Back to Journals » Cancer Management and Research » Volume 13
Anatomical Resection Improves Disease-Free Survival After Lung Metastasectomy of Colorectal Cancer
Authors Liu T, Chang W , Wang H, Lin Q, Wei Y , Tang W, Liu Y, Chen Y, Niu Z, Jiang Y, Ren L, Xu J
Received 6 October 2021
Accepted for publication 10 December 2021
Published 30 December 2021 Volume 2021:13 Pages 9429—9437
DOI https://doi.org/10.2147/CMAR.S341543
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Seema Singh
Tianyu Liu,1,* Wenju Chang,1,2,* Hao Wang,3,* Qi Lin,1,2,* Ye Wei,1,2 Wentao Tang,1 Yu Liu,1 Yijiao Chen,1 Zhengchuan Niu,1 Yudong Jiang,1 Li Ren,1,2 Jianmin Xu1,2
1Colorectal Cancer Center, Department of General Surgery, Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China; 2Shanghai Engineering Research Center of Colorectal Cancer Minimally Invasive Technology, Shanghai, People’s Republic of China; 3Department of Thoracic Surgery; Zhongshan Hospital, Fudan University, Shanghai, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Jianmin Xu; Li Ren
Colorectal Cancer Center, Department of General Surgery, Zhongshan Hospital, Fudan University, 180 Fenglin Road, Shanghai, 200032, People’s Republic of China
Tel/Fax +86-21-64041990
Email [email protected]; [email protected]
Purpose: This study aimed to evaluate the role of anatomical resection (AR) in lung metastasectomy (LM) of colorectal cancer (CRC) and to investigate clinically relevant prognostic factors.
Patients and Methods: The medical records of 350 consecutive patients who underwent LM of CRC from 2011 to 2019 were reviewed. The patients were designated into AR group (lobectomy and segmentectomy), and non-anatomical resection (NAR) group (wedge resection), respectively. Kaplan–Meier method was used to analyze disease-free survival (DFS), pulmonary-specific disease-free survival (PDFS) and overall survival (OS). Cox proportional hazards regression model was performed to analyze the factors associated with DFS, PDFS and OS.
Results: A total of 92 (31.2%) patients were enrolled in AR group and 203 (68.8%) in non-anatomical resection (NAR) group. AR significantly improved the 3-year DFS (64.1% vs 46.8%, HR 0.587, 95% CI 0.397– 0.867, P = 0.007) and PDFS (75.0% vs 60.1%, HR 0.565, 95% CI 0.356– 0.899, P = 0.016) compared with NAR. However, the extent of resection did not significantly impact the 3-year OS (AR 92.4% vs NAR 85.7%, HR 0.511, 95% CI 0.224– 1.165, P = 0.110). In multivariate analysis, AR was identified as a protective factor for DFS (HR 0.576, 95% CI 0.356– 0.934, P = 0.025) and PDFS (HR 0.631, 95% CI 0.409– 0.973, P = 0.037). Preoperative abnormal CA19-9 was identified as the only prognostic factor for OS.
Conclusion: AR was superior to NAR for DFS and PDFS after LM from CRC.
Keywords: pulmonary metastasis, lobectomy, wedge resection, prognosis
Introduction
The most common extra-abdominal metastasis of colorectal cancer (CRC) was lung metastasis,1 which initially appears in approximately 24.5% to 29.2% of metastatic CRC.2,3 It has been widely acknowledged that lung metastasectomy (LM) could provide survival benefits for selected CRC patients with limited metastases.4–6 However, it remains unclear whether non-anatomical resections (NAR) or anatomical resections (AR) provide better outcomes after pulmonary metastasectomy of CRC.7,8
Owing to high rate of lung recurrence, NAR (wedge resection) is preferred by many specialists for pulmonary parenchymal sparing and preservation of pulmonary function,9 which allows repeated resections. Indeed, repeated lung resection can be performed safely without increasing post-operative morbidity10 and can lead to good outcomes.11,12 However, NAR may be associated with increased risk of local recurrence.13 On the other hand, AR (segmentectomy or lobectomy) may seem to be so aggressive in most cases that it becomes a less dominant surgical approach in clinical practice. Intriguingly, some recent studies have reported that AR could be associated with lower recurrence rate and provide better survival outcomes after LM of CRC compared with NAR,14,15 especially in CRC patients harboring KRAS mutations,16 while no evidence suggesting the opposite conclusion has been reported. To date, the evidence remains too limited and lacking of high-quality clinical trials, the debate about the extent of resection in LM is still out there,17,18 which calls for more related data to answer the question.
