Back to Journals » Clinical, Cosmetic and Investigational Dermatology » Volume 15
Androgen Receptor Inhibitors in the Treatment of Acne Vulgaris: Efficacy and Safety Profiles of Clascoterone 1% Cream
Received 1 January 2022
Accepted for publication 27 April 2022
Published 15 July 2022 Volume 2022:15 Pages 1357—1366
DOI https://doi.org/10.2147/CCID.S289750
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Jeffrey Weinberg
Carol Sanchez,1 Jonette Keri1,2
1University of Miami Department of Cutaneous Surgery and Dermatology, Miami, FL, USA; 2Miami Veterans Affairs Medical Center, Miami, FL, USA
Correspondence: Carol Sanchez, University of Miami Department of Cutaneous Surgery and Dermatology, 7815 NW 104 AVE, Doral, Miami, FL, 33178, USA, Email [email protected]
Abstract: The purpose of this narrative review is to provide a summary of the clinical trials on the efficacy and safety of clascoterone 1% cream (Winlevi) to grant providers an understanding of which patients will benefit most from this novel topical antiandrogen medication. Clascoterone 1% cream (Winlevi) offers a new and exciting treatment approach for a difficult and common skin condition such as acne vulgaris. This topical androgen antagonist is the first of its kind but will hopefully provoke investigations into other androgen receptor antagonists with similar or better efficacy.
Keywords: hormonal acne, anti-androgens, CB-03-01, Winlevi
A Letter to the Editor has been published for this article.
Introduction
Acne vulgaris is an inflammatory skin condition that typically affects teens and adolescents, although it can expand to affect other age groups.1 There are several pathophysiologic pathways in the emergence of acne vulgaris. These pathways involve follicular hyperkeratinization, overactive sebaceous glands, Cutibacterium acnes colonization, and inflammation. Therefore, the role of current acne treatments is to target these specific pathogenic factors.
Anti-androgen medications target overactive sebaceous glands, leading to a reduction in sebum secretion rate.2 Physicians have long been using hormonal agents with anti-androgen effects to treat acne vulgaris. The most commonly prescribed medications are combined oral contraceptives (COCs) and spironolactone. COCs were first introduced in the 1960s for contraceptive use. It was not until 1997 that the first COC, Ortho Tri-cyclen, was approved for acne vulgaris.3 Since then, there have been three more COCs approved by the FDA. These include Estrostep (approved in 2001), Yaz (approved in 2007), and Beyaz (approved in 2010).3 The estrogen in the FDA-approved medications is ethinyl estradiol. Progestins in the formulations for COCs approved for acne differ but include norgestimate, norethindrone acetate, and drospirenone.3 COCs alter acne lesions through disparate pathways. The anti-androgenic effect of COCs is mainly through estrogen’s testosterone synthesis inhibition mechanism.4 However, progestins in birth control also have a function in anti-androgenic effects through antagonistic mechanisms.4 First, COCs, primarily through estrogen, increase the levels of sex-hormone binding globulin, which leads to a decrease in free testosterone available for receptors. Simultaneously, luteinizing hormone inhibition leads to decreased production of testosterone.5 In addition, COCs inhibit the enzyme 5-alpha-reductase, which is responsible for the conversion of testosterone into dihydrotestosterone (DHT). COCs have also been proposed to block testosterone and DHT binding to their corresponding receptors, thus limiting their androgenetic effects on the skin.6
There has been a wide range of clinical trials to assess the efficacy of COCs for acne. Most of these trials showed evidence of reduced acne lesion count in patients on COCs. In trials in which acne lesion count was not reduced, researchers still established an effect on sebum production. COCs were associated with a significant reduction in sebum production in the face.4 Despite significant efficacy in treating acne, COCs carry a more extensive side effect profile than topical therapies. For instance, although infrequent, COCs confer a cardiovascular risk, especially in patients with higher baseline risk. This cardiovascular risk increases with the length of contraceptive use.4 Another uncommon risk to acknowledge when counseling patients for treatment is a cancer risk, specifically breast cancer, which remains controversial.4 More commonly, patients report headaches, menstrual irregularities, and mood lability after initiation of COCs.7
