Back to Journals » Journal of Inflammation Research » Volume 15
ANCA Associated Vasculitis Subtypes: Recent Insights and Future Perspectives
Authors Austin K, Janagan S , Wells M, Crawshaw H, McAdoo S, Robson JC
Received 11 November 2021
Accepted for publication 4 March 2022
Published 21 April 2022 Volume 2022:15 Pages 2567—2582
DOI https://doi.org/10.2147/JIR.S284768
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Ning Quan
Keziah Austin,1,* Shalini Janagan,2,* Matthew Wells,3 Helena Crawshaw,4 Stephen McAdoo,5 Joanna C Robson2,6
1Department of Rheumatology, Royal National Hospital for Rheumatic Diseases, Bath, UK; 2Department of Rheumatology, Bristol Royal Infirmary, University Hospitals Bristol and Weston NHS Foundation Trust, Bristol, UK; 3Department of Rheumatology, North Bristol NHS Trust, Bristol, UK; 4Department of Rheumatology, Gloucestershire Hospitals NHS Foundation Trust, Gloucester, UK; 5Department of Medicine, Imperial College London, London, UK; 6Faculty of Health and Applied Sciences, University of the West of England, Bristol, UK
*These authors contributed equally to this work
Correspondence: Keziah Austin, Department of Rheumatology, Royal National Hospital for Rheumatic Diseases, Bath, UK, Email [email protected]
Abstract: The ANCA associated vasculitides (AAVs) affect a range of internal organs including ear nose and throat, respiratory tract, kidneys, skin and nervous system. They include granulomatosis with polyangiitis (GPA), eosinophilic granulomatosis with polyangiitis (EGPA) and microscopic polyangiitis (MPA). The AAVs are treated with high dose glucocorticoids, immunosuppressants, and targeted biological medications. Since the 1990s classification criteria for the AAVs have been based on clinical features, laboratory tests and basic imaging; an initiative to update the classification criteria incorporating newer tests, for example, anti-neutrophil cytoplasmic antibodies (ANCA) and novel imaging techniques will be published this year. There is also evidence for classification of patients based on ANCA subtype; those with anti-proteinase 3 antibodies (PR3) or anti-myeloperoxidase antibodies (MPO) have differences in response to treatment and clinical outcomes. An update is described within this review. The pathogenesis of AAV involves necrotizing inflammation of small to medium blood vessels involving multiple immunological pathways. We present an update on emerging evidence related to auto-antibodies, complement and lymphocyte pathways. This review describes emerging treatment regimens, including evidence for plasma exchange in severe disease and the inhibitor of the complement C5a receptor (C5aR) inhibitor, Avacopan. Lastly, patient reported outcomes are key secondary outcomes in randomised controlled trials and increasingly clinical practice, we report development in disease specific and glucocorticoid-specific PROs.
Keywords: vasculitis, pathogenesis, patient-reported outcomes, epidemiology
A Letter to the Editor has been published for this article.
A Response to Letter by Dr Merkel has been published for this article.
Introduction
The anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitides (AAV) are a heterogenous group of rare systemic disorders comprising granulomatosis with polyangiitis (GPA, previously Wegener’s granulomatosis), eosinophilic granulomatosis with polyangiitis (EGPA, previously Churg-Strauss syndrome), and microscopic polyangiitis (MPA). They are characterised by necrotising inflammation of small and medium blood vessels, pauci-immune on histopathological analysis, but associated with antibodies directed at two key antigenic targets: leukocyte proteinase 3 (PR3-ANCA) and myeloperoxidase (MPO-ANCA). Patients with GPA tend to be PR3-ANCA positive, whilst MPA patients are predominantly MPO-ANCA positive, though this is not definitive and there is not a clear overlap between ANCA subtype and clinical syndrome. Clinical features are wide-ranging and varied, but affected organ systems include the kidneys, upper and lower respiratory tracts, eyes, peripheral nerves, skin and brain.1
Historically, AAV has been divided into subtypes depending on clinical phenotype. In 1990, the American College of Rheumatology published classification criteria for seven primary vasculitides including GPA (then known as Wegener’s Granulomatosis) and EGPA (then known as Churg-Strauss syndrome).2 The criteria were clinico-pathological, and were developed prior to the recognition of MPA as an AAV subtype, and prior to the discovery and widespread use of ANCA testing as a diagnostic tool.3 Subsequently, the Chapel Hill Consensus Guidelines developed AAV nomenclature to include ANCA serology,4 but with the focus remaining on traditional clinical diagnoses of GPA, EGPA and MPA.
One emerging area of thought is that, given the significant overlap in clinical phenotype between these subtypes, AAV should be classified according to ANCA (PR3 vs MPO) specificity, an aspect of the disease which is increasingly recognised as of vital importance in terms of genetics, morbidity and mortality, and relapse rates.5 This article, with a focus on both clinico-pathological and immunological AAV subtypes, will explore recent insights and future perspectives on pathogenesis, epidemiology, diagnosis, management, disease monitoring and outcomes.
Pathogenesis
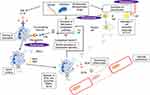
The pathogenesis of AAV has been studied, and there is understanding of disease mechanisms that are shared and distinct across disease phenotypes and serotypes. A summary of pathophysiological mechanisms is included in Figure 1.
Firstly, genetic factors have been confirmed to play an important role. Three large genome-wide association studies (GWAS) have been conducted in AAV, identifying several genetic variants in MHC and non-MHC regions that associate with disease susceptibility.6–8 These show that the different clinical phenotypes of AAV have distinct genetic backgrounds but that there are stronger genetic associations with ANCA serotype than clinical syndrome, suggesting that MPO-ANCA and PR3-ANCA may be defining different diseases (Table 1). In PR3-AAV, there were associations with polymorphisms in HLA-DP, PRTN3 (the gene encoding proteinase-3), and SERPINA1 (the gene encoding a1-antitrypsin, a circulating inhibitor of PR3), strongly implicating the autoimmune response to PR3 in disease pathogenesis. In contrast, MPO-AAV was associated mainly with HLA-DQ polymorphisms. Of note, GWAS in EGPA suggests this disease comprises two genetically and clinically distinct syndromes: MPO-ANCA +ve EGPA is an eosinophilic disorder that shares some of the vasculitic manifestations and the HLA-DQ association of MPO-AAV, whereas ANCA-negative EGPA has a distinct genetic profile associated with mucosal/barrier dysfunction. As these GWAS were conducted in patients of European descent, these findings will require validation in global populations, particularly in light of geo-ethnic variations in disease presentation and serotype.
 |
Table 1 Clinical Phenotypes, Genetic Polymorphisms and Biomarkers Associated with PR3 and MPO ANCA Subtypes |
Environmental factors may have a causal relationship with AAV including infections, harmful microparticles and drugs. The earliest reported cases of AAV were linked to Ross river virus.9 Staphylococcus aureus produces toxic shock syndrome toxin 1 (TSST1) that can exacerbate GPA and provides complementary PR3 or mimicry PR3 peptide, leading to PR3-ANCA.10 Several harmful microparticles such as silica, asbestos, and metal may trigger AAV,11,12 with silica of particular interest.13 Drugs such as hydralazine, minocycline, propylthiouracil, and levamisole-contaminated cocaine are associated with the onset of AAV,14–17 potentially via the induction of neutrophil extracellular traps (see below).18 There are studies following Japanese earthquakes which have suggested an increased incidence of MPO-ANCA-associated vasculitis in subsequent years; one study describes a doubling of MPO-ANCA vasculitis incidence following the Great East Japan Earthquake.19 Moreover, patients post-earthquake demonstrated a more severe phenotype with worse disease severity indices. Additionally, further weight has been added to the idea that cigarette smoking may be associated with development of AAV in a US case-control study of 473 AAV cases, which showed that smoking was associated with increased odds of having AAV. The association was particularly strong amongst MPO-ANCA positive patients.20
The hallmark of AAV remains the ANCAs. ANCAs are autoantibodies directed against cytoplasmic antigens expressed in the primary granules of neutrophils and lysosomes of monocytes, specifically against MPO and PR3.1 In general, P-ANCA recognises MPO and C-ANCA recognizes PR3. PR3-ANCA are most commonly associated with GPA (65%), whereas MPO-ANCA are more commonly associated with MPA (60%) or renal-limited vasculitis (80%)21 (Table 1). Atypical ANCAs, which are not directed against either PR3 or MPO (but detectable by IIF), can be found in a range of non-vasculitic conditions (inflammatory bowel disease, autoimmune disease, and malignancy).22–24
Several clinical and experimental observations support a pathogenic role for ANCA. In particular, MPO-ANCA are convincingly shown to enhance leukocyte-endothelial cell interactions, and to induce glomerulonephritis, in rodent models.25 Animal models of PR3-ANCA have been less forthcoming, which may reflect underlying differences in disease pathogenesis, though a proof-of-concept study using mice reconstituted with a humanised immune system indicate the PR3-ANCA may have pathogenic potential in vivo.26
In humans, the epitope specificity of MPO-ANCA determines their pathogenicity, and may explain why some patients may remain ANCA positive in the absence of clinically active disease. The pathogenic epitopes within PR3-ANCA are not yet clearly defined.27
The widely accepted mechanism of ANCA-mediated injury includes exposure of neutrophils to pro-inflammatory cytokines (eg interleukin (IL)-1β and tumor necrosis factor (TNF)-α) causing translocation of the ANCA-autoantigens (Ags) to the cell surface (“priming”).28 Binding of ANCA causes neutrophil activation, resulting in several pro-inflammatory responses, including the production of reactive oxygen species (ROS), lytic enzymes, matrix metalloproteinases, and NETs, which can cause vascular damage.29
NETs represent a unique cell-death mechanism of neutrophils, an innate defence mechanism to trap and kill invading microbes, in which there is extracellular release of chromatin fibres and antibacterial cytoplasmic proteins, including the ANCA-autoantigens PR3 and MPO.30 NETs are immunogenic and participate in the tolerance breakdown toward the ANCA autoantigens.31,32 This may account for the association of some infections with triggered AAV onset. The production of NETs also leads to damage of endothelial cells and may participate in activation of the alternative complement pathway.33,34
The complement system was originally thought not to play a part in AAV due to paucity of complement deposition in vessel walls in ANCA vasculitis and glomerulonephritis as compared to the significant deposition of complement seen with immune complex disease and anti-glomerular basement membrane disease.35,36 Xiao et al hypothesized through murine models that ANCA-induced activation of neutrophils results in the release of factors that activate the alternative complement pathway amplification loop, leading to recruitment and activation of more neutrophils, resulting in the severe necrotizing leukocytoclastic inflammation that is characteristic of acute ANCA disease.37
More recently, the important role of C5a/C5a receptor (C5aR/CD88) interactions have been demonstrated to be important primers of neutrophils for activation by ANCA.38 C5a is a powerful chemoattractant for neutrophils, and ligation by C5a of C5aR/CD88 activates neutrophils. Oral administration of CCX168, a small molecule antagonist of human C5aR/CD88, ameliorated anti-MPO–induced necrotizing crescentic GN in mice expressing human C5aR/CD88.39 CCX168 (Avacopan) has successfully progressed to clinical trials in AAV, where it has shown potential as promise as a replacement for glucocorticoids.40,41 Complement factor H (FH) is a key regulator of the alternative complement pathway. Studies show plasma levels of FH were inversely associated with the disease activity and renal damage of AAV.42 Neutrophils bound by complement factor H inhibit ANCA-induced neutrophil activation.43 However, FH from patients with active AAV exhibited a deficient ability in inhibiting ANCA-induced neutrophil activation. MPO released from neutrophils upon activation by ANCA, binds to FH and inhibits the complement regulatory activity of FH. Therefore, it indicates that FH deficiency may amplify the feedback loop between activation of neutrophils and the alternative complement pathway, thus contributing to the development of AAV42.