The objective of this study was to compare the clinical outcomes between CRC patients who received NAR and AR in LM, and to investigate the factors associated with survival outcomes following LM of CRC.
Materials and Methods
Patients
We retrospectively reviewed the medical records of 350 consecutive patients who underwent LM for CRC with curative intent in Zhongshan Hospital, Fudan University from January 2011 to December 2019. Inclusion criteria of this study were as follows: the pulmonary lesions were accessed to be metastases from CRC and confirmed by at least one pathologist, the LM was performed with curative intent. The exclusion criteria included other extrahepatic metastases, palliative resection, previous history of other malignant tumors, mutation of BRAF, ablation or other palliative local treatment of the metastases, and prior LM received before the first visit.
According to the extent of resection, AR group was defined as lobectomy and segmentectomy, while NAR group was defined as wedge resection. Written informed consent was obtained from all patients before the start of the study. This study was approved by the Institutional Ethics Committee of Zhongshan Hospital, Fudan University, and was conducted in accordance with the Declaration of Helsinki.
Pulmonary Surgery
Surgical approach was determined by the thoracic surgeons based on the localization of the identified lesions and the principle of achieving an R0 resection. Specifically, wedge resection was preferentially adopted under the premise of R0 resection could be achieved. Segmentectomy and lobectomy were performed only in cases with central or large lesions that were not accessible by wedge resection.
Hilar or mediastinal lymphadenectomy was not routinely performed in our practice. In general, chest CT and PETCT scan were routinely used to evaluate the lymph node metastases prior to LM. Lymphadenectomy was performed in the presence of suspicious lymph nodes on radiographic imaging.
Follow-Up
The patients were followed up according to clinical practice guidelines. The following information was recorded: age, sex, location and staging of primary lesion, disease-free interval (DFI) length, preoperative carcino-embryogenic antigen (CEA) and cancer antigen 19-9 (CA19-9) levels, history of liver metastases, surgical approach of LM, dissection of lymph nodes, KRAS, NRAS and BRAF statues, overall survival (OS), disease-free survival (DFS) and pulmonary-specific disease-free survival (PDFS).
The KRAS/NRAS/BRAF mutation status was preferentially reviewed in pulmonary samples obtained from surgical specimens, unless the status was just detected in the primary tumor. AmoyDx™ KRAS/NRAS/BRAF Mutations Detection Kit (AmoyDx, Xiamen, China) was used to detect KRAS/NRAS/BRAF mutation status, and the detection process had been described in our previous study.19
Considering the combination of liver metastasis often occurred in our CRC patients with lung metastasis, the DFI was precisely defined in three different ways: DFI-1 was defined as the interval between the primary CRC surgery and the diagnosis of either a thoracic or a liver metastasis via imaging; DFI-2 was the interval between the primary CRC surgery and the diagnosis of a thoracic metastasis via imaging. DFI-3 was the interval from the surgery of primary colorectal lesions and liver metastases, if any, to the diagnosis of a thoracic metastasis via imaging. DFS was defined as the time from the LM to the diagnosis of recurrence/metastasis or the last follow-up. PDFS was defined as the time from the LM to lung recurrence or the last follow-up. OS was defined as the time from LM to death or the last follow-up.
Statistical Analysis
Statistical analysis was processed via SPSS software version 22.0. The Chi-square test and Fisher’s exact test were used for categorical data. Survival curves were estimated with Kaplan–Meier method and compared using Log rank test. Univariable and multivariable Cox proportional-hazards regression analyses were carried out to identify the prognostic factors for OS, DFS and PDFS, respectively. All hypothesis tests were bilateral and a P-value <0.05 was considered statistically significant. The survival curve was plotted with GraphPad Prism software (Version 8.2.1).
Results
Characteristics of Patients
A total of 295 CRC patients were enrolled in this study. Of the 295 patients, 203 (68.8%) received NAR and 92 (31.2%) received AR, including 73 (24.7%) received lobectomy and 19 (6.4%) received segmentectomy (Figure 1). The maximum diameter of pulmonary lesions of the AR group was significantly greater than that of the NAR group (P = 0.000). As for lymphadenectomy, it was performed in more patients in the AR group than in the NAR group (79.3% vs 21.2%, P = 0.000), which was attributed to the difficulty of achieving complete resection or sampling of lymph nodes via wedge resection. However, no significant difference was observed in lymph node metastasis between the two groups. Bilobar resection was performed in 26 (12.8%) patients in the NAR group and 1 (1.1%) in the AR group (P = 0.001). The KRAS/NRAS/BRAF status was reviewed in 248 patients, of which, 232 was reviewed in pulmonary specimens and 97 in primary specimens. No significant difference was observed in other parameters, and all details are listed in Table 1.