Apart from COCs, spironolactone is a different therapeutic agent used for its anti-androgenic properties. However, it has not received United States Food and Drug Administration (FDA) approval for acne and thus is used off-label. According to the American Academy of Dermatology (AAD), it is considered an alternative treatment in all cases of acne vulgaris from mild to severe.8 Dating back to the 1980s, prospective studies on oral spironolactone have shown statistically significant efficacy against acne vulgaris in women.8–11 Although studies have shown efficacy in spironolactone for acne, they have been small in sample size. A recent retrospective study of 110 patients concluded that spironolactone therapy provided patients with improvement in their acne.11 Similar to COCs, the systemic exposure of oral anti-androgens poses risks for potential side effects. Common side effects are menstrual irregularities, dizziness, breast enlargement, and nausea. Less common side effects associated with spironolactone include hyperkalemia and estrogen-sensitive cancers. However, cancer development has not been well-established in humans and instead has been shown in animal studies.8 Many of the risks conferred by hormonal agents arise from their systemic exposure. In addition, male patients do not tolerate associated gynecomastia when taking spironolactone for acne.12
On the contrary, systemic exposure can be limited through the use of topical applications. They reduce the risk for potential side effects, although they have sometimes been considered as less efficacious in more severe cases of acne vulgaris.13
Topical spironolactone has been postulated for use in acne vulgaris. In one small single-blinded study, topical spironolactone 2% solution was shown to significantly reduce comedone count compared to no effect on comedones by clindamycin.14 Additionally, compared to clindamycin, the topical application of spironolactone resulted in a more significant reduction of patients’ Acne Severity Index (ASI). Similar results have been achieved in other clinical trials.15,16
Another way to target the increased levels of DHT in acne pathogenesis is through inhibition of 5-alpha-reductase, the enzyme responsible for peripherally converting testosterone to DHT.16 Finasteride is a 5-alpha-reductase inhibitor that is mainly used in benign prostatic hyperplasia (BPH) and male pattern hair loss. However, there have been studies examining its use for acne vulgaris. For example, in one clinical trial done on hyperandrogenic women with moderate-severe acne, finasteride was beneficial for decreasing Cook Score (an acne severity index that uses photographs to document acne severity and is scored from 0–8), although less beneficial than other anti-androgens such as flutamide.17 In a more recent prospective study, the efficacy of finasteride was compared to montelukast for moderate acne in women. The study results showed that finasteride is more efficacious in treating moderate acne than montelukast. Specifically, at the end of the study period (12 weeks), 88% of patients on finasteride had near-total clarity of their acne compared to 76% of patients in the montelukast group. For patient satisfaction, the finasteride group reported 80% satisfaction at the end of the 12 weeks, whereas the montelukast group had a 66.7% satisfaction rate.16
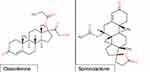
A novel topical antiandrogens for use in acne vulgaris is Cortexolone 17α-propionate (Clascoterone or CB-03-01). Clascoterone 1% cream (Winlevi) is a steroidal topical androgen receptor inhibitor hypothesized to work through competitive inhibition of DHT receptors within the sebaceous glands and hair follicles.7,15 The structure of clascoterone resembles other hormonal agents used for acne vulgaris (Figure 1). The primary metabolite of clascoterone is cortexolone or 11-deoxycortisol, an inactive form.18 In regards to excretion, clascoterone is hypothesized to be hydrolyzed in the skin’s epidermis upon topical application. During in vitro studies, clascoterone 1% cream (Winlevi) was significantly more efficacious in inhibiting inflammatory cytokine production from sebocytes than spironolactone.1 Clascoterone 1% cream (Winlevi) was approved for male and female patients, 12 years and older, by the United States FDA on August 27, 2020.7,15 This novel treatment provides physicians with more options when treating such common skin conditions such as acne. It gives practitioners an anti-androgen treatment, which can be used in males without systemic side effects. Notably, there are no human studies on clascoterone 1% cream (Winlevi) use during pregnancy and breastfeeding. However, animal studies conducted on rats showed organ malformations when subcutaneous doses at 8 to 39 times the recommended dose for humans were used.19
With more options in acne treatment, it is imperative that prescribing physicians understand the populations and outcomes in which this drug was studied. Therefore, the objective of this narrative review is to provide a summary of the clinical trials on the efficacy and safety of clascoterone 1% cream (Winlevi) to grant providers an understanding of which patients will benefit most from this novel topical anti-androgen medication.