In addition to targeting the C5a receptor on neutrophils, drugs targeting the ANCA-induced neutrophil response have shown therapeutic potential in experimental studies. These include agents targeting the ANCA autoantigen MPO; those inhibiting intracellular signalling pathways initiated by ANCA binding, such a spleen tyrosine kinase; and those aimed at preventing NETosis, with inhibitors of PAD4 (a critical enzyme in NET generation).44,45 Of note, these agents have been tested in pre-clinical models of MPO-AAV only, owing to their ready availability, and so their potential utility in PR3-AAV is less clear.
In addition to the distinct genetic and clinical features that associate with ANCA serotype, evaluation of circulating cytokines in patients with active disease suggest stronger associations of selected serum biomarkers with either PR3-AAV or MPO-AAV, than with clinical phenotype of GPA or MPA (Table 1). PR3-AAV had higher levels of 9 proteins (IL-6, GM-CSF, IL-15, IL-18, CXCL8/IL-8, CCL17/TARC, IL-18BP, sIL-2Rα, NGFβ) compared to MPO-AAV, whereas MPO-AAV had higher levels of 4 proteins (sIL6R, sTNFRII, NGAL, sICAM-1) compared to PR3-AAV.46 In contrast, when patients were classified according to the clinical diagnosis with either GPA or MPA, there was less association noted with the same biomarkers: serum levels of 6 markers were higher in GPA compared to MPA (IL-6, GM-CSF, IL-15, IL-18, sIL-2Ra, NGFβ), and 3 were higher in MPA compared to GPA (Osteopontin, sTNFRII, NGAL). Differences in these circulating immune mediators were more strongly associated with ANCA specificity than with clinical diagnosis, suggesting that the heterogeneity in the AAV subtypes extends beyond the clinical phenotypes identified by the conventional clinical classification (GPA versus MPA).46
Though AAV is primarily an antibody-associated disease, it is important to consider the pathogenic role for T cell responses in AAV. Persistent T cell activation, especially of Th17 cells, contributes to the autoimmune pathogenesis of AAV through malfunctioning of the T-reg compartment resulting in failure to suppress the activation of autoAg-specific T cells.28,47 However, the mechanisms that initiate and maintain T cell activation in AAV remain unclear.
Epidemiology
Studying the epidemiology of ANCA-associated vasculitis has historically been challenging due to the rarity of the conditions and lack of diagnostic criteria. The presence of vasculitis mimics also makes diagnosis for the purpose of epidemiological study challenging – for example one study found that 21% of patients with non-vasculitis diseases fulfilled the 1990 ACR classification criteria.48
Whilst AAV remains a rare autoimmune disease, there has been a trend towards increasing reported incidence of AAV over time. In the 1980s, the mean pooled incidence rate for GPA was 4.65/million, increasing to 8.33/million in the 1990s and 9.2/million in the 2000s.49 Though less studied, incidence rates of MPA have also risen with time. It is unclear whether increased AAV rates reflect a true increase in incidence, or whether increased clinician awareness, diagnostic capability with the advent of ANCA testing, and improving classification and diagnostic criteria are bigger factors.
Incidence also appears to increase with age, and the peak age at onset has also shifted to an older age bracket than previously described. UK data between 1988–1997 described the peak age for primary systemic vasculitis (including PAN as well as AAV) as 65–74.50 A recent study in the UK Midlands demonstrated increasing incidence rates with age, with an incidence rate ratio of 31.1 for the >85 year age group compared with the 16–39 age group.51
Geographic variations have long been described in AAV, which may be a combination of genetic and ancestral factors, and the effect of location in terms of latitude and UV radiation. Variations in the incidence of PR3-ANCA and MPO-ANCA vasculitis with latitude are well documented, with Northern Hemisphere rates of GPA higher in more northerly locations, and MPA more common towards the equator, for example in Southern Europe, China and Japan.52–55 A large multicentre European study describes increasing PR3-ANCA positivity with increasing latitude and decreasing UV radiation.56 MPO-ANCA vasculitis appears significantly more common in Japan compared with the UK, as well as in Chinese and Southern European populations.53,54 Seasonality has been repeatedly reported as a factor in AAV epidemiology, with a higher incidence during winter months.55,57
It has been previously suggested that ANCA-associated vasculitis is more prevalent in populations with European ancestry and less common in black and minority ethnic groups. However, a UK study from a multi-ethnic population has shown no significant difference in age- and sex-matched incidence between white and black and minority ethnic populations.51
Clinical Features and Disease Associations
The clinical features of the three AAV clinico-pathologic subtypes – MPA, GPA and EGPA – have been clearly described. In addition to general features such as constitutional symptoms and purpuric skin rash, the more common disease specific manifestations are summarised in Table 2.
 |
Table 2 Core Clinical Features of MPA, GPA and EGPA |
Interestingly, age at AAV onset has been recently shown to associate with clinical features as well as outcomes 6 months after onset. A study of 1338 patients enrolled in the Diagnostic and Classification Criteria for Primary Systemic Vasculitis (DCVAS) study showed several differences between younger (under 65 years) and older (65 years and above) AAV cohorts. Younger patients were more likely to have MSK, cutaneous and ENT involvement. Older patients were more likely to have systemic, neurologic, cardiovascular, renal involvement and higher damage indices. Older patients were more likely to be MPO-ANCA positive, and older age was an independent risk factor for death within 6 months.58
In recent years, further work has explored the relationship between ANCA subtype and clinical manifestations. Some have concluded that ANCA subtype does not significantly influence clinical features, for example a pooled analysis of GPA patients concluded there were no important clinical differences in terms of disease manifestations and rates of relapse when comparing MPO-ANCA positive GPA patients and PR3-ANCA positive GPA patients.59 Risk of relapse was associated more strongly with disease type (ie GPA vs MPA) than ANCA subtype.
However, other studies have recently explored and described possible links between ANCA subtype and clinical features. One retrospective cohort study from a German vasculitis centre found that patients who had MPO-ANCA positive GPA phenotype were more likely to have limited disease without severe organ involvement, compared with PR3-ANCA positive GPA patients and MPO-ANCA positive MPA patients.60 The same cohort had higher prevalence of subglottic stenosis, but a lower overall need for aggressive immune suppression including cyclophosphamide and rituximab regimens. Similar observations have been made in a large Chinese study, which observed that renal involvement in MPO-ANCA positive patients was significantly higher in those with an MPA phenotype rather than a GPA phenotype.61 This adds weight to the argument that AAV should be classified according to ANCA subtype in addition to clinical phenotype, since MPO-ANCA GPA seems to be a unique subset with milder (in particular renal) disease.