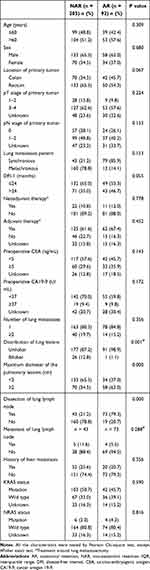 |
Table 1 Baseline Characteristics of Patients (n = 295) |
 |
Figure 1 Flowchart of the included patients. |
Disease-Free Survival
With a median follow-up of 34.6 months overall, recurrence and metastasis were observed in 37 (40.2%) patients in the AR group and 115 (56.7%) in the NAR group. The DFS was significantly improved in patients who underwent AR (median DFS not reached) when compared to who received NAR (median DFS 26.2 months, 95% CI 20.9–31.6) (Figure 2A. Log rank P = 0.009). The 3-year DFS of the AR and NAR group were 64.1% and 46.8%, respectively (HR = 0.587, 95% CI 0.397–0.867, P = 0.007). In univariate analysis, DFS was associated with DFI-3, preoperative CA19-9 level, maximum diameter of the pulmonary lesions, the history of liver metastasis and type of LM, but not some previously reported factors, such as RAS status or preoperative CEA level. In multivariate analysis, AR was the only prognostic factor of better outcome for patients (HR = 0.576, 95% CI 0.356–0.934, P = 0.025), while the history of liver metastasis was an independent prognostic factor of worse outcome (HR = 1.987, 95% CI 1.330–2.969, P = 0.001) (Table 2).
 |
Table 2 Uni- and Multivariate Analyses of DFS |
Pulmonary-Specific Disease-Free Survival
During follow-up, 27 (29.3%) patients in the AR group and 87 (42.9%) in the NAR group experienced pulmonary recurrence. The PDFS was significantly improved in the AR group compared with the NAR group (Figure 2B. Log rank P = 0.025), with the corresponding 3-year PDFS being 75.0% and 60.1%, respectively (HR 0.565, 95% CI 0.356–0.899, P = 0.016). In univariate and multivariate analyses, PDFS was associated with the history of liver metastasis and type of LM. AR was an independent prognostic factor of prolonged PDFS (HR = 0.631, 95% CI 0.409–0.973, P = 0.037). The history of liver metastasis was an independent prognostic factor of worse outcome (HR = 1.563, 95% CI 1.048–2.332, P = 0.028) (Table 3).
 |
Table 3 Uni- and Multivariate Analyses of PDFS |
Overall Survival
A total of 43 deaths occurred during follow-up, including 10 (10.9%) in the AR group and 33 (16.3%) in the NAR group. The extent of resection in LM did not significantly affect the OS (Figure 2C. Log rank P = 0.222). The 3-year OS in the AR group and the NAR group were 92.4% and 85.7%, respectively (HR = 0.511, 95% CI 0.224–1.165, P = 0.110). In univariate analysis, preoperative abnormal CA19-9 level, KRAS status, the history of liver metastasis and pulmonary lymph node metastasis were significantly associated with worse OS, while in multivariate analysis, only preoperative abnormal CA19-9 level was identified (HR = 3.522, 95% CI 1.430–8.678, P = 0.006) (Table 4).
 |
Table 4 Uni- and Multivariate Analyses of OS |
Discussion
In the era of precision therapy, there has been much debate on whether AR or NAR in LM of CRC should be endorsed to achieve better outcomes.7,8 This study indicated that compared with NAR, AR was associated with better DFS and PDFS following LM of CRC patients, while no statistically significant difference was observed on OS between the two surgical approaches.
