mL EP tube that removed the buckle cap. The 1.5mL EP tube is more anastomosed to the diameter of the rat’s hind limbs so that the O-shaped rubber ring is more easily placed on the hind limb of the rats and does not cause injuries easily. Miecamps et al30 placed the O-ring with an inner diameter of 2mm in the hind limbs of 8-week-old mice, successfully transferred and replicated the model in mice.
Tie Method
The key to the success of the CPIP model is the blockage of the arterial blood flow to the extremities without necrosis of the surrounding tissue. A rigorous perusal of existing literature showed that when the rats with the same strain were used to make the CPIP model, the inner diameter of the O-ring used in different studies was the same, with significant variance in the body weight of the of the rats.31–34 The changes in the diameter of the hind limb and the inner diameter of the O-ring can affect the ultimate pressure. However, it is difficult to quantitatively measure the pressure induced by the O-ring on the hind limbs of the rats and mice. The modeling method seems to be used empirically. Seo et al35 developed a new method for the production of the CPIP model by rubber bands ligation instead of the O-ring, through the application of a 300g uniform force on the hind limbs of mice with a rubber band and push-pull gauge manometer. The tie method showed the following advantages: (i) higher model success rate and (ii) more persistent edema and mechanical allodynia symptom, with an average mechanical allodynia maintenance time of 55 days, although these symptoms later appeared in the O-ring group.
Generally, the modeling method of the CPIP model is easy to operate, with a relatively short modelling time. The tie method further delayed the time of symptom remission in the CPIP model, thus enhancing the suitability of the CPIP model for a longer study period. However, the mechanism of mechanical allodynia is different from that of the thermal allodynia.36 Seo et al35 did not measure the cold allodynia and the thermal allodynia. The occurrence of cold allodynia and thermal allodynia and the length of symptom maintenance in CPIP model with tie method needs to be investigated in future studies.
The Tibial Fracture/Cast Immobilization Model
Distal limb fracture is the most common cause of CRPSI.37 It is reported that the incidence of CRPSI after tibial fracture is about 30%.38 Through the simulation of the pathogenesis of CRPSI induced by extremities fracture, Guo et al14 developed a rat tibial fracture/cast immobilization model. Under anesthesia, the closed fractures of the distal tibia of the rats were made with hemostatic forceps, and then the fractured hind limb was fixed with a cast for four weeks. The occurrence of edema in the later stage entailed that a window was opened at the dorsum of the paw and the ankle. Buprenorphine and saline were given to relieve pain and to prevent dehydration after the operation. This model replicates similar symptoms to those of CRPSI patients, such as abnormal nociception, inflammatory symptoms, dyskinesia, nutritional and cognitive changes.39 Compared with other models, the duration of symptoms in the model of tibial fracture/cast immobilization model is relatively longer. Mechanical allodynia could last for nearly five months, but no thermal allodynia was observed in this model.14 The model was then applied to mice. Compared with rats, the cast fixation time of the hind limbs was three weeks.15 Limb immobilization may be a risk factor in the development of CRPSI. Fixing a cast on the forearms of healthy volunteers for four weeks could cause changes in the skin temperature, mechanical pain sensitivity, and cold pain sensitivity.40 Guo et al41 investigated the contribution of immobilization alone to nociceptive behavior, neuropeptide signals, and changes in inflammatory level after fracture in rats, and observed that cast immobilization alone showed similar effect to cast immobilization after fracture, including i) hindlimb CRPS-like symptoms, ii) the levels of substance P(SP) and calcitonin gene-related peptide in sciatic nerve and SP NK1 receptor in skin of rats were increased, iii) keratinocyte proliferation and inflammatory mediator expression on the skin of the hind paw, iv) nerve growth factor are up-regulated and c-Fos was activated in the spinal cord. Rats fixed with intramedullary pinning in tibial fracture had nociceptive changes at 1-week post-fracture, and these changes are alleviated 4 weeks after the fracture, however, there was no exaggerated neuropeptide signal and the expression of inflammatory mediator. Shi et al42 found that the reversals of pain behavior, up-regulated neuropeptide signaling, and inflammatory changes after four weeks of exercise in the tibial fractured cast mice.These findings give evidence that immobilization contributes to the development of CRPSI, and exercise helps to suppress or alleviate CRPSI-like symptoms. The CRPSI-like symptoms of rats immobilized with the cast for four weeks become relieved within two weeks. Interestingly, Ohmichi et al43 anesthetized healthy rats and fixed them with a cast for two weeks to generate a chronic post-cast pain (CPCP). After two weeks of not utilizing the hind limb, the CPCP rats developed local inflammation, thermal hyperalgesia, tactile allodynia, cold allodynia, and mechanical hyperalgesia was maintained for at least ten weeks.43–45 Unlike rats fixed with a cast alone for four weeks, the symptoms in the CPCP rats did not ease rapidly. The reasons for this difference, such as the time length of plaster fixation, the tightness of the fixation, and postoperative window decompression, still need further study.