In terms of renal disease, prevalence was historically thought to be similar in PR3-positive and MPO-positive patients. A recent US study has described a higher risk of end-stage renal failure in PR3-ANCA positive patients, but that renal involvement as a whole was commoner in MPO-ANCA positive patients, who had overall lower eGFR at presentation62 – the latter observation is consistent with other recent studies in which MPO-ANCA is associated with risk of renal disease.63,64
Cutaneous manifestations in AAV – most frequently petechiae and purpura – are well described, but manifestations according to ANCA subtype and AAV phenotype less well. Recent work has explored skin disease within AAV in more detail, as well as its prognostic implications: those who are PR3-ANCA positive or ANCA negative, or have an GPA or EGPA (rather than MPA) phenotype, and who also have skin involvement, have been found to be more likely to have severe systemic manifestations such as alveolar haemorrhage and glomerulonephritis.65,66
Pulmonary involvement may also relate to ANCA subtype. A 2017 study of 140 GPA and MPA patients described pulmonary involvement on CT scans performed at or after the onset of AAV. Central airways disease was more prevalent in PR3-ANCA positive patients, whilst features more common in MPO-ANCA positive patients include usual interstitial pneumonia and bronchiectasis.67 Further work has emphasised the association between bronchiectasis and MPO-ANCA positivity, and described an association between MPO-positive AAV patients with bronchiectasis and increased frequency of peripheral nerve involvement (but reduced frequency of renal involvement).68
In terms of comorbidities, a UK case-control study has identified associations with pre-existing bronchiectasis and other autoimmune diseases in patients with GPA.69
Morbidity, Mortality and Co-Morbidities
Despite progressively falling mortality rates in AAV,70 long term outcomes in the AAVs remain suboptimal. A recent meta-analysis has indicated a 2.7-fold increase in mortality in patients with AAV compared with the general population.71 GPA alone conferred a similar mortality risk at 2.63-fold, mortality risk was highest in older cohorts, and there has been a trend towards improvement over time.
Further studies have attributed premature mortality in AAV to cardiovascular disease (CVD), infection, malignancy and renal causes of death. In terms of specific ANCA subtypes, a recent cohort study in the USA determined standardised mortality ratios (SMRs) for AAV patients, and specifically compared MPO-ANCA positive and PR3-ANCA positive cases. Both subsets’ highest SMR (compared with the general population) was for infection, and both had elevated SMR for malignancy. MPO-ANCA patients (when compared with PR3-ANCA patients) had a higher risk of death attributed to CVD (adjusted hazard ratio 5.0).72
There is also significant morbidity attached to AAV as a whole, which until recently has been poorly quantified. A systematic review comprising 1375 studies and almost 14,000 AAV patients showed a relative risk of 1.65 for all cardiovascular events, 1.60 for ischaemic heart disease, and 1.20 for cerebrovascular accidents.73
Malignancy risk in AAV has long been accepted to be higher than the general population, largely attributed to cyclophosphamide exposure,74,75 with malignancy risk in rituximab-treated patients largely similar to the general population.76 There has been debate as to whether AAV patients are truly at higher risk of solid organ malignancies, or whether cancer rates are driven by non-melanoma skin cancer. Some studies have concluded that the risk of non-skin cancers in AAV is non-significant,77 whilst others suggest that rates of solid organ malignancies such as pancreatic and bladder cancers are significantly elevated in AAV patients over long-term follow up.78 A large nationwide study in Korea has more recently explored cancer risk further. This propensity-matched study of 1982 patients with AAV showed an overall increased cancer incidence in patients with AAV, with a hazard ratio 1.32. AAV patients were at increased risk of haematological, skin, bladder and lung cancers.79 Cancer risk was higher in GPA than MPA or EGPA. Older age at diagnosis as well as cyclophosphamide use were other variables independently associated with increased risk. Rituximab use was not associated with increased risk.
Recent studies have demonstrated an association between AAV and venous thromboembolism (VTE), with incidence of VTE in AAV patients around three times higher than in the general population.80 A more recent observational study confirmed high rates of VTE in AAV patients, and described disease activity (measured by BVAS) and age as independent predictors of AAV-related VTE.81 MPO-ANCA positivity showed a positive trend with VTE, but did not reach statistical significance. Furthermore, a large multinational study has recently shown high rates of VTE within the initial phase of active disease, but did not indicate any difference between GPA, MPA and EGPA.82 Neutrophil extracellular traps and associated tissue factor have been postulated as a possible mechanism for the increased propensity to VTE in AAV patients.83
Diagnosis and Classification
Diagnosis
Initial presentation of AAV is often non-specific with constitutional symptoms before the development of organ-specific features. This can cause diagnostic delay, with the median time to diagnosis of vasculitis being seven months in one 2021 cohort.84
The diagnostic process is dependent upon recognition of a clinical phenotype, exclusion of vasculitis mimics, and assessment of the extent of disease. This can help identify an appropriate site for biopsy. The aim should always be to gain timely serological, imaging and histological evidence of AAV prior to commencing treatment (for example glucocorticoids) to maximise yield and sensitivity of tests.85 When indirect immunofluorescence (IIF) is combined with ELISA testing e.g. a cANCA pattern with confirmatory ELISA for PR3, ANCA testing is highly specific.86 More recently, however, recommendations have changed to encourage use of antigen-specific assays for PR3- and MPO-ANCA first line over indirect immunofluorescence due to their high diagnostic performance, and large variability between two tested IIF methods.85
International consensus guidelines recommend testing ANCA in any patient with clinical features suggesting AAV, as well as any patient with anti-GBM disease or idiopathic interstitial pneumonia. Routine testing is not recommended in other conditions, with some exceptions, for example atypical ANCA testing by IIF in case of diagnostic uncertainty to help differentiate ulcerative colitis from Crohn’s disease.87
Histopathological findings of necrotising vasculitis in any organ or pauci-immune glomerulonephritis are the most specific findings for AAV but diagnostic utility varies from tissue to tissue. Renal biopsy is most sensitive and specific in GPA and lung biopsy in EGPA.88,89 ENT biopsy specimens may only show acute or chronic inflammatory features, with more specific findings of vasculitis and granulomas evident in 21% of GPA specimens and necrotizing vasculitis or eosinophilic granuloma evident in under 10% of EGPA specimens.90 Whilst not specific, the exclusion of immune complexes or vascular IgA deposition on immunofluorescence of tissue specimens can rule out other forms of small vessel vasculitis.
Classification
The American College of Rheumatology (ACR) first published classification criteria for systemic vasculitis including GPA and EGPA in 1990.91 These have served to identify homogenous cases of vasculitis for recruitment into research studies and clinical trials from amongst a disease population that is otherwise heterogeneous in terms of clinical features and outcomes.92 Classification criteria are traditionally weighted toward a high specificity over sensitivity and are not intended for routine clinical or diagnostic use.93 Conversely, diagnostic criteria are intended for clinical use and should have a high positive predictive value of disease with both high specificity and sensitivity.93 This is challenging to develop for conditions such as vasculitis that lack a “gold standard” test for diagnosis.
In 2010, the European League Against Rheumatism (EULAR) published on points to consider for the future development of classification criteria for vasculitis.94 Some shortcomings in the 1990 criteria were identified; MPA was not recognised as a specific condition having been formally defined in 199495 and ANCA serology was not incorporated into the GPA or MPA criteria but is now a key investigation.96 Reflecting this, when applied to a contemporary patient cohort, the 1990 classification criteria performed poorly with one third of patients not correctly classified, particularly in those with GPA or EGPA.97 Finally, advances in both modern imaging technology, for example the routine use of computer tomography, temporal artery ultrasounds and positron emission tomography (PET) scans, and statistical methodology have highlighted the need for updated classification criteria for all forms of primary systemic vasculitis.
The DCVAS collaboration is an international project funded by the ACR and EULAR, established with the aim of publishing updated diagnostic and classification criteria for primary systemic vasculitis, including AAV.98 Final data was submitted for 6991 patients from 136 sites in 32 countries.99 The data has been used to develop and validate new classification criteria for GPA, EGPA and MPA, which has aimed to incorporate clinical, serological, cross sectional radiological and histological findings. The criteria will however be for classification, and should only be applied in patients who have had a diagnosis of small to medium vessel-vasculitis made, after all potential vasculitis mimics have been excluded. The AAV classification criteria was published in 2022.100,101,102
Management
AAV can cause organ and life-threatening disease. Treatment involves immunosuppressive medication with potential for significant adverse effects. Treatment can be divided into two main phases, induction of remission (in new or relapsing disease) and maintenance of remission. Current recommended management is summarised in Table 3.