Several studies have compared the oncological outcomes between NAR and AR, the conclusions happened to be similar with what was found in this study.14,15 For example, Shiono et al reported that segmentectomy was superior to wedge resection for lower resection-margin recurrence rate (2.0% vs 7.3%, P = 0.035) and better 5-year OS (80.1% vs 68.5%).15 The GECMP-CCR study compared major resection (lobectomy and pneumonectomy) with lesser resection (wedge resection and segmentectomy), and found that major resection could improve DFS (median not reached vs 23.9 months, HR 0.5, P < 0.001).14 On the other hand, no direct evidence in favor of NAR on this issue has been reported as far as we know. Being different from the previous studies, the present study defined AR as lobectomy and segmentectomy, which seems more reasonable as they were performed according to anatomical structure. Although the OS of AR group was higher than that of NAR group, however, no statistical difference was reach. The short follow-up time may be one of the reasons to explain this result. In addition, not all patients received adjuvant treatment in our hospital, which may affect the prognosis of CRC patients received LM.20–23
For whether to recommend AR or NAR in LM of CRC, the conclusion of this study, along with those of the several previous studies,14–16 kind of challenges the current practice. In such an era highly endorsing minimally invasive procedures in clinical practice, wedge resection is preferred whenever possible and has become the dominant surgical approach for pulmonary metastases of CRC, especially with video-assisted thoracoscopic surgery (VATS) being widely used, while segmentectomy and lobectomy are less frequently performed, according to the previous studies and our own experience.14–16 Moreover, less than lobectomy was also recommended by the Expert Consensus of the Society of Thoracic Surgeons, while in which, lobectomy was just occasionally indicated.17 Given the contradiction between the generally accepted concept and the findings of the previous studies, the debate on the extent of resection is still out there. In this case, it is of great importance to provide more evidences on this issue to make it clear.
Based on what mentioned above, for selection of AR or NAR in clinical practice, it will be worthy to step back and go deep into the fundamentals to reconsider it. For one thing, the localization of identified pulmonary metastases basically determines whether NAR can be performed, but in fact, it is difficult to assure that all the metastases have been found during the preoperative evaluation and the surgery, especially those metastasized to the lymph nodes. For another, the possibility of complete lymph node dissection is a major difference between AR and NAR, and remains a crucial issue in decision-making. It is acknowledged that lymph node metastasis of pulmonary metastases could affect prognosis, and lymph node sampling or dissection during LM has been recommended.17 In this case, NAR giving little accessibility to perform lymph node resection, seems not appropriate enough even if it seems to be able to achieve R0 resection. Besides, our data regarding lymph node sampling have also suggested some disturbing issues of NAR. As shown in Table 1, obviously more patients in the AR group received lymph node dissection than those in the NAR group, but even with less sampling, the cases with metastasis-positive lymph nodes were still more in the NAR group than in the AR group (11.6% vs 5.5%), which kind of suggests that NAR could possibly fail to eradicate all metastasis-positive lymph nodes. Taken together, we believe that in physiologically appropriate patients, AR is a better choice for LM of CRC than NAR.
Reviewing the previous literatures, we firstly noticed that the KRAS status may be the prognostic factor of LM of CRC.24,25 As reported by a multicenter retrospective study performed by Renaud et al, segmentectomy improved both the OS and time to pulmonary recurrence in LM of CRC harboring KRAS mutations.16 In the present study, KRAS mutation was found in 145 patients and NRAS mutation in 10 patients. We observed the association between KRAS mutation and OS in univariate analysis. However, multivariable analysis did not identify NRAS or KRAS status as prognosis factor of DFS, PDFS or OS. Second, the DFI was a prognostic factor in some previous studies.5,26 In this study, we calculated the DFIs using three kinds of method to explore the influence of calculation method. As a result, none of the DFIs was identified as prognostic factor in multivariable analysis. Third, the 3-year OS of all patients was 87.8%, with 92.4% in AR group and 85.7% in NAR group, which was slightly higher than previous studies.27–29 One reason to explain the better outcome was that the subjects in this study were highly selected. The subjects with extrahepatic metastasis were excluded, and all the liver metastases received R0 resection. Another reason may be the higher proportion of adjuvant chemotherapy than previous studies.27,28
A few limitations must be considered when interpreting this study. Since the present study was a retrospective study with not pre-established criteria of the type of resection, a patient selection bias could not be avoided, for example, the maximum diameter of the pulmonary metastases and the dissection of lymph node. However, the outcomes of the AR group were better than those of the NAR group despite of larger lesion was a risk factor for poor prognosis.30 The proportion of the dissection of lymph node was similar with previous studies.15,31 In addition, more bilobar resection could be found in NAR group. To preserve more pulmonary function, wedge resection could be preferred in bilobar distributed patients due to much more lung parenchyma needed to be removed by anatomical resection. However, the distribution of lung lesions did not significantly affect the survivals. With limited data and no dramatical superiority of either type of resection being reported, the research on this issue may probably remain observational on the near future, and more data need to be provided before launching a large-scale randomized controlled trial.