The longer duration of symptoms in the tibial fracture/cast immobilization model makes it suitable for long-term experimental studies. However, the modeling method is relatively complex, with a considerable extension of the modeling period. Also, it is vulnerable to the impact of the technology adopted by the researcher. During the modeling, the shedding of the cast and the occurrence of amputated limb decreased the success rate of the model.46
The Passive Transfer-Trauma Model
The IgG-Transfer-Trauma Model
With the clinical observation that intravenous immunoglobulin can relieve pain in patients with CRPS, there is more and more evidence that CRPS may be an autoimmune disease.47
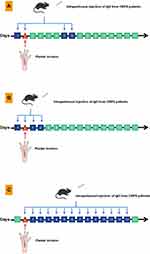
The diagnostic criteria for autoimmune diseases include the reproduction of clinical features in recipient animals through the passive transfer of pathogenic antibodies.48 Myasthenia gravis and pemphigus are typical autoimmune diseases. Studies have proven that the injection of serum IgG from patients with myasthenia gravis and pemphigus into rodents can replicate clinical features and the pathological changes similar to those of patients.49,50 Goebel et al51 injected IgG from CRPS patients into the abdominal cavity of mice for the first time, resulting in abnormal exploratory behavior and motor function in mice. However, no typical CRPSI manifestations such as pain sensitivity and limb swelling were detected. Limb injury plays an important role in the pathogenesis of CRPSI. Most CRPSI patients have a clear history of tissue injury. Some of the known triggers are: fractures, surgery, sprains, contusions, and crush injuries.8 Goebel et al47 proposed that CRPS is a new autoimmune prototype: an injury-triggered, regionally-restricted autoantibody-mediated autoimmune disorder with a minimally-destructive course(IRAM). Autoantibodies in the body may become pathogenic in the context of local trauma. Through the combination of the passive transfer of serum IgG from CRPS patients with the incisional pain model, Tékus et al established a CRPS-IgG-transfer-trauma model.52,53 The serum IgG of patients with chronic CRPS (average course of disease 5.3 years) was isolated, purified, and injected into the abdominal cavity of mice at a discontinuous time point. On the first injection, a cut involving skin fascia and the muscle was made on the hind paw of mice to cause trauma (Figure 1A).53 Finally, the model replicated the critical clinical features of CRPS, limb swelling, and mechanical hyperalgesia, and the concentration of P substance in the experimental group increased significantly. However, the duration of the symptoms in this model is short, and the degree is so mild that its practicability is limited, which may be due to the rapid metabolic consumption of IgG in rats. Helyes et al54 improved the CRPS-IgG-transfer-trauma model and established an enhanced passive IgG transfer trauma model by increasing the frequency of serum IgG injections which involves the replacement of the intermittent injections every few days with continuous daily intraperitoneal injections to compensate for the rapid metabolism of human IgG in rats (Figure 1C). The enhanced passive IgG transfer trauma model showed more intense and stable mechanical hyperalgesia, but the upper transfer limit of this model was 13 days due to the adverse effects of serum disease after the infusion of xenogeneic serum.54 Different from Helyes and Tékus et al, Cuhadar et al55 established the passive transfer-trauma model by injecting the IgG from CRPS patients into the abdominal cavity of mice for four consecutive days and making a plantar incision on the day of the second injection (Figure 1B). Their findings showed that in addition to mechanical hyperalgesia, mice also developed cold allodynia and thermal allodynia, and the degree of hyperalgesia of the model was IgG dose-dependent and correlated with the pain level of the donor CRPS patients.