 |
Table 3 Summary of ACR 2021 and EULAR 2017 Guidelines for the Management of AAV |
Historically patients with GPA/MPA and positive PR3/MPO have been grouped together for clinical trials. Emerging evidence shows influence of serotype on disease outcomes eg PR3-ANCA cases responded better to Rituximab and MPO-ANCA cases tended to have better responses to avacopan, leading to the exciting prospect of whether trials should explore “personalised” treatment according to ANCA subtype.41,103
Remission Induction
The EULAR recommendations for remission induction include glucocorticoids (GC) plus cyclophosphamide (CYC) or Rituximab (RTX) in organ or life threatening disease.85 Methotrexate (MTX) or mycophenolate mofetil (MMF) are recommended for non-organ threatening disease. In 2021, the American College of Rheumatology (ACR) published treatment guidelines; for patients with active, severe GPA/MPA, treatment with RTX was conditionally recommended over CYC for remission induction with reduced dose GCs to standard protocol.104 RTX is considered less toxic than CYC and is generally better-tolerated. Patients with relapsing disease and PR3-ANCA showed better outcomes on long term follow up in the RAVE trial.103
In patients with relapsing disease, a prospective observational cohort of patients enrolled into the induction phase of the RITAZAREM trial showed that rituximab in conjunction with glucocorticoids demonstrated a high level of efficacy for the reinduction of remission.105 Remission induction with MTX and GCs was preferred over other combinations in active non-severe GPA patients.104 In patients with EGPA with severe disease, ACR recommends GCs with CYC/RTX and in patients with non-severe EGPA GC with mepolizumab, MTX, Azathioprine (AZP), MMF or RTX is preferred in order of preference. Induction therapy leads to remission in 90% of patients.106
Early mortality in elderly patients with AAV was associated with severe infections in the initial phase of treatment.107 Initial high dose steroids and renal impairment at diagnosis were identified as predictive risk factors for early severe infection. Treatment with methylprednisolone (MP), as well as the total dose of MP delivered, was associated with a higher and earlier onset of infection and severe infection episodes that were particularly prominent in the first month following commencement of therapy.108 Indeed, recent trials show that a reduced-dose regimen of oral glucocorticoids was non-inferior to a standard-dose regimen and associated with fewer infections.109 The recently published LoVAS trial showed that a reduced-dose glucocorticoid plus rituximab regimen was noninferior to a high-dose glucocorticoid plus rituximab regimen with regard to induction of disease remission at 6 months in newly diagnosed elderly ANCA patients.110
A retrospective analysis of 129 sequential patients with newly diagnosed or relapsing active ANCA vasculitis showed a 3-drug regimen comprising rituximab, a 2-month course of oral, low-dose cyclophosphamide, and an accelerated prednisone taper was efficacious in inducing remission.111 A study among a cohort of patients at Hammersmith Hospital showed a combination of rituximab, intravenous cyclophosphamide and a rapidly tapered oral prednisolone was efficacious in early disease control in renal AAV and remained effective in 94% of patients at 6 months.112 Prospective studies are required to compare these regimens with current standard treatment protocols, to consider the benefits of further reduction of glucocorticoids in the management of AAV.
Studies have also investigated whether glucocorticoids can be replaced without compromising efficacy. The orally administered C5aR inhibitor avacopan can replace high-dose glucocorticoids effectively and safely in patients with AAV with added benefit by avoiding glucocorticoid side effects, and improved preservation of renal function.40 The ADVOCATE trial showed that Avacopan was noninferior but not superior to tapered prednisone with respect to remission at week 26 and was superior to prednisone with respect to sustained remission at week 52.41
EULAR recommendations suggest the use of PLEX as add-on therapy in AAV patients with severe diffuse alveolar haemorrhage (DAH) or serum creatine level of ≥ 500 mmol/L.85 PEXIVAS showed that in patients with severe ANCA-associated vasculitis plasma exchange did not result in a lower incidence of death or ESKD than no plasma exchange.109 A meta-analysis did not support the wide use of PLEX for the management of AAV, as it did not lead to decreased overall mortality rates, though there was encouraging data indicating a potentially decreased incidence of ESRD in the long-term among patients receiving PLEX.113 Significant differences in outcome were noted in a cohort of patients requiring dialysis at outset and those presenting with serum creatinine >500 μmol/L but not requiring dialysis.114 As a result, further work to explore the benefit of PLEX in patients with severe kidney involvement or pulmonary haemorrhage is warranted.
Remission Maintenance
EULAR and ACR recommendations for remission maintenance therapy are RTX, MTX, AZA, MMF or leflunomide (LEF) in GPA/MPA patients.85,104 For maintenance therapy in AAV, rituximab was superior to azathioprine at maintaining remission of ANCA associated vasculitis especially for patients with granulomatosis with polyangiitis and PR3-ANCA and this superiority lasted at 60 months follow up.115,116 MAINRITSAN 2 compared fixed dose regimen (two 500 mg doses of RTX a fortnight apart after remission induction followed by 500 mg every 6 months until month 18) with an individually tailored RTX maintenance regimen based on monitoring of ANCA and B cells and showed AAV relapse rates were not significantly different between the groups but the cumulative Rituximab dose received in the individually tailored group was much less.117 BSR guidelines recommend fixed interval dosing with RTX, either 500 mg or 1000 mg administered every 6 months for a period of 2 years.118 The REMAIN trial showed that ANCA positivity at initiation of maintenance (but not ANCA serotype) predicted subsequent relapse and a longer maintenance regimen may need to be considered for such patients.119
Novel Therapies
Several new therapeutic targets are being identified and tested in trials. A multi-centre double blinded placebo-controlled Phase 3 trial showed that in patients with relapsing or refractory EGPA, treated with mepolizumab, an anti-IL-5 monoclonal antibody, demonstrated longer remission with a lower frequency of relapse.120 An open label small prospective study of 20 patients with non-severe relapsing GPA treated with abatacept provided support for the therapeutic efficacy of abatacept in non-severe GPA.121 This has led to the ABROGATE trial which is currently underway and will conclude in 2023.122 Humanised monoclonal anti-CD52 antibodies (alemtuzumab, CAMPATH-1H) selectively deplete lymphocytes and were shown to induce remission in difficult to treat AAV patients.123 An open-label Phase IV trial, Alemtuzumab for ANCA-Associated Refractory Vasculitis (ALEVIATE) is ongoing.124 Case reports show a place for Eculizumab, a monoclonal antibody against C5 which was efficacious in rapidly inducing remission in aggressive AAV patients in whom religious beliefs prohibited the receipt of blood products precluding the use of plasma exchange and cyclophosphamide.125
These developments will hopefully lead to less toxic, patient-tailored therapy by targeting specific immune mediators, rather than the broad immunosuppressive effect of such treatments as glucocorticoids and cyclophosphamide.
Outcome Measures in AAV
Disease activity in AAV vasculitis has traditionally been monitored using clinician-based assessments including biochemical markers of inflammation or renal function and validated clinician-led assessment tools including Birmingham Vasculitis Activity Scale (BVAS)126 and the Vasculitis Damage Index (VDI).127 However, qualitative data suggests that patients with AAV can continue to report poor Health-Related Quality of Life (HRQoL) scores despite low BVAS or VDI scores. As AAV has progressed from a life-limiting illness to a chronic, relapsing and remitting condition (for many patients), the importance of HRQoL and the patient perspective has become increasingly important. In particular, the majority of patients experience profound fatigue128 which has repeatedly been found to impact significantly upon HRQoL.129,130 Increased rates of depression, anxiety and pain can also impact upon both work and social life and are important factors affecting the patient’s view of their quality of life.130
Patient-reported outcome (PRO) data are validated tools that measure a patient’s perspective of their condition. Patient-reported outcome measures (PROMs) evaluate the patients’ perceived HRQoL and health status and patients are therefore involved in the evolution of PROMs.131 PRO measures can be either generic and used across a variety of conditions or disease-specific. A recent review in 2020 found that 41% of RCTs evaluating vasculitis were now using PRO data.132
Since 2011, the OMERACT vasculitis working group has highlighted the importance of developing a disease-specific PROM in vasculitis.133 In 2018 Robson et al published the validation of the ANCA-associated vasculitis patient-reported outcomes (AAV-PRO) questionnaire.134 This is a 29-item questionnaire encompassing the impact of AAV and its associated treatments on the patients’ HRQoL. Domains include: “organ specific symptoms”, “treatment side effects” and “concerns about the future”. Younger patients and women scored higher (i.e. poorer scores) across the subscales for AAV-PRO. Participants were able to assess their own activity of the disease in around 70% of cases as “active” or “in remission”. This has recently been cross-validated in Italy and it has now been translated into 27 languages.135 AAV-PRO is therefore validated to be included in clinical trials to provide an important patient perspective. During validation of the AAV-PRO, there was a significant difference in scores between patients’ self-report of active disease versus inactive disease on all six domains on known groups analysis, indicating that the disease specific PRO may be useful as a measure of disease activity from the patients’ perspective, in addition to capturing impact on HRQoL.
Future areas of interest include treatment-specific outcome measures. In AAV, as with many autoimmune conditions, glucocorticoids are a cornerstone of treatment. Despite it being well recognised that steroids can produce a number of potential side effects136 a glucocorticoid-specific PROM is not yet in use. The OMERACT Glucocorticoid Working Group has identified this as a key area for future work137 and Bridgewater et al have recently presented a conceptual framework that will be used in the development of a steroid-specific PROM.138 This will be of particular interest for AAV patients given the frequent need for long-term steroids.
Measures of Disease Activity
PR3-ANCA serological testing may be helpful in terms of predicting relapse in AAV patients, as evidenced by a recent analysis of data from the Rituximab versus Cyclophosphamide for ANCA-associated Vasculitis (RAVE) trial. An increase in PR3-ANCA antibody level was associated with subsequent severe relapse, particularly in patients with renal involvement and alveolar haemorrhage.139 Similarly, the MAINRITSAN2 trial107 used ANCA to guide retreatment with rituximab. Additionally, a recent single centre cohort study in the Netherlands found that patients who achieved and maintained PR3-ANCA negativity after remission induction with rituximab were significantly less likely to relapse. Therefore, in this specific cohort of PR3-ANCA patients treated with rituximab particularly, there may be value in closely monitoring antibody levels.140
Conclusions
Significant developments have been made in understanding the underlying pathogenesis of AAV, and refinement of optimal treatment regimens which maximise control of active disease, whilst reducing adverse effects and impact on health-related quality of life. Advances have been made particularly in identifying the nuances between MPO- and PR3-ANCA vasculitis. Better understanding of the pathogenesis, genetics and cytokine profiles of each subtype may mean future therapies can be tailored to the patient’s specific ANCA profile as well as (or even instead of) their clinical phenotype. There may therefore be a move in practice away from broad immunosuppressive regimes, including high dose glucocorticoids and cyclophosphamide, towards more personalised management strategies. Specific ANCA subtype may influence disease monitoring, approach to (particularly cardiovascular) morbidity, and patient-reported outcome measures.