Experimental Studies
In the United States (US), the first clinical trial on clascoterone 1% cream (Winlevi) started in June 2012.20 Since then, there have been a total of 6 clinical trials completed on the use of clascoterone (Winlevi) for acne vulgaris.20 As described above, orally administered androgen receptor inhibitors have shown efficacy in treating the effects that testosterone and DHT have on the skin and skin appendages but they have potential systemic consequences. On the contrary, topical androgen antagonists have the potential to exhibit activity locally with reduced risks of systemic exposure. Before human studies, Celasco et al examined the systemic effects of cortexolone 17a-propionate in rats and found that while there were strong local antagonistic effects, there was no evidence of systemic antagonistic effects. For 21 days, the researchers applied the experimental drug to the right flank with a microsyringe at concentrations of 100–200- 400 ug/animal dissolved in 0.05 mL of acetone containing 4 ug of testosterone propionate (TP).21 There was a dose-dependent flank organ inhibition, with increased doses resulting in greater inhibition and the highest inhibition of 84% was achieved with the 400 ug dose. Moreover, the researchers did not find evidence of irritation or damaging effects to the skin, thus concluding that it is well-tolerated when topically applied. To assess the systemic effects, the researchers used a separate group of rats, which were injected subcutaneously for seven days at a daily dose of 0.2–1.5 mg/animal in 0.2 mL of a suspending vehicle. After the seven days with injections, the animals were euthanized and the ventral prostate, seminal vesicles, and levator ani muscles were isolated in order to assess the effects of the experimental drug on them. The animals were simultaneously injected with TP on the contralateral side. In conclusion, cortexolone 17a-propionate was unable to completely antagonize the effects of the TP.21 The drug was also devoid of toxic effects on the rats evident by the lack of spontaneous deaths or clinical symptoms during their study. The researchers hypothesize that the absence of systemic effects is, in part, due to a fast hydrolysis by tissues into the inactive parent compound cortexolone.21
Clinical Trials
The findings from a pilot study in 2011 reinforced the use of clascoterone 1% cream for acne. A total of 77 men with facial acne were enrolled in this randomized, double-blind study. In summary, clascoterone 1% cream (Winlevi) was significantly better than placebo in reducing inflammatory lesion count (ILC), acne severity index (ASI), and total lesion count (TLC). Compared to tretinoin 0.05% cream, clascoterone 1% cream (Winlevi) was clinically more effective, but not statistically significant, in reducing TLC and ASI.22 Contrastingly, CB-03-01 was significantly better in reducing inflammatory lesion count compared to tretinoin cream at week 6 (p= 0.0374) The application involved applying the cream nightly for 8 weeks. Additionally, clascoterone 1% cream (Winlevi) also demonstrated a quicker time to the beneficial effects than placebo and tretinoin. Treatment with clascoterone 1% cream (Winlevi) resulted in a 50% improvement of TLC in a median of 43.5 days, whereas the median time for 50% improvement of TLC for tretinoin and placebo were 57 and 58 days, respectively (p= 0.0199). For ILC, the time to 50% improvement was a median of 36.5, 44, and 58 days for clascoterone 1% cream (Winlevi), tretinoin, and placebo, respectively (p= 0.0490). Compared to tretinoin, clascoterone 1% cream (Winlevi) exhibited better tolerability as evidenced by the irritancy score. Patients treated with tretinoin and placebo experienced worsening in their irritancy score in the first 2 weeks of treatment compared to clascoterone 1% cream (Winlevi) (p= 0.0412). Although this study included a small sample of patients, it reinforced findings from previous studies on animals and fortified the need for larger-scale clinical trials.22