The Tibia Fracture Passive Transfer Model
The Tibial fracture/cast immobilization model is one of the most commonly used CRPSI animal models. Using this model, Li et al observed that B cells and IgM are indispensable in the full expression of CRPS-like symptoms in the mouse tibial fracture/cast immobilization model, which suggests the important role of autoimmunity.56 Subsequently, the tibial fracture/cast immobilization model was further extended to the tibia fracture passive transfer model. The tibia fracture passive transfer model has two modes: the mouse-mouse and the human-mouse mode.21,57 In the mouse-mouse transfer mode, both wild-type mice and muMT mice lacking B cells and immunoglobulin were subjected to tibial fractures and 3-week cast immobilization, and then the serum or IgM obtained from wild-type mice was injected into 3-week post fracture muMT mice.21 In the human-mouse transfer mode, muMT mice lacking B cells and immunoglobulins were treated with tibial fractures and cast immobilization for 3 weeks, and then serum or IgM from patients with early CRPS (1–12 month post injury) was injected into the muMT mice.57 In both transfer modes, muMT mice showed hyperalgesia and unweighting for about 2 weeks.21,57 Unlike the IgG-transfer-trauma model, the immunoglobulin subtype responsible for pronociception in the tibia fracture passive transfer model is IgM instead of IgG. The reason for the difference may be related to the pathophysiological process of CRPSI, the injury model used, the IgG dose of or the injection method.58 The emergence of the CRPSI tibia fracture passive transfer model provides direct evidence for autoimmune support for nociceptive sensitization and contributes to the in-depth exploration and discussion of the following CRPSI related issues, including the regulatory effect of neuropeptides on adaptive immunity after limb injury and immobilization, the time course of adaptive immunity after trauma, and the autoimmune signaling pathway that mediates nociceptive sensitization.21,58–61
The establishment of the passive transfer-trauma model is of great significance in the exploration of the role of autoimmunity in the pathophysiological process of CRPSI. In the tibial fracture/cast immobilization model, the symptoms of rodents are more consistent with the warm CRPS subtype. The fracture rats immobilized with a cast for 4 weeks were relieved after 5 months, similar to most CRPSI patients whose symptoms disappeared within one year.14 Results from the tibia fracture passive transfer model may suggest that IgM-mediated autoimmunity is responsible for the early stage of CRPSI. The successful establishment of the IgG-transfer-trauma model caused by transferring serum IgG from chronic CRPS patients into mice may indicate that IgG-mediated autoimmunity is responsible for chronic and persistent CRPSI. Some scholars have hypothesized that chronic CRPS patients may have ongoing symptoms due to their inability to recover immune tolerance with time.21 However, the IgG or IgM in these models often comes from different patients, making it difficult to standardize the experiment. The rapid metabolic consumption of human serum IgG and IgM in rodents and the influence of serum diseases limit the application of this model in long-term studies.