Disclosure
Keziah Austin and Shalini Janagan are co-first authors for this study. Dr Joanna C Robson reports grants and/or personal fees from Vifor Pharma, Vifor Pharma, and Vifor Pharma, outside the submitted work; and led the development of the AAV PRO, GCA PRO and Steroid PRO. The authors report no other conflicts of interest in this work.
References
1. Geetha D, Jefferson JA. ANCA-Associated Vasculitis: core Curriculum 2020. Am J Kidney Dis. 2020;75(1):124–137. doi:10.1053/j.ajkd.2019.04.031
2. Fries JF, Hunder GG, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Summary. Arthritis Rheum. 1990;33:1135–1136. doi:10.1002/art.1780330812
3. van der Woude FJ, Rasmussen N, Lobatto S, et al. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet. 1985;1(8426):425–429. doi:10.1016/S0140-6736(85)91147-X
4. Jennette JC. Overview of the 2012 revised International Chapel Hill Consensus Conference nomenclature of vasculitides. Clin Exp Nephrol. 2013;17(5):603–606. doi:10.1007/s10157-013-0869-6
5. Cornec D, Cornec-le Gall E, Fervenza FC, Specks U. ANCA-associated vasculitis - clinical utility of using ANCA specificity to classify patients. Nat Rev Rheumatol. 2016;12(10):570–579. doi:10.1038/nrrheum.2016.123
6. Li W, Huang H, Cai M, Yuan T, Sheng Y. Antineutrophil cytoplasmic antibody-associated vasculitis update: genetic pathogenesis. Front Immunol. 2021;26(12):970.
7. Merkel PA, Xie G, Monach PA, et al.; for Vasculitis Clinical Research Consortium. Identification of functional and expression polymorphisms associated with risk for antineutrophil cytoplasmic autoantibody-associated vasculitis. Arthritis Rheumatol. 2017;69(5):1054–1066. doi:10.1002/art.40034
8. Lyons PA, Rayner TF, Trivedi S, et al. Genetically distinct subsets within ANCA-associated vasculitis. N Engl J Med. 2012;367(3):214–223. doi:10.1056/NEJMoa1108735
9. Davies DJ, Moran JE, Niall JF, Ryan GB. Segmental necrotising glomerulonephritis with antineutrophil antibody: possible arbovirus aetiology? Br Med J. 1982;285(6342):606. doi:10.1136/bmj.285.6342.606
10. Popa ER, Stegeman CA, Abdulahad WH, et al. Staphylococcal toxic-shock-syndrome-toxin-1 as a risk factor for disease relapse in Wegener’s granulomatosis. Rheumatology. 2007;46(6):1029–1033. doi:10.1093/rheumatology/kem022
11. Gómez-Puerta JA, Gedmintas L, Costenbader KH. The association between silica exposure and development of ANCA-associated vasculitis: systematic review and meta-analysis. Autoimmun Rev. 2013;12(12):1129–1135. doi:10.1016/j.autrev.2013.06.016
12. Lane SE, Watts RA, Bentham G, Innes NJ, Scott DGI. Are environmental factors important in primary systemic vasculitis? A case–control study. Arthritis Rheum. 2003;48(3):814–823. doi:10.1002/art.10830
13. Giorgiutti S, Dieudonne Y, Hinschberger O, et al. Prevalence of antineutrophil cytoplasmic antibody-associated vasculitis and spatial association with quarries in a region of northeastern France: a capture-recapture and geospatial analysis. Arthritis Rheumatol. 2021;73:2078–2085. doi:10.1002/art.41767
14. Dobre M, Wish J, Negrea L. Hydralazine-induced ANCA-positive pauci-immune glomerulonephritis: a case report and literature review. Ren Fail. 2009;31(8):745–748. doi:10.3109/08860220903118590
15. Sethi S, Sahani M, Oei LS. ANCA-positive crescentic glomerulonephritis associated with minocycline therapy. Am J Kidney Dis. 2003;42(2):E27–31. doi:10.1016/S0272-6386(03)00671-1
16. Dolman KM, Gans RO, Vervaat TJ, et al. Vasculitis and antineutrophil cytoplasmic autoantibodies associated with propylthiouracil therapy. Lancet. 1993;342(8872):651–652. doi:10.1016/0140-6736(93)91761-A
17. Jin Q, Kant S, Alhariri J, Geetha D. Levamisole adulterated cocaine associated ANCA vasculitis: review of literature and update on pathogenesis. J Community Hosp Intern Med Perspect. 2018;8(6):339–344. doi:10.1080/20009666.2018.1536242
18. Nakazawa D, Tomaru U, Suzuki A, et al. Abnormal conformation and impaired degradation of propylthiouracil-induced neutrophil extracellular traps: implications of disordered neutrophil extracellular traps in a rat model of myeloperoxidase antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheum. 2012;64(11):3779–3787. doi:10.1002/art.34619
19. Takeuchi Y, Saito A, Ojima Y, et al. The influence of the Great East Japan earthquake on microscopic polyangiitis: a retrospective observational study. PLoS One. 2017;12(5):e0177482. doi:10.1371/journal.pone.0177482
20. McDermott G, Fu X, Stone JH, et al. Association of cigarette smoking with antineutrophil cytoplasmic antibody-associated vasculitis. JAMA Intern Med. 2020;180(6):870–876. doi:10.1001/jamainternmed.2020.0675
21. Hagen EC, Daha MR, Hermans J, et al. Diagnostic value of standardized assays for anti-neutrophil cytoplasmic antibodies in idiopathic systemic vasculitis: EC/BCR Project for ANCA Assay Standardization. Kidney Int. 1998;53:743–745. doi:10.1046/j.1523-1755.1998.00807.x
22. Bossuyt X. Serologic markers in inflammatory bowel disease. Clin Chem. 2006;52(2):171–181. doi:10.1373/clinchem.2005.058560
23. Weiner M, Segelmark M. The clinical presentation and therapy of diseases related to anti-neutrophil cytoplasmic antibodies (ANCA). Autoimmun Rev. 2016;15(10):978–982. doi:10.1016/j.autrev.2016.07.016
24. Philipponnet C, Garrouste C, Le Guenno G, et al. Antineutrophilic cytoplasmic antibody-associated vasculitis and malignant hemopathies, a retrospective study of 16 cases. Joint Bone Spine. 2017;84(1):51–57. doi:10.1016/j.jbspin.2016.01.012
25. Little MA, Smyth L, Salama AD, et al. Experimental autoimmune vasculitis: an animal model of anti-neutrophil cytoplasmic autoantibody-associated systemic vasculitis. Am J Pathol. 2009;174(4):1212–1220. doi:10.2353/ajpath.2009.080458
26. Little MA, Al-Ani B, Ren S, et al. Anti-proteinase 3 anti-neutrophil cytoplasm autoantibodies recapitulate systemic vasculitis in mice with a humanized immune system. PLoS One. 2012;7(1):e28626. doi:10.1371/journal.pone.0028626
27. Roth AJ, Ooi JD, Hess JJ, et al. Epitope specificity determines pathogenicity and detectability in ANCA-associated vasculitis. J Clin Invest. 2013;123(4):1773–1783. doi:10.1172/JCI65292
28. von Borstel A, Sanders JS, Rutgers A, Stegeman CA, Heeringa P, Abdulahad WH. Cellular immune regulation in the pathogenesis of ANCA-associated vasculitides. Autoimmunity Rev. 2018;17(4):413–421. doi:10.1016/j.autrev.2017.12.002
29. Nakazawa D, Shida H, Tomaru U, et al. Enhanced formation and disordered regulation of NETs in myeloperoxidase-ANCA–associated microscopic polyangiitis. J Am Soc Nephrol. 2014;25(5):990–997. doi:10.1681/ASN.2013060606
30. Brinkmann V, Reichard U, Goosmann C, et al. Neutrophil extracellular traps kill bacteria. Science. 2004;303(5663):1532–1535. doi:10.1126/science.1092385
31. Kessenbrock K, Krumbholz M, Schonermarck U, et al. Netting neutrophils in autoimmune small-vessel vasculitis. Nat Med. 2009;15(6):623–625. doi:10.1038/nm.1959
32. Sangaletti S, Tripodo C, Chiodoni C, et al. Neutrophil extracellular traps mediate transfer of cytoplasmic neutrophil antigens to myeloid dendritic cells toward ANCA induction and associated autoimmunity. Blood. 2012;11(120):3007–3018. doi:10.1182/blood-2012-03-416156
33. Saffarzadeh M, Juenemann C, Queisser MA, et al. Neutrophil extracellular traps directly induce epithelial and endothelial cell death: a predominant role of histones. PLoS One. 2012;7(2):e32366. doi:10.1371/journal.pone.0032366
34. Tsukui D, Kimura Y, Kono H. Pathogenesis and pathology of anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis. J Transl Autoimmun. 2021;4:100094. doi:10.1016/j.jtauto.2021.100094
35. Jennette JC, Wilkman AS, Falk RJ. Anti-neutrophil cytoplasmic autoantibody-associated glomerulonephritis and vasculitis. Am J Pathol. 1989;135(5):921–930.