Conclusion
This study has provided the evidence on AR improving survival outcomes following LM in CRC patients, specifically for DFS and PDFS. However, whether AR can benefit OS remains questionable, which calls for more high-quality evidences on it.
Acknowledgments
This study was funded by the National Natural Science Foundation of China (82072678, 81602035, 81472228); Shanghai Science and Technology Committee Project (19511121301, 18140903200); Clinical Research Plan of SHDC (SHDC2020CR5006, SHDC2020CR1033B, SHDC2020CR3037B); Youth Science Fund of Zhongshan Hospital (2019ZSQN28).
Disclosure
There was no financial and personal relationship with other people and organizations that could inappropriately influence this work. The authors report no conflicts of interest in this work.
References
1. Mitry E, Guiu B, Cosconea S, et al. Epidemiology, management and prognosis of colorectal cancer with lung metastases: a 30-year population-based study. Gut. 2010;59(10):1383–1388. doi:10.1136/gut.2010.211557
2. Wang Z, Wang X, Yuan J, et al. Survival benefit of palliative local treatments and efficacy of different pharmacotherapies in colorectal cancer with lung metastasis: results From a Large Retrospective Study. Clin Colorectal Cancer. 2018;17(2):e233–e255. doi:10.1016/j.clcc.2017.12.005
3. Tampellini M, Ottone A, Bellini E, et al. The role of lung metastasis resection in improving outcome of colorectal cancer patients: results from a large retrospective study. oncologist. 2012;17(11):1430–1438. doi:10.1634/theoncologist.2012-0142
4. Mise Y, Kopetz S, Mehran RJ, et al. Is complete liver resection without resection of synchronous lung metastases justified? Ann Surg Oncol. 2015;22(5):1585–1592. doi:10.1245/s10434-014-4207-3
5. Yokoyama S, Mitsuoka M, Kinugasa T, et al. Survival after initial lung metastasectomy for metastatic colorectal cancer in the modern chemotherapeutic era. BMC Surg. 2017;17(1):54. doi:10.1186/s12893-017-0252-8
6. Moorcraft SY, Ladas G, Bowcock A, Chau I. Management of resectable colorectal lung metastases. Clin Exp Metastasis. 2016;33(3):285–296. doi:10.1007/s10585-015-9774-6
7. Vogelsang H, Haas S, Hierholzer C, et al. Factors influencing survival after resection of pulmonary metastases from colorectal cancer. Br J Surg. 2004;91(8):1066–1071. doi:10.1002/bjs.4602
8. Lin B-R, Chang T-C, Lee Y-C, et al. Pulmonary resection for colorectal cancer metastases: duration between cancer onset and lung metastasis as an important prognostic factor. Ann Surg Oncol. 2009;16(4):1026–1032. doi:10.1245/s10434-008-0286-3
9. Park JS, Kim HK, Choi YS, et al. Outcomes after repeated resection for recurrent pulmonary metastases from colorectal cancer. Ann Oncol. 2010;21(6):1285–1289. doi:10.1093/annonc/mdp475
10. Forster C, Ojanguren A, Perentes JY, et al. Is repeated pulmonary metastasectomy justified? Clin Exp Metastasis. 2020;37(6):675–682. doi:10.1007/s10585-020-10056-w
11. Hishida T, Tsuboi M, Okumura T, et al. Does repeated lung resection provide long-term survival for recurrent pulmonary metastases of colorectal cancer? Results of a Retrospective Japanese Multicenter Study. Ann Thorac Surg. 2017;103(2):399–405. doi:10.1016/j.athoracsur.2016.08.084
12. Menna C, Berardi G, Tierno SM, et al. Do repeated operations for recurrent colorectal lung metastases result in improved survival? Ann Thorac Surg. 2018;106(2):421–427. doi:10.1016/j.athoracsur.2018.02.065
13. Shiono S, Ishii G, Nagai K, et al. Predictive factors for local recurrence of resected colorectal lung metastases. Ann Thorac Surg. 2005;80(3):1040–1045. doi:10.1016/j.athoracsur.2004.12.033
14. Hernández J, Molins L, Fibla JJ, et al. Role of major resection in pulmonary metastasectomy for colorectal cancer in the Spanish prospective multicenter study (GECMP-CCR). Ann Oncol. 2016;27(5):850–855. doi:10.1093/annonc/mdw064
15. Shiono S, Okumura T, Boku N, et al. Outcomes of segmentectomy and wedge resection for pulmonary metastases from colorectal cancer. Eur J Cardiothorac Surg. 2017;51(3):504–510. doi:10.1093/ejcts/ezw322
16. Renaud S, Seitlinger J, Lawati YA, et al. Anatomical resections improve survival following lung metastasectomy of colorectal cancer harboring KRAS mutations. Ann Surg. 2019;270(6):1170–1177. doi:10.1097/SLA.0000000000002829
17. Handy JR, Bremner RM, Crocenzi TS, et al. Expert consensus document on pulmonary metastasectomy. Ann Thorac Surg. 2019;107(2):631–649. doi:10.1016/j.athoracsur.2018.10.028
18. Liao H, Xiao Z. The role of anatomic resection in pulmonary metastasectomy. Ann Thorac Surg. 2019;108(6):1925–1926. doi:10.1016/j.athoracsur.2019.02.051
19. Zheng P, Ren L, Feng Q, et al. Patients with RAS wild-type right-sided unresectable liver-confined mCRC also benefit from cetuximab plus chemotherapy in first-line treatment. Am J Cancer Res. 2018;8(11):2337–2345.