The Needlestick-Nerve-Injury (NNI) Model
Some scholars hypothesized that similar to CRPSII, CRPSI, is also caused by nerve injury. In particular, unlike the primary peripheral nerve injury of CRPSII, the CRPSI nerve injury occurs in small nerve fibers mainly composed of myelinated Aδ fibers and unmyelinated C fibers.62 Histological analysis of the skin and skeletal muscle tissues of the affected limbs in patients with CRPSI showed that the axon density of small nerve fibers decreased significantly.63,64 Although the pathological results of the affected limbs in the patients with CRPS are encouraging, these studies are retrospective and cannot determine causality. In order to prospectively prove that small nerve fiber injury can induce similar CRPSI symptoms. Siegel et al65 proposed the needlestick-nerve-injury model by slightly damaging the distal nerve to reduce the density of the small nerve fibers. After fully exposing the sciatic trifurcation of the rat under anesthesia, the needle bevel of the syringe passed vertically through the tibial nerve axis. On the 7th day after the operation, intradomain (tibial nerve innervation) and extraterritorial (sural nerve innervation) mechanical hyperalgesia were common in the bilateral hind paws, and the prevalence of hyperalgesia was not related to the diameter of the needle. In addition, abnormal tonic posture and the edema of the hind paw of rats with nerve injury was found. On the 14th day after operation, mechanical hyperalgesia and abnormal tonic posture of the hind limbs were relieved in some NNI rats. Similar to the pathological changes of the endoneurium in CRPSI patients, Waller axonal degeneration, endoneurium edema, microvascular and inflammatory changes occurred in the distal tibia of the NNI rats.63,64,66
Conclusions
This paper summarizes the factors influencing CRPSI animal models and the current progress in the development of CRPSI animal models. Although CRPS has long been proposed, the understanding and treatment of this debilitating disease remains inadequate. Presently, there are four CRPSI animal models namely: the CPIP model, the tibial fracture/cast immobilization model, the passive transfer-trauma model, and the NNI model. Not only do these animal models promote the exploration of the pathogenesis and effective treatment strategies but also provide a valuable tool for prospective verification of new scientific hypotheses. The selection of suitable animal models for research purposes and programs is crucial for the smooth development of experimental research. In the design and implementation of experimental programs and the interpretation of experimental results, researchers should consider the influencing factors in animal models. CRPSI animal models are jointly affected by genes and the environment. Significant differences were observed in the anatomical structure and the tolerance to different noxious stimuli among different experimental animals. Taking gender as a variable in the CRPSI studies, there were significant differences in some results between the two sexes. The potential pathogenesis of CRPSI between the two sexes is not consistent. To guarantee the comprehensiveness and the reliability of the conclusions from the research, CRPSI experimental studies should include female experimental animals. Feeding animals during the experiment requires more refinement. Fasting or not, food composition and social environment in the cage can result in fluctuations in the indicators. Each model has its advantages and disadvantages. Furthermore, the continuous improvement of the modeling method enhances the success rate and the stability of the model. Among the four main CRPSI animal models, CPIP and tibial fracture/ cast immobilization models are currently widely used. It should be mentioned that CRPSI animal models cannot simulate all the clinical characteristics of CRPSI patients. Similar to the spontaneous remission of most CPRSI patients within one year after injury, symptoms in CRPSI animal models do not persist, and the duration of the symptoms in each model varies. Therefore, establishing an animal model that is consistent with the pathological process of persistent CRPSI needs further exploration to meet the increasingly urgent scientific research needs of the pathogenesis, diagnosis, and treatment of the disease.
Acknowledgments
This work was supported by the Key Research and Development Program of Shanxi Province, China (201803D31138) and Special Fund Project for Guiding Local Scientific and Technological Development by The Central Government (YDZX20191400002563).
Disclosure
The authors reported no conflicts of interest for this work.
References
1. Lloyd ECO, Dempsey B, Romero L. Complex Regional Pain Syndrome. Am Fam Phys. 2021;104(1):49–55.
2. Ott S, Maihofner C. Signs and symptoms in 1043 patients with complex regional pain syndrome. J Pain. 2018;19(6):599–611.
3. Cutts S, Gangoo S, Srinivasan SH, Modi N, Pasapula C, Power D. Complex regional pain syndrome: an evolving perspective. Postgrad Med J. 2021;97(1146):250–255.
4. Kessler A, Yoo M, Calisoff R. Complex regional pain syndrome: an updated comprehensive review. Neuro Rehabilitation. 2020;47(3):253–264.
5. Misidou C, Papagoras C. Complex regional pain syndrome: an update. Mediterr J Rheumatol. 2019;30(1):16–25.
6. Bean DJ, Johnson MH, Kydd RR. The outcome of complex regional pain syndrome type 1: a systematic review. J Pain. 2014;15(7):677–690.
7. de Mos M, Huygen FJ, van der Hoeven-borgman M, Dieleman JP, Ch Stricker BH, Sturkenboom MC. Outcome of the complex regional pain syndrome. Clin J Pain. 2009;25(7):590–597.