36. Jennette JC. Implications for pathogenesis of patterns of injury in small-and medium-sized-vessel vasculitis. Cleve Clin J Med. 2002;69(Suppl2):SII33–8. doi:10.3949/ccjm.69.Suppl_2.SII33
37. Xiao H, Schreiber A, Heeringa P, Falk RJ, Jennette JC. Alternative complement pathway in the pathogenesis of disease mediated by anti-neutrophil cytoplasmic autoantibodies. Am J Pathol. 2007;170(1):52–64. doi:10.2353/ajpath.2007.060573
38. Chen M, Jayne DRW, Zhao M-H. Complement in ANCA-associated vasculitis: mechanisms and implications for management. Nat Rev Nephrol. 2017;13(6):359–367. doi:10.1038/nrneph.2017.37
39. Xiao H, Dairaghi DJ, Powers JP, et al. C5a receptor (CD88) blockade protects against MPO-ANCA GN. J Am Soc Nephrol. 2014;25(2):225–231. doi:10.1681/ASN.2013020143
40. Jayne DR, Bruchfeld AN, Harper L, et al.; for CLEAR Study Group. Randomized trial of C5a receptor inhibitor avacopan in ANCA-associated vasculitis. J Am Soc Nephrol. 2017;28(9):2756–2767. doi:10.1681/ASN.2016111179
41. Jayne DR, Merkel PA, Schall TJ, Bekker P; ADVOCATE Study Group. Avacopan for the treatment of ANCA-associated vasculitis. N Engl J Med. 2021;384(7):599–609. doi:10.1056/NEJMoa2023386
42. Chen SF, Wang FM, Li ZY, Yu F, Zhao MH, Chen M. Plasma complement factor H is associated with disease activity of patients with ANCA-associated vasculitis. Arthritis Res Ther. 2015;17(1):129. doi:10.1186/s13075-015-0656-8
43. Chen SF, Wang FM, Li ZY, Yu F, Chen M, Zhao MH. Complement factor H inhibits anti-neutrophil cytoplasmic autoantibody-induced neutrophil activation by interacting with neutrophils. Front Immunol. 2018;19(9):559. doi:10.3389/fimmu.2018.00559
44. Antonelou M, Michaëlsson E, Evans RDR, et al; for RAVE-ITN Investigators. Therapeutic myeloperoxidase inhibition attenuates neutrophil activation, ANCA-mediated endothelial damage, and crescentic GN. J Am Soc Nephrol. 2020;31(2):350–364. doi:10.1681/ASN.2019060618
45. McAdoo SP, Prendecki M, Tanna A, et al. Spleen tyrosine kinase inhibition is an effective treatment for established vasculitis in a pre-clinical model. Kidney Int. 2020;97(6):1196–1207. doi:10.1016/j.kint.2019.12.014
46. Berti A, Warner R, Johnson K, et al; for RAVE-ITN Research Group. Brief report: circulating cytokine profiles and antineutrophil cytoplasmic antibody specificity in patients with antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2018;70(7):1114–1121. doi:10.1002/art.40471
47. Abdulahad WH, Stegeman CA, Kallenberg CGM. Review article: the role of CD4+ T cells in ANCA-associated systemic vasculitis. Nephrology. 2009;14(1):26–32. doi:10.1111/j.1440-1797.2008.01069.x
48. Rao JK, Allen NB, Pincus T. Limitations of the 1990 American College of Rheumatology classification criteria in the diagnosis of vasculitis. Ann Intern Med. 1998;129(5):345–352. doi:10.7326/0003-4819-129-5-199809010-00001
49. Mohammad AJ. An update on the epidemiology of ANCA-associated vasculitis. Rheumatology. 2020;59(Suppl 3):iii42–iii50. doi:10.1093/rheumatology/keaa089
50. Watts RA, Lane SE, Bentham G, Scott DG. Epidemiology of systemic vasculitis: a ten-year study in the United Kingdom. Arthritis Rheum. 2000;43(2):414–419. doi:10.1002/1529-0131(200002)43:2<414::AID-ANR23>3.0.CO;2-0
51. Pearce FA, Lanyon PC, Grainge MJ, et al. Incidence of ANCA-associated vasculitis in a UK mixed ethnicity population. Rheumatology. 2016;55(9):1656–1663. doi:10.1093/rheumatology/kew232
52. Watts RA, Gonzalez-Gay MA, Lane SE, Garcia-Porrua C, Bentham G, Scott DG. Geoepidemiology of systemic vasculitis: comparison of the incidence in two regions of Europe. Ann Rheum Dis. 2001;60(2):170–172. doi:10.1136/ard.60.2.170
53. Pearce FA, Craven A, Merkel PA, Luqmani RA, Watts RA. Global ethnic and geographic differences in the clinical presentations of anti-neutrophil cytoplasm antibody-associated vasculitis. Rheumatology. 2017;56(11):1962–1969. doi:10.1093/rheumatology/kex293
54. Fujimoto S, Watts RA, Kobayashi S, et al. Comparison of the epidemiology of anti-neutrophil cytoplasmic antibody-associated vasculitis between Japan and the. U K Rheumatology. 2011;50(10):1916–1920. doi:10.1093/rheumatology/ker205
55. Li J, Cui Z, Long JY, et al. The frequency of ANCA-associated vasculitis in a national database of hospitalized patients in China. Arthritis Res Ther. 2018;20(1):226. doi:10.1186/s13075-018-1708-7
56. Weiner M, Bjørneklett R, Hrušková Z, et al. Proteinase-3 and myeloperoxidase serotype in relation to demographic factors and geographic distribution in anti-neutrophil cytoplasmic antibody-associated glomerulonephritis. Nephrol Dial Transplant. 2019;34(2):301–308. doi:10.1093/ndt/gfy106
57. Draibe J, Rodó X, Fulladosa X, et al.; for GLOMCAT. Seasonal variations in the onset of positive and negative renal ANCA-associated vasculitis in Spain. Clin Kidney J. 2018;11(4):468–473. doi:10.1093/ckj/sfx127
58. Monti S, Craven A, Klersy C, et al. Association between age at disease onset of anti-neutrophil cytoplasmic antibody-associated vasculitis and clinical presentation and short-term outcomes. Rheumatology. 2021;60(2):617–628. doi:10.1093/rheumatology/keaa215
59. Miloslavsky EM, Lu N, Unizony S, et al. Myeloperoxidase-antineutrophil cytoplasmic antibody (ANCA)-positive and ANCA-negative patients with granulomatosis with polyangiitis (Wegener’s): distinct patient subsets. Arthritis Rheumatol. 2016;68(12):2945–2952. doi:10.1002/art.39812
60. Schirmer JH, Wright MN, Herrmann K, et al. Myeloperoxidase-antineutrophil cytoplasmic antibody (ANCA)-positive granulomatosis with polyangiitis (Wegener’s) is a clinically distinct subset of ANCA-associated vasculitis: a retrospective analysis of 315 patients from a German vasculitis referral center. Arthritis Rheumatol. 2016;68(12):2953–2963. doi:10.1002/art.39786
61. Chang DY, Li ZY, Chen M, Zhao MH. Myeloperoxidase-ANCA-positive granulomatosis with polyangiitis is a distinct subset of ANCA-associated vasculitis: a retrospective analysis of 455 patients from a single center in China. Semin Arthritis Rheum. 2019;48(4):701–706. doi:10.1016/j.semarthrit.2018.05.003
62. Aljuhani M, Makati D, Hoff A, et al. Antibody subtypes and titers predict clinical outcomes in ANCA-associated vasculitis. Rheumatol Int. 2021;41(5):965–972. doi:10.1007/s00296-021-04802-w
63. Berti A, Cornec-le Gall E, Cornec D, et al. Incidence, prevalence, mortality and chronic renal damage of anti-neutrophil cytoplasmic antibody-associated glomerulonephritis in a 20-year population-based cohort. Nephrol Dial Transplant. 2019;34(9):1508–1517. doi:10.1093/ndt/gfy250
64. Solans-Laqué R, Fraile G, Rodriguez-Carballeira M, et al. Clinical characteristics and outcome of Spanish patients with ANCA-associated vasculitides: impact of the vasculitis type, ANCA specificity, and treatment on mortality and morbidity. Medicine. 2017;96(8):e6083. doi:10.1097/MD.0000000000006083
65. Micheletti RG, Chiesa Fuxench Z, Craven A, Watts RA, Luqmani RA, Merkel PA; for DCVAS Investigators. Cutaneous manifestations of antineutrophil cytoplasmic antibody-associated vasculitis. Arthritis Rheumatol. 2020;72(10):1741–1747. doi:10.1002/art.41310
66. Frumholtz L, Laurent-Roussel S, Aumaître O, et al.; for French Vasculitis Study Group. Clinical and pathological significance of cutaneous manifestations in ANCA-associated vasculitides. Autoimmun Rev. 2017; 16(11):1138–1146. doi:10.1016/j.autrev.2017.09.009
67. Mohammad AJ, Mortensen KH, Babar J, et al. Pulmonary involvement in antineutrophil cytoplasmic antibodies (ANCA)-associated vasculitis: the influence of ANCA subtype. J Rheumatol. 2017;44(10):1458–1467. doi:10.3899/jrheum.161224
68. Néel A, Espitia-Thibault A, Arrigoni PP, et al. Bronchiectasis is highly prevalent in anti-MPO ANCA-associated vasculitis and is associated with a distinct disease presentation. Semin Arthritis Rheum. 2018;48(1):70–76. doi:10.1016/j.semarthrit.2017.12.002
69. Pearce FA, Lanyon PC, Watts RA, et al. Novel insights into the aetiology of granulomatosis with polyangiitis-a case-control study using the clinical practice research datalink. Rheumatology. 2018;57(6):1002–1010. doi:10.1093/rheumatology/kex512
70. Scherlinger M, Mertz P, Sagez F, et al. Worldwide trends in all-cause mortality of auto-immune systemic diseases between 2001 and 2014. Autoimmun Rev. 2020;19(6):102531. doi:10.1016/j.autrev.2020.102531
71. Tan JA, Dehghan N, Chen W, Xie H, Esdaile JM, Avina-Zubietaa JA. Mortality in ANCA-associated vasculitis: ameta-analysis of observational studies. Ann Rheum Dis. 2017;76(9):1566–1574. doi:10.1136/annrheumdis-2016-210942
72. Wallace ZS, Fu X, Harkness T, Stone JH, Zhang Y, Choi H. All-cause and cause-specific mortality in ANCA-associated vasculitis: overall and according to ANCA type. Rheumatology. 2020;59(9):2308–2315. doi:10.1093/rheumatology/kez589
73. Houben E, Penne EL, Voskuyl AE, et al. Cardiovascular events in anti-neutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis of observational studies. Rheumatology. 2018;57(3):555–562. doi:10.1093/rheumatology/kex338
74. Knight A, Askling J, Granath F, Sparen P, Ekbom A. Urinary bladder cancer in Wegener’s granulomatosis: risks and relation to cyclophosphamide. Ann Rheum Dis. 2004;63(10):1307–1311. doi:10.1136/ard.2003.019125
75. Faurschou M, Sorensen IJ, Mellemkjaer L, et al. Malignancies in Wegener’s granulomatosis: incidence and relation to cyclophosphamide therapy in a cohort of 293 patients. J Rheumatol. 2008;35(1):100–105.