To further assess safety in human subjects, a phase two trial was conducted in which researchers investigated the pharmacokinetic properties and hypothalamic-pituitary-adrenal (HPA) axis response to clascoterone 1% cream. The study participants consisted of a cohort of 42 subjects who were 12 years of age and older and were diagnosed with moderate-severe acne according to the Investigator’s Global Assessments [IGA] (grade 3–4). The participants were divided into two cohorts. Cohort 1 was those subjects who were older than 18 years of age whereas Cohort 2 consisted of participants aged 12–18. The medication was applied twice daily for 14 days after which a Cosyntropin Stimulation Test was completed to assess HPA axis suppression and compared to the response on day 1 of the trial. Post-stimulation cortisol levels less than 18 ug/dL were considered to be suppressed levels. Simultaneously, the pharmacokinetics of the medication was assessed through concentration-time profiles of clascoterone and cortexolone (the clascoterone metabolite) in plasma. On day 14, 42.7% of the 42 subjects showed an abnormal HPA axis suppression evidenced by post-stimulation cortisol levels between 14.9 ug/dL and 17.7 ug/dL. However, the cortisol levels returned to normal four weeks after the Cosyntrophin Stimulation Test. Additionally, although cortisol levels were in an abnormal range, there were no clinical signs or symptoms of adrenal suppression in these participants.23 In regards to the pharmacokinetic properties of clascoterone 1% cream (Winlevi), steady-state concentrations in plasma were reached by the fifth day with the mean maximum concentration of 4.4 ug/dL and 4.7 ug/dL in the two cohorts, respectively. The levels of cortexolone stayed below levels of quantitation throughout the study period. Secondary endpoints of the study included local and systemic reactions, clinical laboratory testing, physical exam, and vital signs. There were local skin reactions noted of mild intensity with one case of moderate pruritus. In total, there were nine adverse reactions with two of them being classified as definitely related to the topical medication and four as probably related.23
In a similar study, varying doses of the topical medication were studied for its efficacy and safety. Researchers studied clascoterone cream 0.1%, 0.5%, and 1% twice daily, as well as 1% daily and vehicle (daily and twice daily). Of note, the vehicle cream was formulated to contain and feel the same as the experimental drug but without the active ingredient cortelexone-17a-propionate. Both topical medications (experimental and vehicle) contained cetyl alcohol, citric acid monohydrate, edetate disodium, monoglycerides, mineral oil, polysorbate 80, propylene glycol, purified water, and vitamin E. Efficacy was determined using IGA, total acne lesion counts, and subject satisfaction with treatment. Safety was assessed through local and systemic adverse events, physical exam, vital signs, lab tests, local skin reactions, and electrocardiograms. Subjects were considered to have a successful treatment if their IGA score achieved a 0 or 1, which corresponds to clear or mostly clear, and/or if there was a two-grade improvement in the IGA score from baseline. At the end of 12 weeks, treatment success was highest for the groups who received 1% and 0.1% twice daily. The group receiving the 1% dose achieved the greatest median change from baseline in inflammatory and non-inflammatory lesions with a change in −13.5 and −17.5, respectively. In regards to safety, adverse events were mostly classified as mild and did not differ among different treatment groups or vehicle. For example, there was more than one adverse event in 18.6% of participants in the 1% twice-daily treatment group, while 22.7% participants in the vehicle group and 25% in the 0.1% twice-daily groups experienced at least 1 adverse event. The most common adverse event was a local skin reaction consisting of mild erythema, specifically 32.6% of subjects experienced this local skin reaction throughout the study period. Although these findings show that all tested doses of the clascoterone cream were well-tolerated, they conclude that the most favorable dose of clascoterone cream would be 1%.24