8. Bruehl S. Complex regional pain syndrome. BMJ. 2015;351:h2730.
9. Antunovich D, Tuck N, Reynolds LM, Bean D. I don’t identify with it”: a qualitative analysis of people’s experiences of living with Complex Regional Pain Syndrome. Pain Med. 2021. doi:10.1093/pm/pnab094
10. Taylor SS, Noor N, Urits I, et al. Complex regional pain syndrome: a comprehensive review. Pain Ther. 2021;10:1–8.
11. Challa SR. Surgical animal models of neuropathic pain: pros and cons. Int J Neurosci. 2015;125(3):170–174.
12. Harden RN, Bruehl S, Stanton-Hicks M, Wilson PR. Proposed new diagnostic criteria for complex regional pain syndrome. Pain Med. 2007;8(4):326–331.
13. Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89(2):707–758.
14. Guo TZ, Offley SC, Boyd EA, Jacobs CR, Kingery WS. Substance P signaling contributes to the vascular and nociceptive abnormalities observed in a tibial fracture rat model of complex regional pain syndrome type I. Pain. 2004;108(1–2):95–107.
15. Guo T, Wei T, Shi X, et al. Neuropeptide deficient mice have attenuated nociceptive, vascular, and inflammatory changes in a tibia fracture model of complex regional pain syndrome. Mol Pain. 2012;8:85.
16. Mogil JS. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13(12):859–866.
17. Tsang A, Von Korff M, Lee S, et al. Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. J Pain. 2008;9(10):883–891.
18. Meyer-Friessem CH, Attal N, Baron R, et al. Pain thresholds and intensities of CRPS type I and neuropathic pain in respect to sex. Eur J Pain. 2020;24(6):1058–1071.
19. Tang C, Li J, Tai WL, et al. Sex differences in complex regional pain syndrome type I (CRPS-I) in mice. J Pain Res. 2017;10:1811–1819.
20. Tajerian M, Sahbaie P, Sun Y, et al. Sex differences in a Murine model of complex regional pain syndrome. Neurobiol Learn Mem. 2015;123:100–109.
21. Guo TZ, Shi X, Li WW, Wei T, Clark JD, Kingery WS. Passive transfer autoimmunity in a mouse model of complex regional pain syndrome. Pain. 2017;158(12):2410–2421.
22. Guo TZ, Shi X, Li WW, Wei T, Clark JD, Kingery WS. Sex differences in the temporal development of pronociceptive immune responses in the tibia fracture mouse model. Pain. 2019;160(9):2013–2027.
23. Elma O, Lebuf E, Marnef AQ, et al. Diet can exert both analgesic and pronociceptive effects in acute and chronic pain models: a systematic review of preclinical studies. Nutr Neurosci. 2021;1–23. doi:10.1080/1028415X.2021.1934956
24. Ross-Huot MC, Laferriere A, Gi CM, Khorashadi M, Schricker T, Coderre TJ. Effects of glycemic regulation on chronic postischemia pain. Anesthesiology. 2011;115(3):614–625.
25. Cucinello-Ragland JA, Mitchell-Cleveland R, Bradley Trimble W, et al. Alcohol amplifies cingulate cortex signaling and facilitates immobilization-induced hyperalgesia in female rats. Neurosci Lett. 2021;761:136119.
26. Tran H, Sagi V, Jarrett S, Palzer EF, Badgaiyan RD, Gupta K. Diet and companionship modulate pain via a serotonergic mechanism. Sci Rep. 2021;11(1):2330.
27. Coderre TJ, Bennett GJ. A hypothesis for the cause of complex regional pain syndrome-type I (reflex sympathetic dystrophy): pain due to deep-tissue microvascular pathology. Pain Med. 2010;11(8):1224–1238.
28. Coderre TJ, Xanthos DN, Francis L, Bennett GJ. Chronic post-ischemia pain (CPIP): a novel animal model of complex regional pain syndrome-type I (CRPS-I; reflex sympathetic dystrophy) produced by prolonged hindpaw ischemia and reperfusion in the rat. Pain. 2004;112(1–2):94–105.
29. Hu Q, Zheng X, Chen R, et al. Chronic post-ischemia pain model for complex regional pain syndrome Type-I in rats. J Vis Exp. 2020;155:e60562.