76. van Daalen EE, Rizzo R, Kronbichler A, et al. Effect of rituximab on malignancy risk in patients with ANCA-associated vasculitis. Ann Rheum Dis. 2017;76(6):1064–1069. doi:10.1136/annrheumdis-2016-209925
77. Holle JU, Gross WL, Latza U, et al. Improved outcome in 445 patients with Wegener’s granulomatosis in a German vasculitis center over four decades. Arthritis Rheum. 2011;63(1):257–266. doi:10.1002/art.27763
78. Heijl C, Westman K, Höglund P, Mohammad AJ. Malignancies in patients with antineutrophil cytoplasmic antibody-associated vasculitis: a population-based cohort study. J Rheumatol. 2020;47(8):1229–1237. doi:10.3899/jrheum.181438
79. Choi ST, Ahn SV, Lee PH, Moon CM. The cancer risk according to three subtypes of ANCA-associated vasculitis: a propensity score-matched analysis of a nationwide study. Semin Arthritis Rheum. 2021;51(4):692–699. doi:10.1016/j.semarthrit.2021.03.014
80. Englund M, Merkel PA, Tomasson G, Segelmark M, Mohammad AJ. Comorbidities in patients with antineutrophil cytoplasmic antibody-associated vasculitis versus the general population. J Rheumatol. 2016;43(8):1553–1558. doi:10.3899/jrheum.151151
81. Liapi M, Jayne D, Merkel PA, Segelmark M, Mohammad AJ. Venous thromboembolism in ANCA-associated vasculitis. A population-based cohort study. Rheumatology. 2021;60(10):4616–4623. doi:10.1093/rheumatology/keab057
82. Moiseev S, Kronbichler A, Makarov E, et al. Association of venous thromboembolic events with skin, pulmonary and kidney involvement in ANCA-associated vasculitis: a multinational study. Rheumatology. 2021;60(10):4654–4661. doi:10.1093/rheumatology/keab071
83. Kambas K, Chrysanthopoulou A, Vassilopoulos D, et al. Tissue factor expression in neutrophil extracellular traps and neutrophil derived macroparticles in antineutrophil cytoplasmic antibody associated vasculitis may promote thromboinflammation and the thrombophilic state associated with the disease. Ann Rheum Dis. 2014;73(10):1854–1863. doi:10.1136/annrheumdis-2013-203430
84. Sreih A, Cronin K, Shaw DG, et al. Diagnostic delays in vasculitis and factors associated with time to diagnosis. Orphanet J Rare Dis. 2021;16(1):184. doi:10.1186/s13023-021-01794-5
85. Yates M, Watts RA, Bajema IM, et al. EULAR/ ERA-EDTA recommendations for the management of ANCA associated vasculitis. Ann Rheum Dis. 2016;75(9):1583–1594. doi:10.1136/annrheumdis-2016-209133
86. Damoiseaux J, Csernok E, Rasmussen N, et al. Detection of antineutrophil cytoplasmic antibodies (ANCAs): a multicentre European Vasculitis Study Group (EUVAS) evaluation of the value of indirect immunofluorescence (IIF) versus antigen-specific immunoassays. Ann Rheum Dis. 2017;76(4):647–653. doi:10.1136/annrheumdis-2016-209507
87. Moiseev S, Cohen tervaert JW, Arimura Y, et al. 2020 international consensus on ANCA testing beyond systemic vasculitis. Autoimmun Rev. 2020;19(9):102618. doi:10.1016/j.autrev.2020.102618
88. Assarod K, Bostad L, Hammerstrom J, Jorstad S, Iverson BM. Renal histopathology and clinical course in 94 patients with Wegener's Granulomatosis. Nephrol Dial Transplant. 2001;16(5):953–960. doi:10.1093/ndt/16.5.953
89. Schnabel A, Holl-Ulrich K, Dalhoff K, Reuter M, Gross WL. Efficacy of transbronchial biopsy in pulmonary vasculitides. Eur Respir J. 1997;10(12):2738–2743. doi:10.1183/09031936.97.10122738
90. Padoan R, Campaniello D, Felicetti M, Cazzador D, Schiavon F. Ear, nose and throat in ANCA-associated vasculitis: a comprehensive review. Vessel Plus. 2021;5:41.
91. Hunder GG, Arend WP, Bloch DA, et al. The American College of Rheumatology 1990 criteria for the classification of vasculitis. Introduction. Arthritis Rheum. 1990;33(8):1065–1067. doi:10.1002/art.1780330802
92. Aggarwal R, Ringold S, Khanna D, et al. Distinctions between diagnostic and classification criteria? Arthritis Care Res. 2015;67(7):891–897. doi:10.1002/acr.22583
93. June RR, Aggarwal R. The use and abuse of diagnostic/classification criteria. Best Pract Res Clin Rheumatol. 2014;28(6):921–934. doi:10.1016/j.berh.2015.04.004
94. Basu N, Watts R, Bajema I, et al. EULAR points to consider in the development of classification and diagnostic criteria in systemic vasculitis. Ann Rheum Dis. 2010;69(10):1744–1750. doi:10.1136/ard.2009.119032
95. Jennette JC, Falk RJ, Andrassy K, et al. Nomenclature of the systemic vasculitides. Proposal of an international consensus conference. Arthritis Rheum. 1994;37(2):187–192. doi:10.1002/art.1780370206
96. Sinicio RA, Radice A. Antineutrophil cytoplasmic antibodies (ANCA) testing: detection methods and clinical application. Clin Exp Rheumatol. 2014;32(3 Suppl 82):S112–7.
97. Seeliger B, Sznajd RJC. Are the 1990 American College of Rheumatology classification criteria still valid? Rheumatology. 2017;56(7):1154–1161. doi:10.1093/rheumatology/kex075
98. Craven A, Robson J, Ponte C, et al. ACR/EULAR-endorsed study to develop Diagnostic and Classification Criteria for Vasculitis (DCVAS). Clin Exp Nephrol. 2013;17(5):619–621. doi:10.1007/s10157-013-0854-0
99. Available from: https://research.ndorms.ox.ac.uk/public/dcvas/index.php.
100. Suppiah R, Robson JC, Grayson PC, et al.2022 American College of Rheumatology/ European Alliance of Associations for Rheumatology classification criteria for microscopic polyangitis. Arthritis & Rheumatology. 2022 Mar; 74(3):400-6.
101. Grayson PC, Ponte C, Suppiah R, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for eosinophilic granulomatosis with polyangiitis. Arthritis & Rheumatology. 2022 Mar; 74(3):386-92.
102. Robson JC,Grayson PC, Ponte C, et al. 2022 American College of Rheumatology/European Alliance of Associations for Rheumatology classification criteria for granulomatosis with polyangiitis. Arthritis & Rheumatology. 2022 Mar;74(3):393-9.