20. Cho JH, Kim S, Namgung M, et al. The prognostic importance of the number of metastases in pulmonary metastasectomy of colorectal cancer. World J Surg Oncol. 2015;13(1):222. doi:10.1186/s12957-015-0621-7
21. Brandi G, Derenzini E, Falcone A, et al. Adjuvant systemic chemotherapy after putative curative resection of colorectal liver and lung metastases. Clin Colorectal Cancer. 2013;12(3):188–194. doi:10.1016/j.clcc.2013.04.002
22. Park HS, Jung M, Shin SJ, et al. Benefit of adjuvant chemotherapy after curative resection of lung metastasis in colorectal cancer. Ann Surg Oncol. 2016;23(3):928–935. doi:10.1245/s10434-015-4951-z
23. Shiomi K, Naito M, Sato T, et al. Effect of adjuvant chemotherapy after pulmonary metastasectomy on the prognosis of colorectal cancer. Ann Med Surg. 2017;20:19–25. doi:10.1016/j.amsu.2017.06.026
24. Ghidini M, Personeni N, Bozzarelli S, et al. KRAS mutation in lung metastases from colorectal cancer: prognostic implications. Cancer Med. 2016;5(2):256–264. doi:10.1002/cam4.592
25. Renaud S, Romain B, Falcoz PE, et al. KRAS and BRAF mutations are prognostic biomarkers in patients undergoing lung metastasectomy of colorectal cancer. Br J Cancer. 2015;112(4):720–728. doi:10.1038/bjc.2014.499
26. Rapicetta C, Lococo F, Davini F, et al. Is adjuvant chemotherapy worthwhile after radical resection for single lung metastasis from colorectal cancer? A multicentric analysis evaluating the risk of recurrence. Front Oncol. 2019;9:763. doi:10.3389/fonc.2019.00763
27. Fukada M, Matsuhashi N, Takahashi T, et al. Prognostic factors in pulmonary metastasectomy and efficacy of repeat pulmonary metastasectomy from colorectal cancer. World J Surg Oncol. 2020;18(1):314. doi:10.1186/s12957-020-02076-3
28. Davini F, Ricciardi S, Zirafa CC, et al. Lung metastasectomy after colorectal cancer: prognostic impact of resection margin on long term survival, a retrospective cohort study. Int J Colorectal Dis. 2020;35(1):9–18. doi:10.1007/s00384-019-03386-z
29. Fournel L, Maria S, Seminel M, et al. Prognostic factors after pulmonary metastasectomy of colorectal cancers: a single-center experience. J Thorac Dis. 2017;9(Suppl 12):S1259–S1266. doi:10.21037/jtd.2017.04.44
30. Iizasa T, Suzuki M, Yoshida S, et al. Prediction of prognosis and surgical indications for pulmonary metastasectomy from colorectal cancer. Ann Thorac Surg. 2006;82(1):254–260. doi:10.1016/j.athoracsur.2006.02.027
31. Li H, Hu H, Li B, et al. What is the appropriate surgical strategy for pulmonary metastasis of colorectal cancer? Medicine. 2020;99(30):e21368. doi:10.1097/MD.0000000000021368
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