30. Millecamps M, Laferriere A, Ragavendran VJ, Stone LS, Coderre TJ. Role of peripheral endothelin receptors in an animal model of complex regional pain syndrome type 1 (CRPS-I). Pain. 2010;151(1):174–183.
31. Xu X, Tao X, Huang P, et al. N-methyl-d-aspartate receptor subunit 2B on keratinocyte mediates peripheral and central sensitization in chronic post-ischemic pain in male rats. Brain Behav Immun. 2020;87:579–590.
32. Nahm F, Nahm S, Han W, Gil H, Choi E, Lee P. Increased cerebral nuclear factor kappa B in a complex regional pain syndrome rat model: possible relationship between peripheral injury and the brain. J Pain Res. 2019;12:909–914.
33. Yeo J, Park S. Effect of dexmedetomidine on the development of mechanical allodynia and central sensitization in chronic post-ischemia pain rats. J Pain Res. 2018;11:3025–3030.
34. Kim Y, Kim I, Yoon M. Antiallodynic effect through spinal endothelin-B receptor antagonism in rat models of complex regional pain syndrome. Neurosci Lett. 2015;584:45–49.
35. Seo YH, Gil YW, Choe G, Lee SH, Yoo SH, Park HJ. Comparative study of chronic postischemic pain models in mice: o-ring versus tie method. Pain Phys. 2020;23(1):E51–E60.
36. Lo Vecchio S, Andersen HH, Elberling J, Arendt-Nielsen L. Sensory defunctionalization induced by 8% topical capsaicin treatment in a model of ultraviolet-B-induced cutaneous hyperalgesia. Exp Brain Res. 2021;239(9):2873.
37. Dutton LK, Rhee PC. Complex regional pain syndrome and distal radius fracture: etiology, diagnosis, and treatment. Hand Clin. 2021;37(2):315–322.
38. Sarangi PP, Ward AJ, Smith EJ, Staddon GE, Atkins RM. Algodystrophy and osteoporosis after tibial fractures. J Bone Joint Surg Br. 1993;75(3):450–452.
39. Birklein F, Ibrahim A, Schlereth T, Kingery WS. The rodent tibia fracture model: a critical review and comparison with the complex regional pain syndrome literature. J Pain. 2018;19(10):1102e1101–1102 e1119.
40. Terkelsen AJ, Bach FW, Jensen TS. Experimental forearm immobilization in humans induces cold and mechanical hyperalgesia. Anesthesiology. 2008;109(2):297–307.
41. Guo TZ, Wei T, Li WW, Li XQ, Clark JD, Kingery WS. Immobilization contributes to exaggerated neuropeptide signaling, inflammatory changes, and nociceptive sensitization after fracture in rats. J Pain. 2014;15(10):1033–1045.
42. Shi X, Guo TZ, Li W, et al. Exercise reverses nociceptive sensitization, upregulated neuropeptide signaling, inflammatory changes, anxiety, and memory impairment in a mouse tibia fracture model. Anesthesiology. 2018;129(3):557–575.
43. Ohmichi Y, Sato J, Ohmichi M, et al. Two-week cast immobilization induced chronic widespread hyperalgesia in rats. Eur J Pain. 2012;16(3):338–348.
44. Ohmichi M, Ohmichi Y, Ohishi H, et al. Activated spinal astrocytes are involved in the maintenance of chronic widespread mechanical hyperalgesia after cast immobilization. Mol Pain. 2014;10:6.
45. Ohmichi Y, Ohmichi M, Tashima R, et al. Physical disuse contributes to widespread chronic mechanical hyperalgesia, tactile allodynia, and cold allodynia through neurogenic inflammation and spino-parabrachio-amygdaloid pathway activation. Pain. 2020;161(8):1808–1823.
46. Wu R, Li RH. [Comparison of two methods for establishing rat models of complex regional pain syndrome type 1]. Nan Fang Yi Ke Da Xue Xue Bao. 2011;31(12):1985–1988. Chinese.
47. Goebel A, Blaes F. Complex regional pain syndrome, prototype of a novel kind of autoimmune disease. Autoimmun Rev. 2013;12(6):682–686.