The findings from phase three trials of clascoterone 1% cream (Winlevi) continued to parallel previous findings. In two phase-three trials of patients 9 years and older, patients treated with clascoterone 1% cream (Winlevi) displayed greater treatment success than patients treated with vehicle.25 Treatment success was defined as a 2-point reduction in IGA score in addition to a score of 0 or 1. Other primary endpoints were absolute change from baseline in total lesion counts (TLC), noninflammatory lesion counts (NILC), and inflammatory lesion counts (ILC). In the experimental cohort of one trial, 18.4% of patients achieved treatment success at week 12 compared to 9.0% in the vehicle cohort (p <0.001). In the second trial, 20.3% of patients in the experimental group achieved treatment success at week 12 versus 6.5 of patients receiving the vehicle (p <0.001). Additionally, there was a greater absolute change in the NILC and ILC at week 12 in the experimental cohort in both trials compared to vehicle. Patients in the first trial on experimental treatment achieved an absolute change of −19.4 and −19.3 in NILC and ILC, respectively. Whereas patients on vehicle achieved an absolute change of −13.0 and −15.5 in NILC and ILC, respectively. Patients on the experimental treatment saw a greater percent change in non-inflammatory lesion counts, inflammatory lesion counts, and total lesion counts, which were considered secondary endpoints.25 Similar to the study by Mazzeti et al, the most frequent local skin reaction was erythema. Mild nasopharyngitis, oropharyngeal pain, and headaches were the most common treatment adverse effects in both trials. In addition, both trials cited application site dryness as an adverse effect related to clascoterone 1% cream (Winlevi). Five patients receiving clascoterone 1% cream (Winlevi) and 12 patients receiving vehicle cream discontinued the treatment due to the treatment-emergent adverse effects. The adverse effects that resulted in discontinuation were mild application site hypersensitivity, oropharyngeal pain, sebaceous hyperplasia, facial acute contact dermatitis, and depigmentation of the hair on the nose. Finally, there were no cases of systemic adverse effects in either trial confirming that clascoterone 1% cream (Winlevi) has only local antiandrogenic activity.10 Although clinically significant, these studies did not use adjuvant acne therapies. Future studies should aim to include complementary acne therapies such as topical antibiotics or topical retinoids to determine the most optimal therapeutic approach.
The most recent clinical trial conducted on this novel medication was completed to assess for long-term effects of clascoterone 1% cream (Winlevi) applied twice daily. Thus far, the clinical trials focused on treatments for no longer than 12 weeks. In this trial, the medication was applied twice daily for up to 9 months. Subjects were evaluated for adverse events and local skin reactions at intervals of 1, 3, 6, and 9 months. About 18% of 347 subjects developed 191 treatment-emergent adverse events. Nasopharyngitis, which was the most frequently reported treatment-emergent adverse event, was reported in 20 out of 179 patients. This was similar to the adverse effect cited in the two trials by Herbert et al. The adverse events were similar among both treatment and vehicle groups, coinciding with the previous phase three clinical trials. However, a higher number of adverse events occurred in subjects who were originally assigned to the experimental group. Study discontinuation due to an adverse event was observed in 9 participants. Some of the adverse events experienced by these participants were moderate application site swelling, moderate dryness, and a severe suicide attempt. Although there were adverse events and local skin reactions such as erythema, the authors conclude that the safety profile of the medication could still be considered favorable. These are relatively low frequency of treatment-emergent adverse events and most were mild or moderate.26 Table 1 includes a summary of the data presented.
 |  |  |
Table 1 Summary of Experimental Studies and Clinical Trials |
Discussion
In female and male ≥ 9 year old patients with moderate-severe acne vulgaris, there is strong evidence favoring the clinical efficacy and safety of clascoterone 1% cream (Winlevi) twice daily. In summary, one pilot study, two Phase 2 trials, and three Phase 3 trials shared complementary findings for this new medication. Recently FDA approved, this new medication will slowly start being incorporated by prescribing physicians in the US. It’s currently not approved for use outside the US, and the European Medicines Agency has not listed it as a medication under investigation for human use.