103. Jones RB, Furuta S, Tervaert JWC, et al; for European Vasculitis Society (EUVAS). Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis: 2-year results of a randomised trial. Ann Rheum Dis. 2015;74(6):1178–1182. doi:10.1136/annrheumdis-2014-206404
104. Chung SA, Langford CA, Maz M, et al. 2021 American College of Rheumatology/vasculitis foundation guideline for the management of antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis Rheumatol. 2021;73(8):1088–1105. doi:10.1002/acr.24634
105. Smith RM, Jones RB, Specks U, et al. Rituximab as therapy to induce remission after relapse in ANCA-associated vasculitis. Ann Rheum Dis. 2020;79(9):1243–1249. doi:10.1136/annrheumdis-2019-216863
106. Jayne D, Rasmussen N, Andrassy K, et al.; for European Vasculitis Study Group. A randomized trial of maintenance therapy for vasculitis associated with antineutrophil cytoplasmic autoantibodies. N Engl J Med. 2003;349(1):36–44. doi:10.1056/NEJMoa020286
107. Waki D, Nishimura K, Tokumasu H, et al. Initial high-dose corticosteroids and renal impairment are risk factors for early severe infections in elderly patients with antineutrophil cytoplasmic autoantibody-associated vasculitis: a retrospective observational study. Medicine. 2020;99(8):e19173. doi:10.1097/MD.0000000000019173
108. Chanouzas D, McGregor JA, Nightingale P, et al. Intravenous pulse methylprednisolone for induction of remission in severe ANCA associated vasculitis: a multi-center retrospective cohort study. BMC Nephrol. 2019;20(1):1–8. doi:10.1186/s12882-019-1226-0
109. Walsh M, Merkel PA, Peh C-A, et al; for PEXIVAS Investigators. Plasma exchange and glucocorticoids in severe ANCA-associated vasculitis. N Engl J Med. 2020;382(7):622–631. doi:10.1056/NEJMoa1803537
110. Furuta S, Nakagomi D, Kobayashi Y, et al; for LoVAS Collaborators. Effect of reduced-dose vs high-dose glucocorticoids added to rituximab on remission induction in ANCA-associated vasculitis: a randomized clinical trial. JAMA. 2021;325(21):2178–2187. doi:10.1001/jama.2021.6615
111. Cortazar FB, Muhsin SA, Pendergraft III WF, et al. Combination therapy with rituximab and cyclophosphamide for remission induction in ANCA vasculitis. Kidney Int Rep. 2017;3(2):394–402. doi:10.1016/j.ekir.2017.11.004
112. McAdoo SP, Medjeral-Thomas N, Gopaluni S, et al. Long-term follow-up of a combined rituximab and cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis. Nephrol Dial Transplant. 2019;34(1):63–73. doi:10.1093/ndt/gfx378
113. Bellos I, Michelakis I, Nikolopoulos D. The role of plasma exchange in antineutrophil cytoplasmic antibody-associated vasculitis: a meta-analysis. Clin Rheumatol. 2021;40(4):1447–1456. doi:10.1007/s10067-020-05390-z
114. Gulati K, Edwards H, Prendecki M, et al. Combination treatment with rituximab, low-dose cyclophosphamide and plasma exchange for severe antineutrophil cytoplasmic antibody-associated vasculitis. Kidney Int. 2021;100:
115. Guillevin L, Pagnoux C, Karras A, et al.; for French Vasculitis Study Group. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. N Engl J Med. 2014;371(19):1771–1780. doi:10.1056/NEJMoa1404231
116. Terrier B, Pagnoux C, Perrodeau É, et al.; for French Vasculitis Study Group. Long-term efficacy of remission-maintenance regimens for ANCA-associated vasculitides. Ann Rheum Dis. 2018;77(8):1150–1156. doi:10.1136/annrheumdis-2017-212768
117. Charles P, Terrier B, Perrodeau É, et al; for French Vasculitis Study Group. Comparison of individually tailored versus fixed-schedule rituximab regimen to maintain ANCA-associated vasculitis remission: results of a multicentre, randomised controlled, Phase III trial (MAINRITSAN2). Ann Rheum Dis. 2018;77:1143–1149. doi:10.1136/annrheumdis-2017-212878
118. Tieu J, Smith R, Basu N, et al. Rituximab for maintenance of remission in ANCA-associated vasculitis: expert consensus guidelines. Rheumatology. 2020;59(4):e24–e32. doi:10.1093/rheumatology/kez640
119. Karras A, Pagnoux C, Haubitz M, et al. Randomised controlled trial of prolonged treatment in the remission phase of ANCA-associated vasculitis. Ann Rheum Dis. 2017;76(10):1662–1668. doi:10.1136/annrheumdis-2017-211123
120. Wechsler ME, Akuthota P, Jayne D, et al; for EGPA Mepolizumab Study Team. Mepolizumab or placebo for eosinophilic granulomatosis with polyangiitis. N Engl J Med. 2017;376(20):1921–1932. doi:10.1056/NEJMoa1702079
121. Langford CA, Monach PA, Specks U, et al.; for Vasculitis Clinical Research Consortium. An open-label trial of Abatacept (CTLA4-IG) in non-severe relapsing granulomatosis with polyangiitis (Wegener’s). Ann Rheum Dis. 2014;73(7):1376–1379. doi:10.1136/annrheumdis-2013-204164
122. US National Library of Medicine. ClinicalTrials.gov; 2017. Available from: https://clinicaltrials.gov/ct2/show/NCT02108860.
123. Walsh M, Chaudhry A, Jayne D. Long-term follow-up of relapsing/refractory anti-neutrophil cytoplasm antibody associated vasculitis treated with the lymphocyte depleting antibody alemtuzumab (CAMPATH-1H). Ann Rheum Dis. 2008;67(9):1322–1327. doi:10.1136/ard.2007.081661
124. US National Library of Medicine. ClinicalTrials.gov; 2011. Available from: https://clinicaltrials.gov/ct2/show/NCT01405807.
125. Huizenga N, Zonozi R, Rosenthal J, Laliberte K, Niles JL, Cortazar FB. Treatment of aggressive antineutrophil cytoplasmic antibody-associated vasculitis with eculizumab. Kidney Int Rep. 2019;5(4):542–545. doi:10.1016/j.ekir.2019.11.021
126. Mukhtyar C, Lee R, Brown D, et al. Modification and validation of the Birmingham Vasculitis Activity Score (version 3). Ann Rheum Dis. 2009;68(12):1827–1832. doi:10.1136/ard.2008.101279
127. Exley AR, Bacon PA, Luqmani RA, et al. Development and initial validation of the Vasculitis Damage Index for the standardized clinical assessment of damage in the systemic vasculitides. Arthritis Rheum. 1997;40(2):371–380. doi:10.1002/art.1780400222
128. Basu N, McClean A, Harper L, et al. Explaining fatigue in ANCA-associated vasculitis. Rheumatology. 2013;52(9):1680–1685. doi:10.1093/rheumatology/ket191
129. Hoffman GS, Drucker Y, Cotch MF, Locker GA, Easley K, Kwoh K. Wegener’s granulomatosis: patient-reported effects of disease on health, function, and income. Arthritis Rheum. 1998;41(12):2257–2262. doi:10.1002/1529-0131(199812)41:12<2257::AID-ART22>3.0.CO;2-K
130. Reinhold-Keller E, Herlyn K, Wagner-Bastmeyer R, et al. Effect of Wegener’s granulomatosis on work disability, need for medical care, and quality of life in patients younger than 40 years at diagnosis. Arthritis Rheum. 2002;47(3):320–325. doi:10.1002/art.10458
131. Weldring T, Smith SM. Patient-Reported Outcomes (PROs) and Patient-Reported Outcome Measures (PROMs). Health Serv Insights. 2013;4;6:61–68. doi:10.1016/0006-291x(75)90506-9
132. McClure M, Gopaluni S, Wason J, et al. 322. A randomised, double-blind, controlled, mechanistic study of rituximab and belimumab combination therapy in PR3 ANCA-associated vasculitis (COMBIVAS): study protocol. Rheumatology. 2019;58(2):322. doi:10.1093/rheumatology/kez063.046
133. Robson JC, Milman N, Tomasson G, et al. Exploration, development, and validation of patient-reported outcomes in antineutrophil cytoplasmic antibody-associated vasculitis using the OMERACT process. J Rheumatol. 2015;42(11):2204–2209. doi:10.3899/jrheum.141143
134. Robson JC, Dawson J, Doll H, et al. Validation of the ANCA-associated vasculitis patient-reported outcomes (AAV-PRO) questionnaire. Ann Rheum Dis. 2018;77:1157–1164. doi:10.1136/annrheumdis-2017-212713
135. Treppo E, Palese A, De Vita S, et al. AB0392 Preliminary validation of the Italian version of ANCA-associated vasculitis Patient-reported Outcome (AAV-PRO ita) questionnaire: fatigue and chronic pain as unmet needs by current treatments. Ann Rheum Dis. 2021;80:1224. doi:10.1136/annrheumdis-2021-eular.2123
136. Cheah JTL, Robson JC, Black RJ, et al. The patient’s perspective of the adverse effects of glucocorticoid use: a systematic review of quantitative and qualitative studies. From an OMERACT working group. Semin Arthritis Rheum. 2020;50(5):996–1005. doi:10.1016/j.semarthrit.2020.06.019
137. Black RJ, Robson JC, Goodman SM, et al. A patient-reported outcome measure for effect of glucocorticoid therapy in adults with inflammatory diseases is needed: report from the OMERACT 2016 special interest group. J Rheumatol. 2017;44(11):1754–1758. doi:10.3899/jrheum.161083
138. Bridgewater S, Dawson J, Ndosi M, et al.; for Outcome Measures in Rheumatology (OMERACT) Glucocorticoid Working Group. AB0834 Development of a conceptual framework for a patient reported outcome measure to capture patients’ perception of glucocorticoid therapy during treatment for Rheumatic diseases. Ann Rheum Dis. 2021;80:1441. doi:10.1136/annrheumdis-2021-eular.164
139. Fussner LA, Hummel AM, Schroeder DR, et al.; Rituximab in ANCA-Associated Vasculitis-Immune Tolerance Network Research Group. Factors determining the clinical utility of serial measurements of antineutrophil cytoplasmic antibodies targeting proteinase 3. Arthritis Rheumatol. 2016;68(7):1700–1710. doi:10.1002/art.39637
140. van Dam LS, Dirikgil E, Bredewold EW, et al. PR3-ANCAs predict relapses in ANCA-associated vasculitis patients after rituximab. Nephrol Dial Transplant. 2021;36(8):1408–1417. doi:10.1093/ndt/gfaa066
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.