48. Sommer C. Anti-autonomic nervous system antibodies in CRPS. Pain. 2011;152(12):2675–2676.
49. Anhalt G, Labib R, Voorhees J, Beals T, Diaz L. Induction of pemphigus in neonatal mice by passive transfer of IgG from patients with the disease. N Engl J Med. 1982;306(20):1189–1196.
50. Toyka K, Brachman D, Pestronk A, Kao I. Myasthenia gravis: passive transfer from man to mouse. Science (New York, N.Y.). 1975;190(4212):397–399.
51. Goebel A, Leite MI, Yang L, et al. The passive transfer of immunoglobulin G serum antibodies from patients with longstanding Complex Regional Pain Syndrome. Eur J Pain. 2011;15(5):504e501–506.
52. Tékus V, Hajna Z, Borbély É, et al. A CRPS-IgG-transfer-trauma model reproducing inflammatory and positive sensory signs associated with complex regional pain syndrome. Pain. 2014;155(2):299–308.
53. Brennan TJ, Vandermeulen EP, Gebhart GF. Characterization of a rat model of incisional pain. Pain. 1996;64(3):493–502.
54. Helyes Z, Tékus V, Szentes N, et al. Transfer of complex regional pain syndrome to mice via human autoantibodies is mediated by interleukin-1-induced mechanisms. Proc Natl Acad Sci U S A. 2019;116(26):13067–13076.
55. Cuhadar U, Gentry C, Vastani N, et al. Autoantibodies produce pain in complex regional pain syndrome by sensitizing nociceptors. Pain. 2019;160(12):2855–2865.
56. Li WW, Guo TZ, Shi X, et al. Autoimmunity contributes to nociceptive sensitization in a mouse model of complex regional pain syndrome. Pain. 2014;155(11):2377–2389.
57. Guo TZ, Wei T, Tajerian M, et al. Complex regional pain syndrome patient immunoglobulin M has pronociceptive effects in the skin and spinal cord of tibia fracture mice. Pain. 2020;161(4):797–809.
58. Shi X, Guo TZ, Li WW, et al. C5a complement and cytokine signaling mediate the pronociceptive effects of complex regional pain syndrome patient IgM in fracture mice. Pain. 2021;162(5):1400–1415.
59. Li WW, Guo TZ, Shi X, et al. Neuropeptide regulation of adaptive immunity in the tibia fracture model of complex regional pain syndrome. J Neuroinflammation. 2018;15(1):105.
60. Li WW, Yang Y, Shi XY, et al. Germinal center formation, immunoglobulin production and hindlimb nociceptive sensitization after tibia fracture. Brain Behav Immun. 2020;88:725–734.
61. Li WW, Yang Y, Guo TZ, et al. IL-6 signaling mediates the germinal center response, IgM production and nociceptive sensitization in male mice after tibia fracture. Brain Behav Immun. 2021;94:148–158.
62. Oaklander AL, Fields HL. Is reflex sympathetic dystrophy/complex regional pain syndrome type I a small-fiber neuropathy? Ann Neurol. 2009;65(6):629–638.
63. Oaklander A, Rissmiller J, Gelman L, Zheng L, Chang Y, Gott R. Evidence of focal small-fiber axonal degeneration in complex regional pain syndrome-I (reflex sympathetic dystrophy). Pain. 2006;120(3):235–243.
64. van der Laan L, ter Laak H, Gabreëls-Festen A, Gabreëls F, Goris R. Complex regional pain syndrome type I (RSD): pathology of skeletal muscle and peripheral nerve. Neurology. 1998;51(1):20–25.
65. Siegel SM, Lee JW, Oaklander AL. Needlestick distal nerve injury in rats models symptoms of complex regional pain syndrome. Anesth Analg. 2007;105(6):1820–1829, table of contents.
66. Klein MM, Lee JW, Siegel SM, Downs HM, Oaklander AL. Endoneurial pathology of the needlestick-nerve-injury model of Complex Regional Pain Syndrome, including rats with and without pain behaviors. Eur J Pain. 2012;16(1):28–37.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.