Interestingly, clascoterone 1% cream (Winlevi) has been proposed to be effective in treating other skin conditions such as hidradenitis suppurativa (HS), androgenetic alopecia, and hirsutism. In an experimental study on dermal papilla cells in vitro, clascoterone was shown to decrease transcription of androgen-receptor-dependent genes. It was also shown to be more powerful at inhibiting interleukin-6 (IL-6) synthesis from DHT-stimulated cultures when compared to another androgen receptor antagonist, enzalutamide.24 IL-6 is a cytokine produced in the body in response to infections, tissue injury, and inflammation. It has been associated with the chronic inflammation and autoimmunity pathogenesis behind rheumatoid arthritis and juvenile idiopathic arthritis.27 Der Sakkistian et al explain the rationale for using clascoterone 1% cream in HS is due to its ability to inhibit DHT, which is the androgen most likely responsible for the clinical signs of HS. In one case series, 85% of women on spironolactone demonstrated a clinical response of their HS. Similar or better findings could be achieved with clascoterone due to the fact that this novel medication targets more of the initial pathogenesis of HS such as follicular plugging.28 A similar rationale has been proposed for its use in androgenic alopecia. That is the inhibition of DHT, which is known to play an active role in the development of androgenic alopecia. Moreover, during in vitro studies, clascoterone was found to have similar efficacy to finasteride, which is often used in the treatment of androgenic alopecia. The benefit of clascoterone 1% cream over finasteride and similar medications relates to its local reaction and favorable side effect profile, especially for males.29 Treatment of acne vulgaris commonly requires regimens involving two or more medications. For instance, when treating mild-moderate acne, topical antibiotics are often used concomitantly with benzoyl peroxide or topical retinoids to prevent antibiotic resistance.12 Due to its recent approval for acne, there is a paucity of data on the efficacy and safety of clascoterone 1% cream (Winlevi)when combined with other acne medications. Future studies should focus on addressing the clinical effects that concomitant use of other acne medications has on the efficacy of clascoterone 1% cream (Winlevi). In the AAD 2016 guideline for acne, it was established that there is a considerable amount of gap in knowledge in regards to acne treatment in skin of color.8 The phase 3 clinical trials on clascoterone 1% cream (Winlevi) were composed of mostly White patients (84.4% in one trial and 96.6% in the second trial) with post-inflammatory hyperpigmentation (PIH) rarely being reported. However, for patients with skin of color, PIH is a more common concern when treating acne. In fact, the development of PIH has been associated with irritation from acne treatment. Future studies should investigate how effective this medication may be for people belonging to racial/ethnic groups such as African-Americans, Hispanics, Native Americans, etc. In one survey study, 42% of nonwhite patients cited resolution of PIH as one of the most important goals when undergoing acne therapy.30 Additionally, although two clinical trials focused on patients 9 years old and older and showed beneficial effects, the FDA has approved this medication for patients ≥ 12 years-old. It is possible that this is due to the small number of patients in the cohort of 9–11 year olds. Specifically, there were only 19 patients in the 9–11 year old cohorts.25
There are several limitations to the studies included in this review. First, the sample sizes did not allow for further subanalysis on different populations. It is possible that the results for different groups may differ.22–26 Secondly, none of the clinical trials assessed concomitant acne therapies with clascoterone. There is a possibility that the most optimal results on acne severity are achieved through a compounded action with different medications.22–26
Conclusion
In conclusion, clascoterone 1% cream (Winlevi) offers a new and exciting treatment approach for a difficult and common skin condition such as acne vulgaris. Not only do clinical trials show efficacy for reduction in total acne lesion counts, it has a significantly tolerable safety profile even at nine months of treatment. At this time, this topical androgen antagonist is the first of its kind but will hopefully provoke investigations into other androgen receptor antagonists with similar or better efficacy.
Disclosure
Dr Jonette Keri reports being a consultant for Almirall, being on the advisory board for Ortho dermatologics, research study from Galderma, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Rosette C, Agan FJ, Mazzetti A, Moro L, Gerloni M. Cortexolone 17α-propionate (clascoterone) is a novel androgen receptor antagonist that inhibits production of lipids and inflammatory cytokines from sebocytes in vitro. J Drugs Dermatol. 2019;18(5):412–418.
2. Zouboulis CC, Rabe T. Hormonal antiandrogens in acne treatment. J Dtsch Dermatol Ges. 2010;8(1):S60–S74. doi:10.1111/j.1610-0387.2009.07171.x
3. Harper JC. Should dermatologists prescribe hormonal contraceptives for acne?. Dermatol Ther. 2009;22(5):452–457. doi:10.1111/j.1529-8019.2009.01261.x
4. Trivedi MK, Shinkai K, Murase JE. A review of hormone-based therapies to treat adult acne vulgaris in women. Int J Women’s Dermatology. 2017;3(1):44–52. doi:10.1016/j.ijwd.2017.02.018
5. Thorneycroft IH, Stanczyk FZ, Bradshaw KD, Ballagh SA, Nichols M, Weber ME. Effect of low-dose oral contraceptives on androgenic markers and acne. Contraception. 1999;60(5):255. doi:10.1016/s0010-7824(99)00093-1
6. Park JH, Bienenfeld A, Orlow SJ, Nagler AR. The use of hormonal antiandrogen therapy in female patients with acne: a 10-year retrospective study. Am J Clin Dermatol. 2018;19(3):449–455. doi:10.1007/s40257-018-0349-6
7. Maloney JM, Dietze P, Watson D, et al. Treatment of acne using a 3-milligram drospirenone/20-microgram ethinyl estradiol oral contraceptive administered in a 24/4 regimen: a randomized controlled trial. Obstet Gynecol. 2008;112(4):773–781. doi:10.1097/AOG.0b013e318187e1c5
8. Zaenglein AL, Pathy AL, Schlosser BJ, et al. Guidelines of care for the management of acne vulgaris. J Am Acad Dermatol. 2016;74(5):945–973.e33. doi:10.1016/j.jaad.2015.12.037
9. Goodfellow A, Alaghband-Zadeh J, Carter G, et al. Oral spironolactone improves acne vulgaris and reduces sebum excretion. Br J Dermatol. 1984;111(2):209–214. doi:10.1111/j.1365-2133.1984.tb04045.x
10. Muhlemann MF, Carter GD, Cream JJ, Wise P. Oral spironolactone: an effective treatment for acne vulgaris in women. Br J Dermatol. 1986;115(2):227–232. doi:10.1111/j.1365-2133.1986.tb05722.x
11. Charny JW, Choi JK, James WD. Spironolactone for the treatment of acne in women, a retrospective study of 110 patients. Int J Women’s Dermatology. 2017;3(2):111–115. doi:10.1016/j.ijwd.2016.12.002
12. Sato K, Matsumoto D, Iizuka F, et al. Anti-androgenic therapy using oral spironolactone for acne vulgaris in asians. Aesth Plast Surg. 2006;30(6):689–694. doi:10.1007/s00266-006-0081-0
13. Dhurat R, Shukla D, Lim RK, Wambier CG, Goren A. Spironolactone in adolescent acne vulgaris. Dermatol Ther. 2021;34(1):
14. Noaimi A, Al-Saadi SR. Treatment of acne vulgaris by topical spironolactone solution compared with clindamycin solution. Curēus. 2021;13(8):e17606. doi:10.7759/cureus.17606
15. Afzali BM, Yaghoobi E, Yaghoobi R, Bagherani N, Dabbagh MA. Comparison of the efficacy of 5% topical spironolactone gel and placebo in the treatment of mild and moderate acne vulgaris: a randomized controlled trial. J Dermatolog Treat. 2012;23(1):21–25. doi:10.3109/09546634.2010.488260
16. Rokni GR, Mohammadnezhad F, Saeedi M, et al. Efficacy, tolerability, and safety of montelukast versus finasteride for the treatment of moderate acne in women: a prospective, randomized, single-blinded, active-controlled trial. J Cosmet Dermatol. 2021;20(11):3580–3585. doi:10.1111/jocd.14462
17. Carmina E, Lobo RA. A comparison of the relative efficacy of antiandrogens for the treatment of acne in hyperandrogenic women. Clin Endocrinol. 2002;57(2):231–234. doi:10.1046/j.1365-2265.2002.01594.x
18. Drug bank clascoterone; 2021. Available from: https://go.drugbank.com/drugs/DB12499.
19. Center for drug evaluation and Research Application Number: 213433Orig1s000; 2021.
20. Clinicaltrials.gov. Available from: https://clinicaltrials.gov/ct2/show/NCT01631474?term=clascoterone&cond=acne&draw=2&rank=2.
21. Celasco G, Moro L, Bozzella R, et al. Biological profile of cortexolone 17α-propionate (CB-03-01), a new topical and peripherally selective androgen antagonist. Arzneimittel-Forschung. 2004;54(12):881–886. doi:10.1055/s-0031-1297043
22. Trifu V, Tiplica G, Naumescu E, Zalupca L, Moro L, Celasco G. Cortexolone 17α-propionate 1% cream, a new potent antiandrogen for topical treatment of acne vulgaris. A pilot randomized, double-blind comparative study vs. placebo and tretinoin 0·05% cream. Br J Dermatol. 2011;165(1):177–183. doi:10.1111/j.1365-2133.2011.10332.x
23. Mazzetti A, Moro L, Gerloni M, Cartwright M. Pharmacokinetic profile, safety, and tolerability of clascoterone (cortexolone 17-alpha propionate, CB-03-01) topical cream, 1% in subjects with acne vulgaris: an open-label phase 2a study. J Drugs Dermatol. 2019;18(6):563.
24. Mazzetti A, Moro L, Gerloni M, Cartwright M. A phase 2b, randomized, double-blind vehicle controlled, dose escalation study evaluating clascoterone 0.1%, 0.5%, and 1% topical cream in subjects with facial acne. J Drugs Dermatol. 2019;18(6):570.
25. Hebert A, Thiboutot D, Stein Gold L, et al. Efficacy and safety of topical clascoterone cream, 1%, for treatment in patients with facial acne: two phase 3 randomized clinical trials. JAMA dermatol. 2020;156(6):621–630. doi:10.1001/jamadermatol.2020.0465
26. Eichenfield L, Hebert A, Gold LS, et al. Open-label, long-term extension study to evaluate the safety of clascoterone (CB-03-01) cream, 1% twice daily, in patients with acne vulgaris. J Am Acad Dermatol. 2020;83(2):477–485. doi:10.1016/j.jaad.2020.04.087
27. Tanaka T, Narazaki M, Kishimoto T. IL-6 in inflammation, immunity, and disease. Cold Spring Harb Perspect Biol. 2014;6(10):a016295. doi:10.1101/cshperspect.a016295
28. Der Sarkissian SA, Sun HY, Sebaratnam DF. Cortexolone 17α‐propionate for hidradenitis suppurativa. Dermatol Ther. 2020;33(6):
29. Sun HY, Sebaratnam DF. Clascoterone as a novel treatment for androgenetic alopecia. Clin Exp Dermatol. 2020;45(7):913–914. doi:10.1111/ced.14292
30. Callender VD, Alexis AF, Daniels SR, et al. Racial differences in clinical characteristics, perceptions and behaviors, and psychosocial impact of adult female acne. J Clin Aesthet Dermatol. 2014;7(7):19–31.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.